Introduction
Peanut is a valuable commodity in the United States with approximately 634,800 ha harvested with an estimated value of $1.19 billion (USDA 2016a; USDA 2016b). Weeds compete with peanut for sunlight, moisture, and nutrients throughout the growing season (Wilcut et al., 1994), and negatively affect yield, quality, and economic value (Everman et al., 2008; Walker et al., 1989). Season-long interference from combinations of broadleaf and grass weeds can reduce peanut yield by 60 to 80% and can decrease harvest efficiency in some instances (Everman et al., 2008; Wilcut et al., 1994). In studies investigating the effect of season-long interference from individual weed species, common ragweed (Ambrosia artemisiifolia L.) (Clewis et al., 2001) and Palmer amaranth [Amaranthus palmeri (S.) Wats] (Burke et al., 2007) at a density of 1 plant/m of row, resulted in peanut yield losses of 40 and 28%, respectively. Competition from Texas millet [Urochloa texana (Buckl.)] and Florida beggarweed [Desmodium tortuosum (Sw.) DC.] can also severely reduce peanut yield (Buchanan et al., 1976; Cardina and Brecke, 1991; Wilcut, et al., 1987). The prostrate growth habit of peanut causes peanut to be vulnerable to interference from weeds throughout the season and requires effective season-long management to protect yield and promote efficient digging and vine inversion (Walker et al., 1989; Wilcut et al., 1994).
S-metolachlor is a chloroacetamide herbicide, labeled for either pre-plant incorporated (PPI), preemergence (PRE), postemergence (POST), or lay-by application in peanut (Anonymous, 2017a). Peanut stunting and delayed emergence with S-metolachlor has been noted in previous studies (Cardina and Swann, 1988; Clewis et al., 2007; Grichar and Dotray, 2012). Grichar and Dotray (2012) reported peanut stunting from 0 to 15% with S-metolachlor alone and in combination with paraquat, and stunting increased as application timing was delayed to 28 d compared to 7 d after peanut cracking. Peanut injury by S-metolachlor and the racemic form of metolachlor is dependent on combinations of factors, including herbicide rate, moisture conditions at planting, soil temperature, and rainfall (Cardina and Swann, 1988; Grichar et al., 2004). Cardina and Swann (1988) reported that peanut growth suppression was related to higher metolachlor rates followed by irrigation after planting. Although some level of injury was reported in peanut from metolachlor, negative effects were not observed on peanut market grades and yield unless rates higher than those recommended by the manufacturer were applied. S-metolachlor provides control of a number of troublesome annual broadleaf weeds and yellow nutsedge when applied alone or in combination with sulfentrazone, diclosulam, or flumioxazin (Clewis et al., 2007; Grichar et al., 2008).
Acetochlor is registered for use in field corn (Zea mays L.), cotton (Gossypium hirsutum L.), sorghum [Sorghum bicolor (L.) Moench.], and soybean [Glycine max (L.) Merr.] to control annual grasses and small-seeded broadleaf weeds (Anonymous, 2017b; Cahoon et al. 2015; Geier et al., 2009; Steckel et al., 2002). Acetochlor inhibits geranylgeranyl pyrophosphate cyclization enzymes, which are part of the gibberellin biosynthetic pathway and controls weeds by inhibiting growth of seedling shoots (Shaner, 2014). A microencapsulated (ME) formulation of acetochlor recently received registration for PPI, PRE, and POST application in peanut (Anonymous 2017b). In addition to improving crop safety, this formulation of acetochlor extends herbicide persistence in the soil compared with the emulsifiable concentrate formulation (Parker et al., 2005; Scher et al., 1998). Grichar et al. (2015) reported no negative effect of acetochlor ME application timing and rate on peanut grade and yield in runner and Spanish market type cultivars. Cahoon et al. (2015) documented that acetochlor ME applied PRE caused less than 8% early season injury to cotton and at least 90% control of Palmer amaranth. Early season injury caused by acetochlor was transient and did not negatively impact cotton yield.
One effective approach to manage weeds with long periods of emergence is to use sequential applications of PRE and POST herbicides. Grichar et al. (2008) reported > 80% yellow nutsedge (Cyperus esculentus L.) control at five of six locations in peanut with sequential application of diclosulam PRE followed by S-metolachlor applied POST. Control of Palmer amaranth, common lambsquarters (Chenopodium album L.), and annual morningglory (Ipomoea spp.) in peanut was improved when a POST application of imazapic plus 2,4-DB followed PRE S-metolachlor alone or in combination with diclosulam, flumioxazin, or sulfentrazone, compared with PRE- or POST-only treatments (Clewis et al. 2007). Grichar et al. (2015) reported that the addition of pendimethalin and lactofen to acetochlor, flumioxazin, or S-metolachlor programs improved weed control in peanut.
Peanut producers typically include PPI, PRE, EPOST, and POST herbicide applications in their weed management programs for effective season-long control of nutsedge, annual grass, and broadleaf weeds. Dinitroaniline herbicides, such as ethalfluralin, pendimethalin, or trifluralin, are applied PPI to control many annual grass and small-seeded broadleaf weeds (Grichar and Dotray, 2012; Wilcut et al., 1994). Diclosulam, S-metolachlor, flumioxazin, and sulfentrazone are registered PRE (Clewis et al., 2007; Jordan, 2016). In addition to these herbicides, acifluorfen, bentazon, clethodim, imazapic, imazethapyr, paraquat, sethoxydim, and 2,4-DB are used in peanut for POST weed control (Grey et al., 2003; Wilcut et al., 1994). Herbicide resistance in weeds has increased concerns about proper herbicide stewardship (Wise et al., 2009). The registration of acetochlor will increase herbicide options for weed control in peanut. Considering the need for soil-applied residual herbicides to manage weeds and the limited published information on acetochlor tolerance to peanut, field research was conducted in Georgia and North Carolina to define utility of acetochlor compared with S-metolachlor. The specific objectives of this research were: 1) to determine peanut response to the ME formulation of acetochlor with S-metolachlor when applied at different rates and timings under weed-free conditions and 2) to compare weed control with the ME formulation of acetochlor to control by S-metolachlor alone or in various herbicide programs which includes combinations with acifluorfen, bentazon, flumioxazin, imazapic, lactofen, paraquat, and pendimethalin.
Material and Methods
Field experiments were conducted in Georgia at the Ponder Research Station near Ty Ty, the Attapulgus Research and Education Center near Attapulgus, the Southwest Georgia Research and Education Center near Plains, and in North Carolina at the Peanut Belt Research Station located near Lewiston-Woodville, and the Upper Coastal Plain Research Station near Rocky Mount during 2011 and 2012. Soil was a Tifton loamy sand soil (fine-loamy, kaolinitic, thermic Plinthic Kandiudult) with 1.07 to 1.39% organic matter (OM) and pH 6.0 at Ty Ty, an Orangeburg loamy sand soil (Fine-loamy, kaolinitic, thermic Typic Kandiudult) with 1.07 to 1.19% OM and pH 6.0 at Attapulgus, and a Greenville sandy clay loam soil (Fine, kaolinitic, thermic Rhodic Kandiudult) with 1.0% OM and pH 5.9 to 6.1 at Plains. Soil at Lewiston-Woodville was a Norfolk sandy loam soil (fine-loamy, siliceous, thermic, Aquic Paleudult) with 0.8 to 1.2% OM and pH 5.9. Goldsboro loamy sand soil (fine loamy, mixed, semiactive, thermic, Typic Hapludult) with 1.4% organic matter and pH 6.1 was at Rocky Mount. Soils at these locations are representative of the southeastern United States peanut production region. Experiments were classified as specific combinations of year and/or location within each state. Experiments were conducted in conventionally-prepared, raised seedbeds. Plot size was 4 rows spaced 96-cm apart by 9 m in North Carolina and 2 rows spaced 91-cm apart by 7.6 m in Georgia. Peanut was planted at a depth of 4 to 5 cm at all locations at a rate designed to achieve final in-row populations of 4 and 6 plants/m in North Carolina and Georgia, respectively. Production, irrigation, and pest management practices other than specific treatments were standard for peanut production in Georgia and North Carolina to optimize peanut growth and development (Anonymous, 2017c; Jordan et al., 2016).
Peanut Tolerance
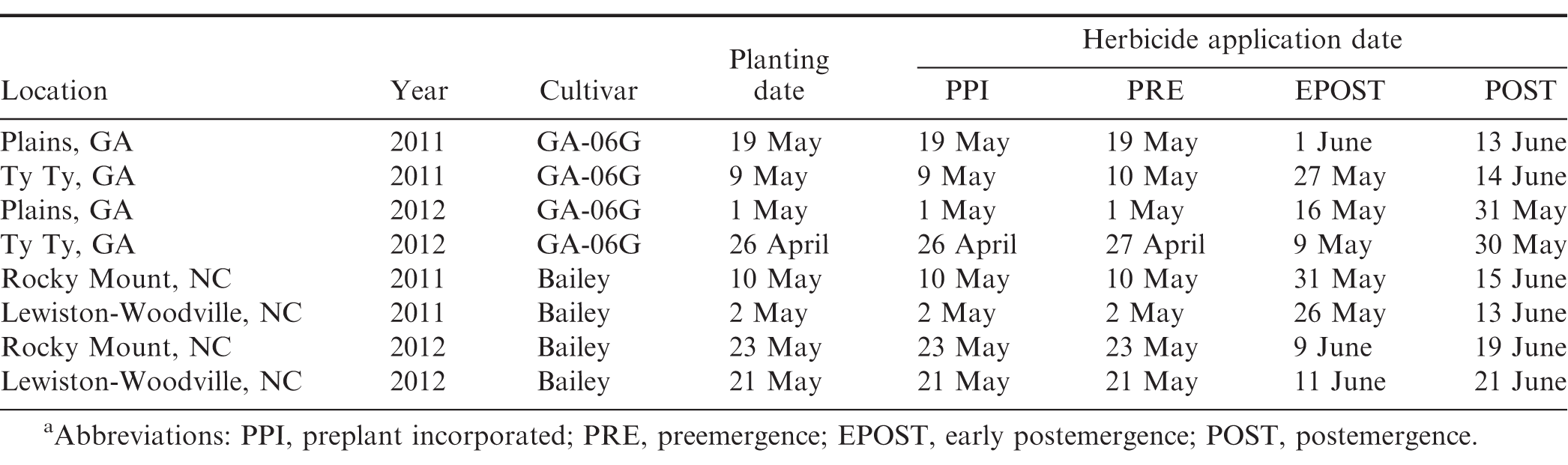
Peanut cultivars (Branch 2007; Mozingo et al. 2006; Isleib et al., 2006, 2011), planting dates, and dates of herbicide application are presented in Table 1. Treatments consisted of a factorial arrangement of 3 levels of herbicide and 4 levels of application timings. Herbicide levels included acetochlor (Warrant herbicide, Monsanto Co., St, Louis, MO) at 1.26 and 2.52 kg/ha and S-metolachlor (Dual Magnum herbicide, Syngenta Crop Protection, Greensboro, NC) at 1.42 kg/ha. These herbicides were applied PPI, PRE, EPOST, or POST. A non-treated control was also included. Herbicides were applied in water using a CO2-pressurized backpack sprayer calibrated to deliver 140 L/ha using 11002 flat fan (TeeJet Technologies, Wheaton, IL) nozzles at 150 kPa at Plains, 11002DG flat fan nozzles at 260 kPa at Ty Ty, and 8002 flat fan nozzles at 200 kPa at Lewiston-Woodville and Rocky Mount. PPI herbicides were incorporated immediately after application with a power-driven rotary tiller (Plains and Ty Ty) or with 2 passes with a field cultivator in opposite directions at Lewiston-Woodville and Rocky Mount to a depth of approximately 5 cm. Herbicides were applied PRE within 24 h after peanut planting. In Georgia, 3 cm of overhead sprinkler irrigation were applied within 1 wk after application of PRE herbicides. Peanut was not irrigated after planting in North Carolina. EPOST applications were made approximately 14 to 24 d after planting when peanut was approximately at V3 to V4 stage of growth (Boote, 1982) while POST applications were made 25 to 38 d after peanut planting when peanut was at R1 stage of growth (Boote, 1982).
All plots were kept weed-free combining hand removal with herbicides applied PRE and POST. Diclosulam (Strongarm herbicide, Dow AgroSciences, Indianapolis, IN) at 0.026 kg ai/ha, flumioxazin (Valor SX, Valent USA Corp., Walnut Creek, CA) at 0.11 kg ai/ha, or pendimethalin (Prowl H2O, BASF Corp., Research Triangle Park, NC) at 1.13 kg ai/ha were applied PRE. Clethodim (Select herbicide, Valent USA Corp., Walnut Creek, CA) at 0.13 kg ai/ha or imazapic (Cadre herbicide, BASF Corp., Research Triangle Park, NC) at 0.70 kg ai /ha with crop oil concentrate applied POST to control annual grasses and broadleaf weeds at Plains and Ty Ty. Pendimethalin at 1.1 kg ai/ha was applied PPI and clethodim at 130 g/ha, and 2,4-DB (Butyrac 200, Winfield Solutions, LLC, St. Paul, MN) at 0.28 kg ai/ha were applied POST for weed control at Lewiston-Woodville and Rocky Mount.
Experiments were conducted in a randomized complete block design with four replications. Visual estimates of peanut injury were determined 2-3 and 6-7 weeks after each application timing (WAT) using a scale of 0 to 100 where 0 = no injury and 100 = plant death. Foliar chlorosis, necrosis, leaf defoliation, and plant stunting were considered when making the visible estimates. Peanut pods were dug and vines inverted based on pod mesocarp color to obtain optimum yield (Williams and Drexler, 1981). Pod yield was determined 4 to 7 d after digging with final yield adjusted to 8% moisture content.
Weed Control
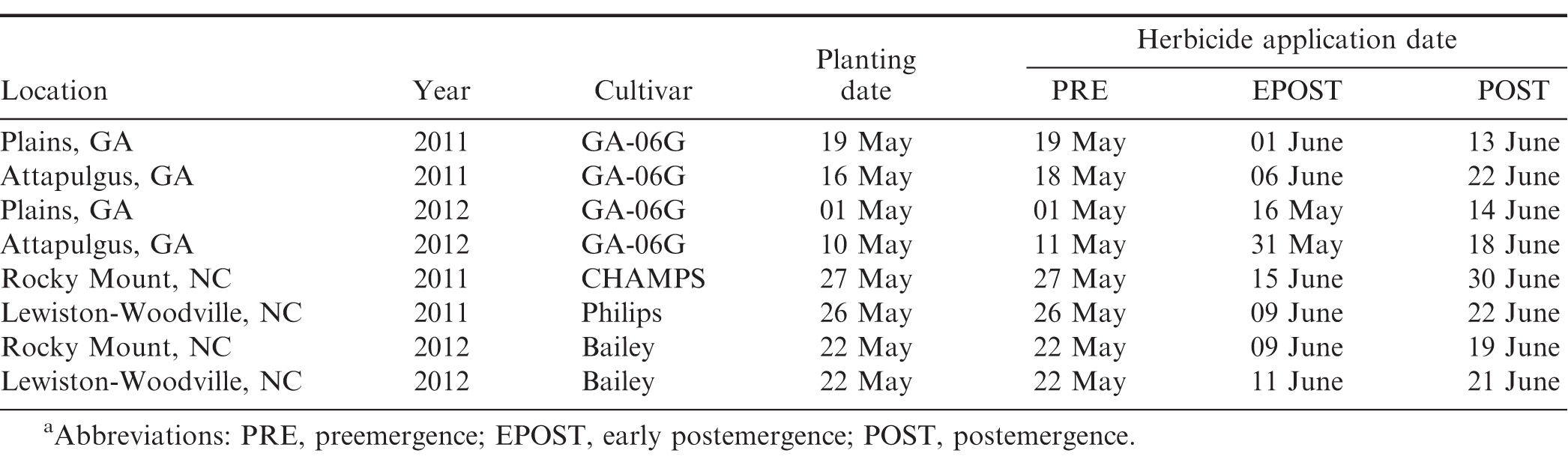
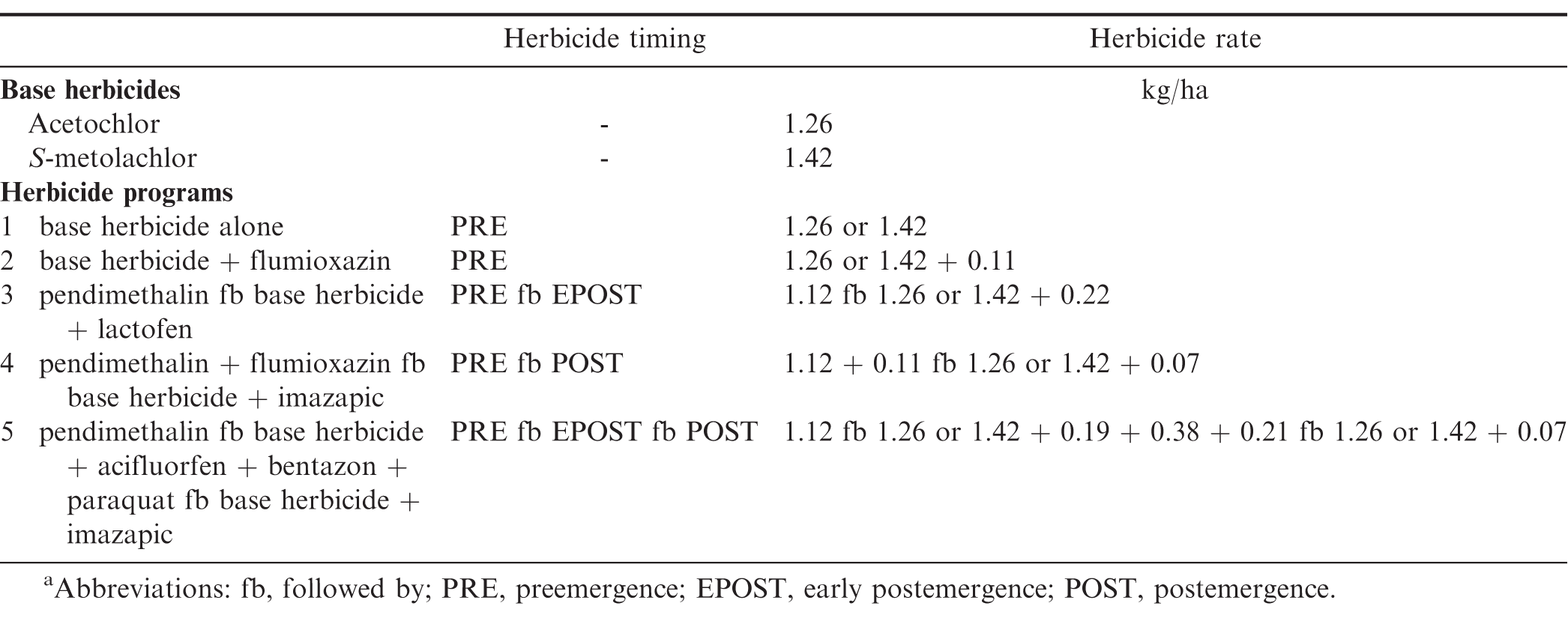
Field experiments were conducted in Georgia at Plains and Attapulgus and in North Carolina at Lewiston-Woodville and Rocky Mount during 2011 and 2012. Peanut cultivars, planting dates, and herbicide application dates are presented in Table 2. Treatments consisted of a factorial arrangement of two base herbicides (S-metolachlor and acetochlor ME) and five herbicide programs with base herbicide applied at different timings (Table 3). A non-treated control was also included. Herbicides were applied using equipment and procedures described in the study evaluating peanut response under weed-free conditions. Preemergence herbicides were applied within 24 h after peanut planting while EPOST and POST herbicides were applied approximately 15 to 22 and 25 to 38 d after peanut was planted, respectively.
Weed control was estimated visually using a scale of 0 (no weed control) to 100 (complete weed control) 14 to 69 d after peanut were planted depending on location. Peanut injury (chlorosis/stunting) was also recorded at 3 to 8 d after POST application at Plains and Lewiston-Woodville both years and Rocky Mount in 2012 while at Attapulgus both years and Rocky Mount 2011 at 8 d after EPOST herbicide application using the scale described previously. Peanut yield was determined as described previously.
Data Analysis
Data for peanut injury were transformed to the arcsine square root before analysis; however, non-transformed means are presented for clarity. Data were subjected to ANOVA using SAS PROC MIXED considering the factorial treatment arrangement (SAS Institute Inc., Cary, NC). Treatment means were separated by Fisher's Protected LSD test at p 0.05. The non-treated check was not included in the weed control or peanut injury analysis but was included in peanut yield analysis. Peanut population, visible injury, and yield data from both peanut tolerance and weed control studies were analyzed separately for Georgia and North Carolina because of variation in cultivar selection, and timing for data collection and weed spectrum. However, percent weed control data were analyzed across states and data were combined if the interaction of experiment by base herbicide by herbicide program was not significant.
Results and Discussion
Peanut Tolerance
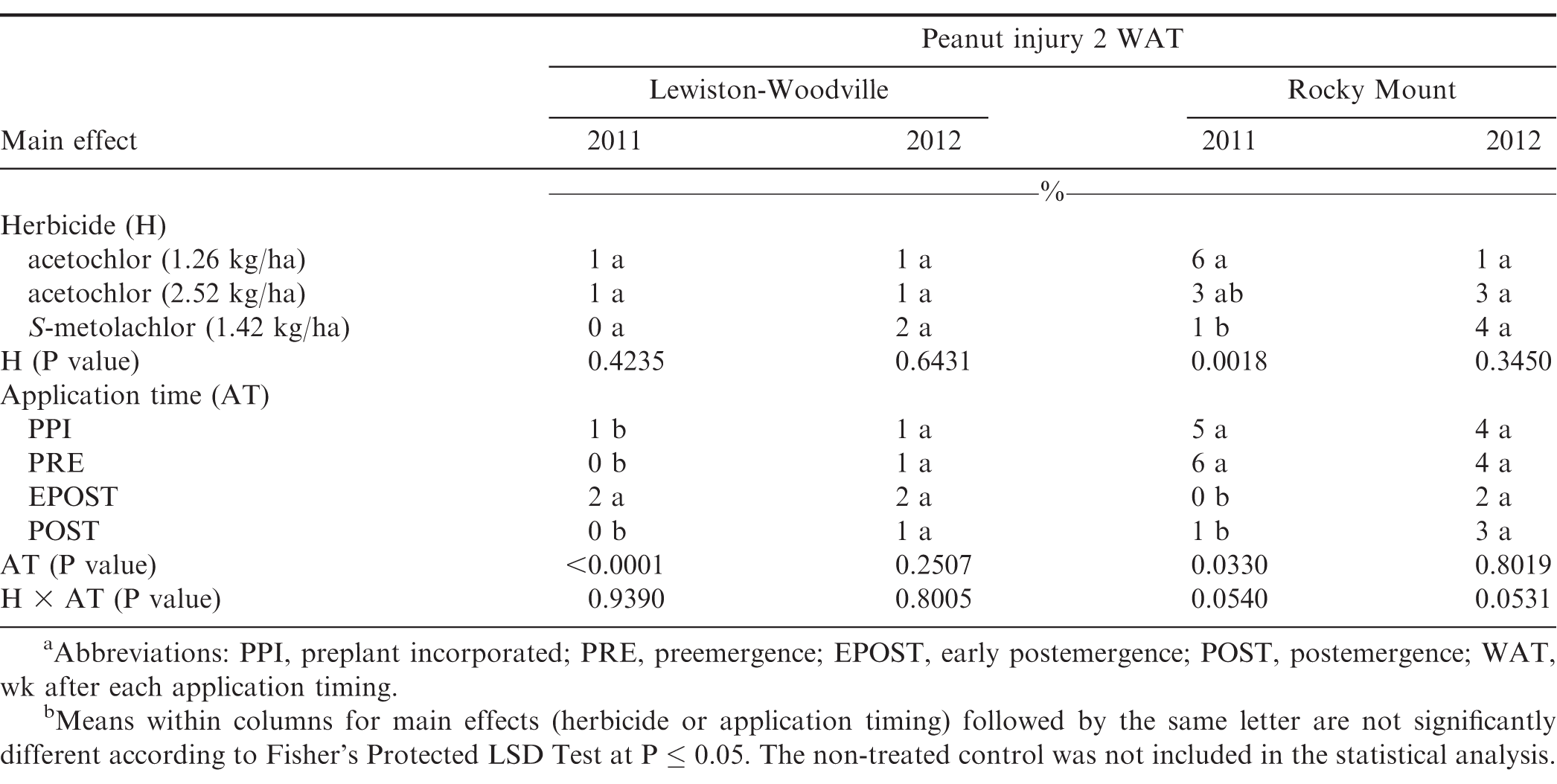
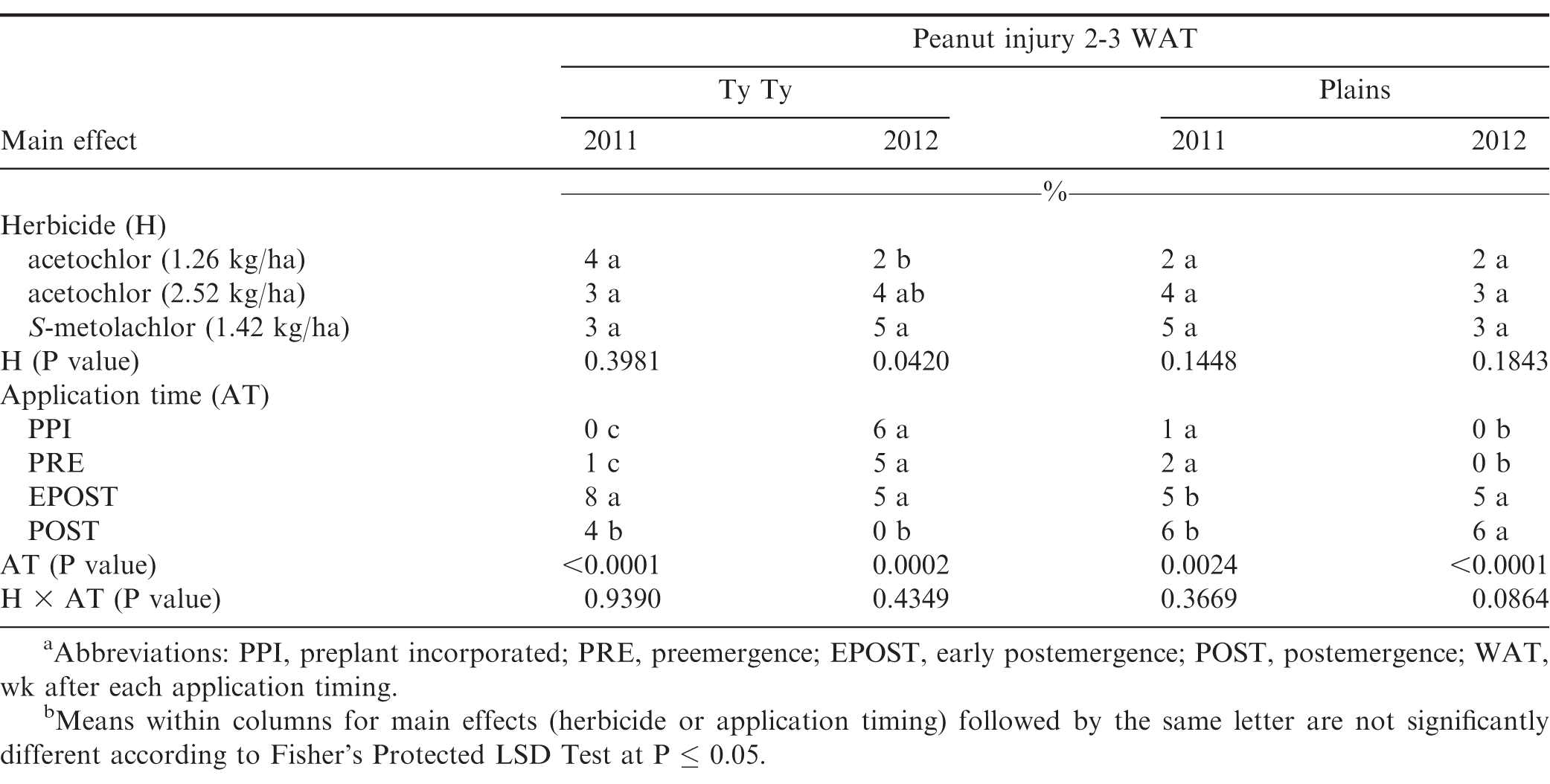
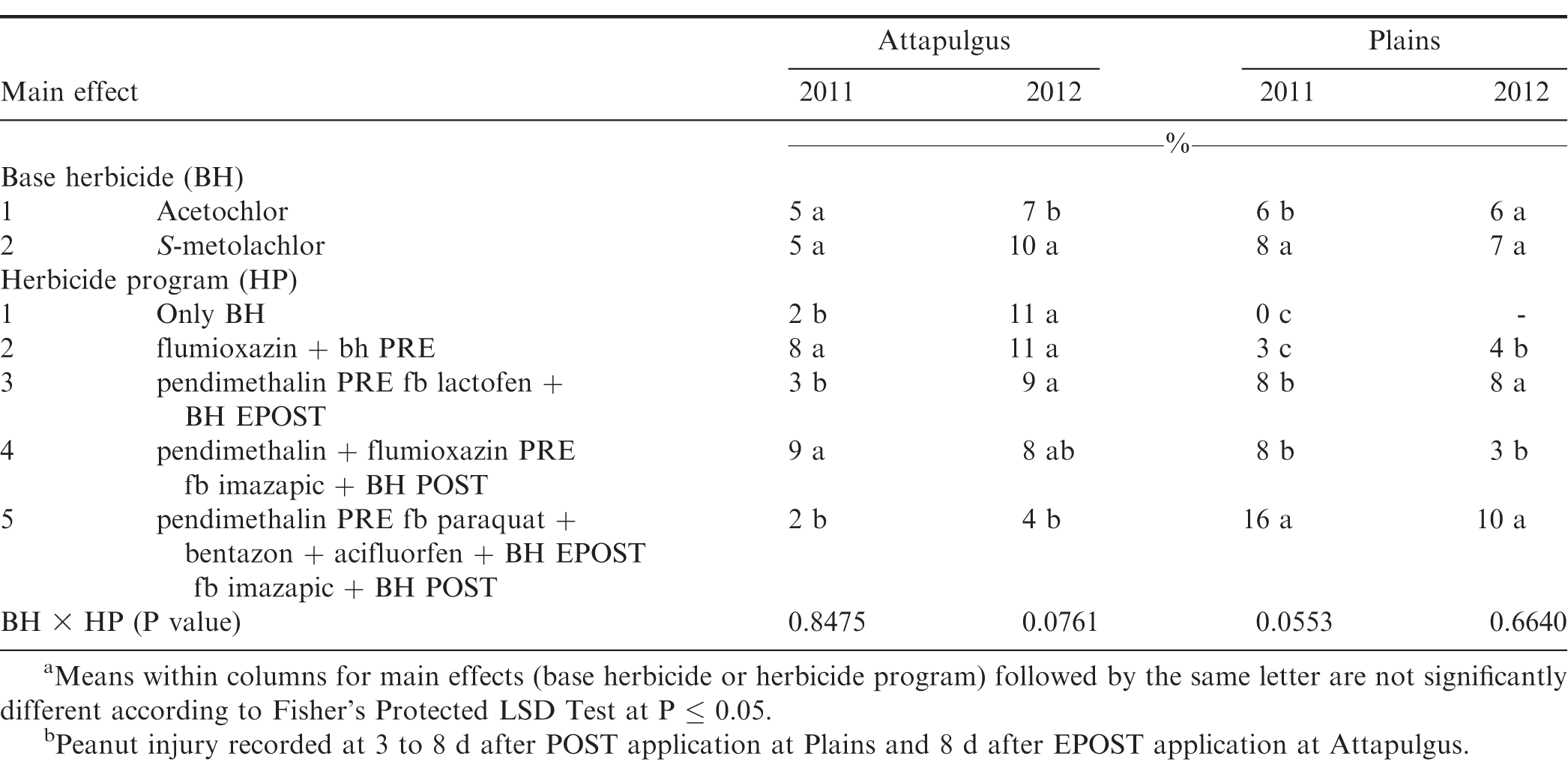
The interaction of experiment by herbicide treatment by application timing was not significant for peanut population and pod yield in Georgia (P=0.31 and 0.38, respectively) and North Carolina (P=0.76 and 0.23, respectively); therefore, data were combined over experiments for both states. However, this three-way interaction was significant for peanut injury at 2 WAT for Georgia (P = 0.0001) and 2-3 WAT for North Carolina (P = 0.009); therefore, data are presented by experiment. Further analysis indicated that the interaction of herbicide by timing of application was not significant for peanut injury, peanut population, and pod yield. Therefore, data for the main effects of herbicide and timing of application are presented.
Peanut injury 2 WAT during 2011 and 2012 at Lewiston-Woodville and 2012 at Rocky Mount was not affected by herbicide treatment (Table 4). In contrast, at Rocky Mount during 2011, S-metolachlor injured peanut less than acetochlor at 1.26 kg/ha. However, similar injury was observed for acetochlor and S-metolachlor when acetolchlor was applied at 2.52 kg/ha. Injury did not exceed 6% regardless of herbicide or rate of acetochlor. Significant but minor differences in injury (6% or less) were observed when comparing method and timing of application at these locations (Table 4). Peanut injury in Georgia did not exceed 5% when comparing herbicides pooled over application timing (Table 5). In 1 of 4 experiments injury by S-metolachlor exceeded that of the lower rate of acetochlor. A consistent trend was not observed when comparing application timings, although injury was 8% or less. Injury was transient, and by 4 to 5 wk at Lewiston-Woodville and Rocky Mount and 6 to 7 wk after treatments at Ty Ty and Plains, injury was 1% or less (data not shown).
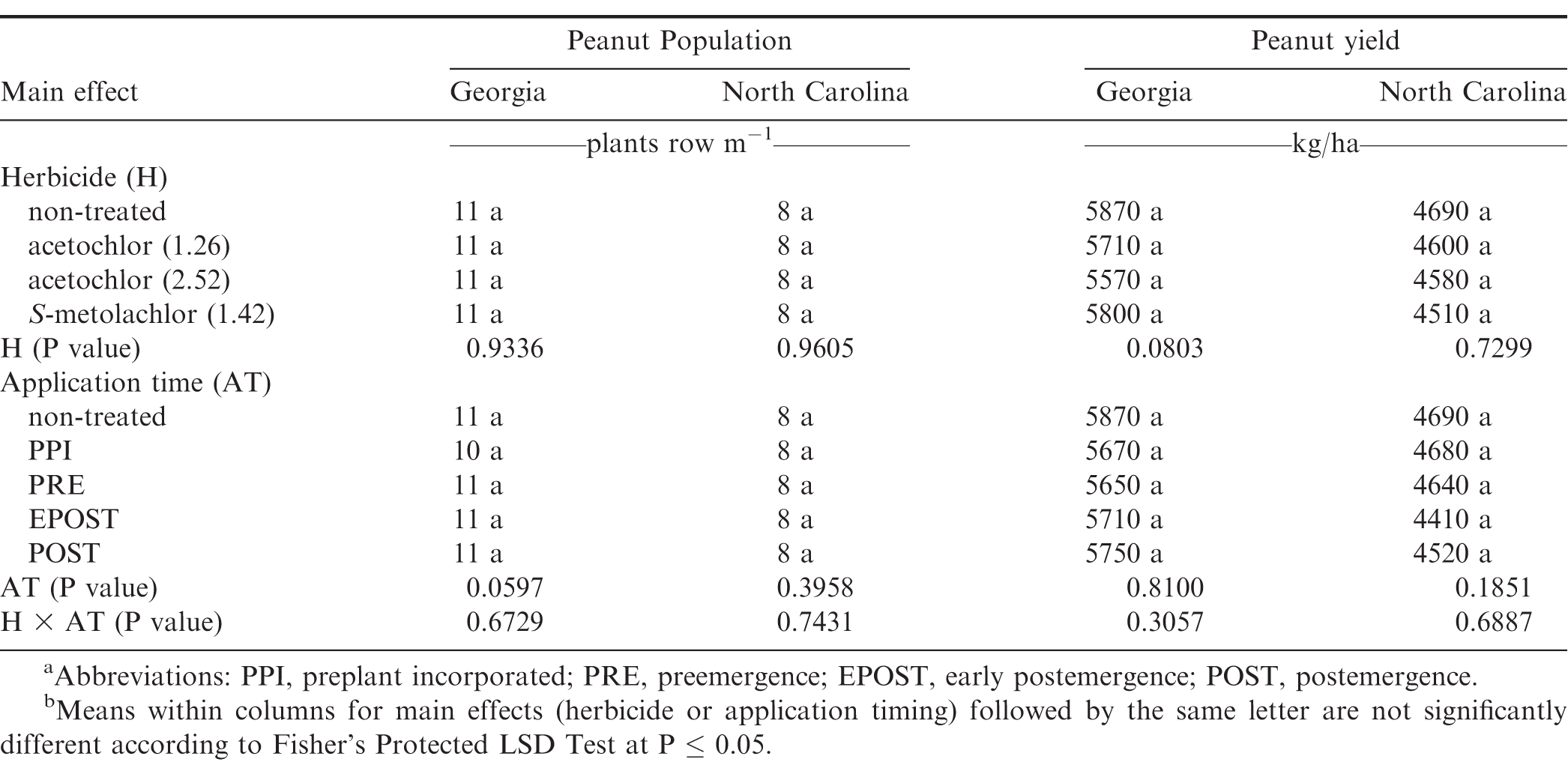
No differences were noted with herbicide treatment or application timing for peanut population or yield in either state or year (Table 6). Visible injury from metolachlor does not always result in lower peanut yield compared with yield of non-treated peanut under weed-free conditions when applied at rates recommended in by the manufacturer (Cardina and Swann, 1988; Clewis et al., 2007; Grichar and Dotray, 2012). In a study similar to the one reported in this article, Grichar et al. (2015) observed in Texas that peanut yield and market grade characteristics were not affected by acetochlor rate or application timing (PPI, PRE, EPOST, POST). The indeterminate growth habit of peanut often enables peanut to recover from early season stress including herbicide injury and yield is often not adversely affected (Carley et al., 2009; Grichar and Dotray, 2012).
Weed Control
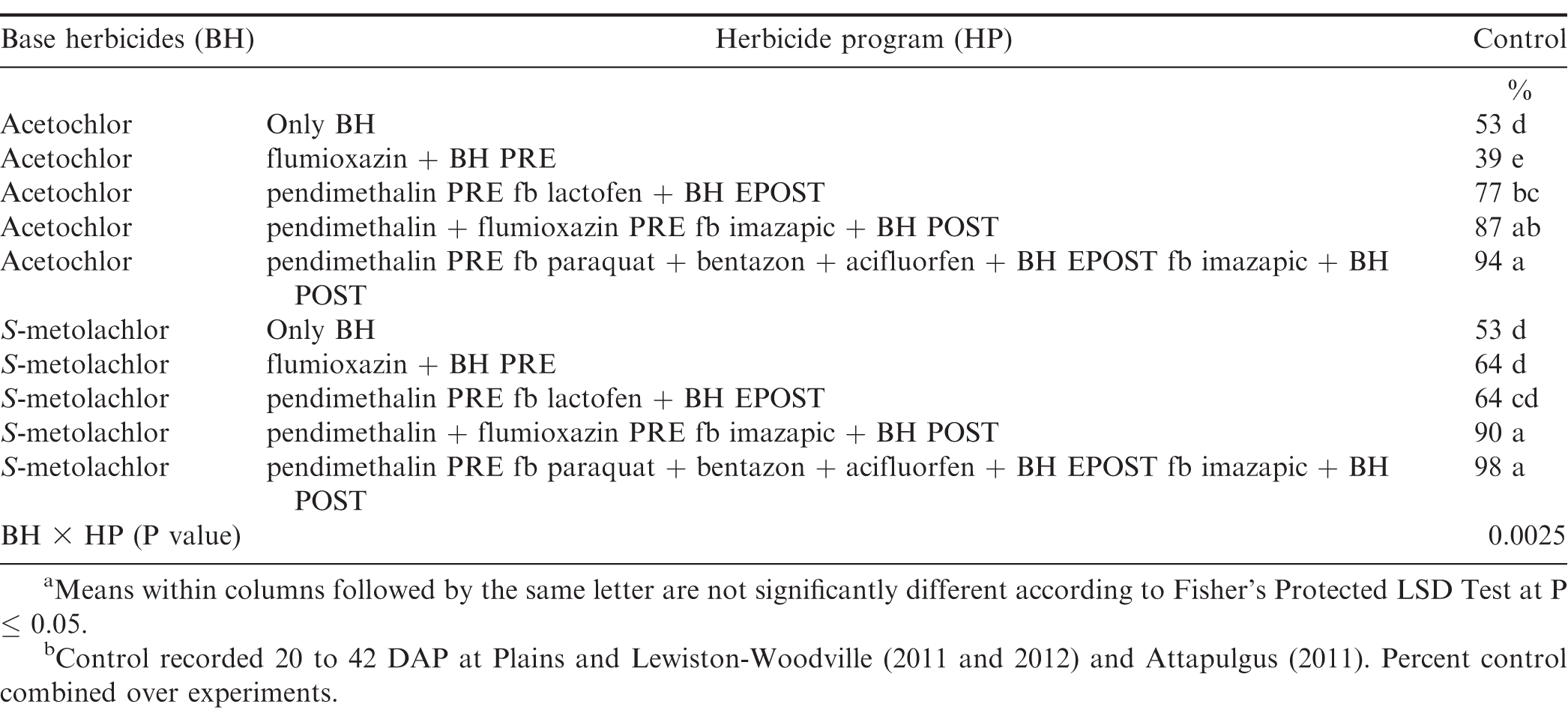
The interaction for experiment by base herbicide by herbicide program was significant for Palmer amaranth control (P=0.001); therefore, data are presented by experiment. In contrast, lack of a significant experiment by base herbicide by herbicide program interaction allowed the combining of data over experiments for broadleaf signalgrass [Urochloa platyphylla (Nash) R.D. Webster] control (P=0.39), pitted morningglory (Ipomoea lacunosa L.) control (P=0.08), sicklepod [Senna obtusifolia (L.) H.S. Irwin & Barneby] control (P=0.26), and Texas millet control (P=0.39). The interaction of base herbicide by herbicide program was not significant for control of Palmer amaranth, broadleaf signalgrass, pitted morningglory, and sicklepod; therefore, data for the main effects are presented (Tables 7 and 8). In contrast, the interaction of base herbicide by herbicide program was significant for Texas millet control (Table 9).
Palmer amaranth
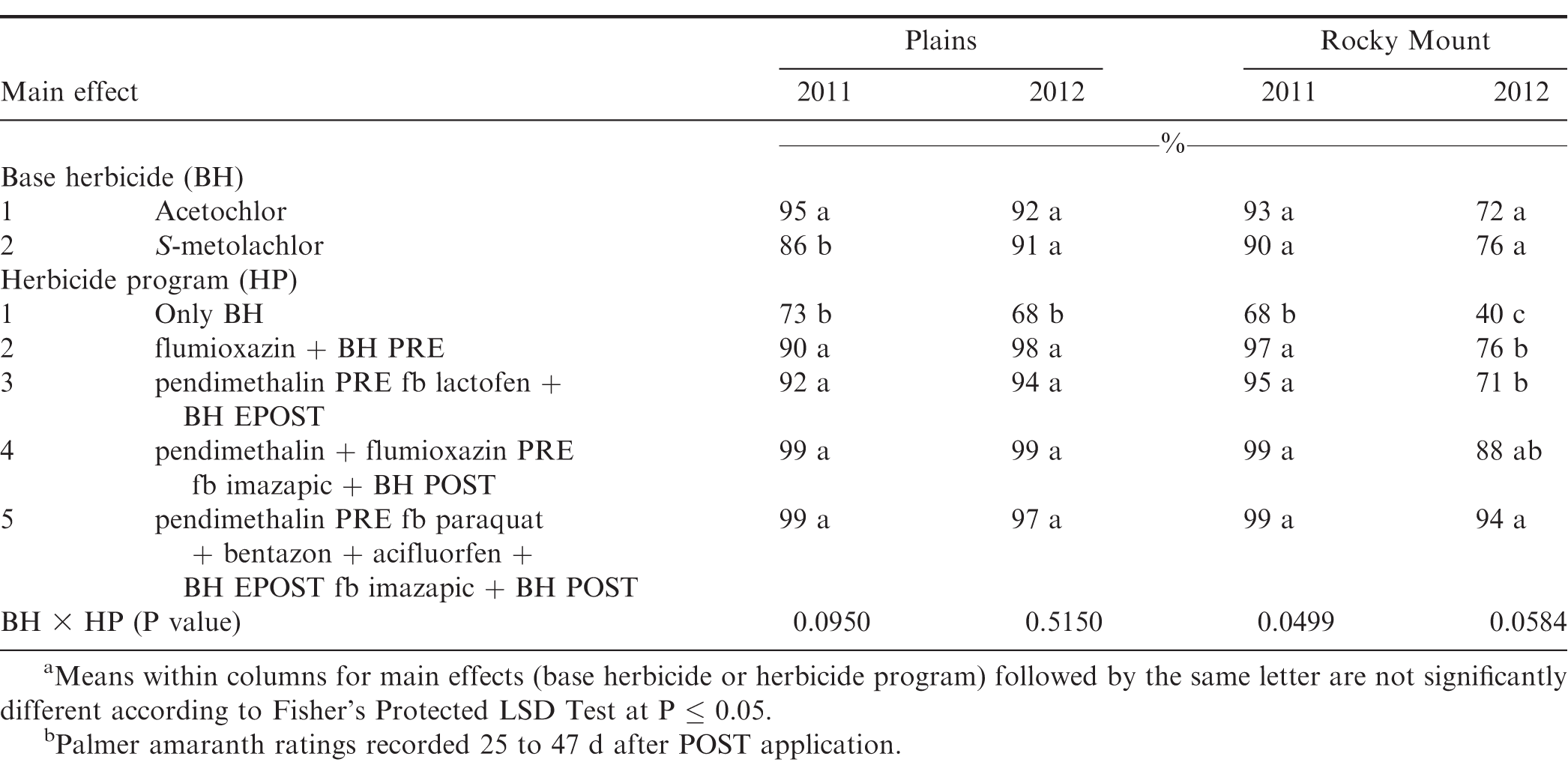
There was no difference between acetochlor and S-metolachlor for Palmer amaranth control except at Plains in 2011, where greater control was obtained from acetochlor compared to S-metolachlor (Table 7). At Plains in 2011 and 2012 and Rocky Mount in 2011, herbicide programs containing only base herbicides provided less Palmer amaranth control compared to other herbicide programs. However, at Rocky Mount in 2012, the herbicide program including pendimethalin PRE fb paraquat plus bentazon plus acifluorfen plus acetochlor or S-metolachlor EPOST fb imazapic plus acetochlor or S-metolachlor POST controlled Palmer amaranth 94% while all other herbicide programs controlled this weed less than 88% (Table 6). Grichar et al. (2005) reported greater control of Palmer amaranth with POST herbicides following PPI application of S-metolachlor was at least 84% compared to only 69% by S-metolachlor. Addition of EPOST or POST herbicides with PPI or PRE herbicides often are beneficial in controlling Palmer amaranth that escapes weed control programs early in the year or when this weed emerges later in the season. Clewis et al. (2007) reported that a EPOST application of paraquat plus bentazon fb the prepackage mixture of acifluorfen plus bentazon POST plus 2,4-DB or imazapic plus 2,4-DB POST controlled Palmer amaranth 93% and 97%.
Broadleaf signalgrass
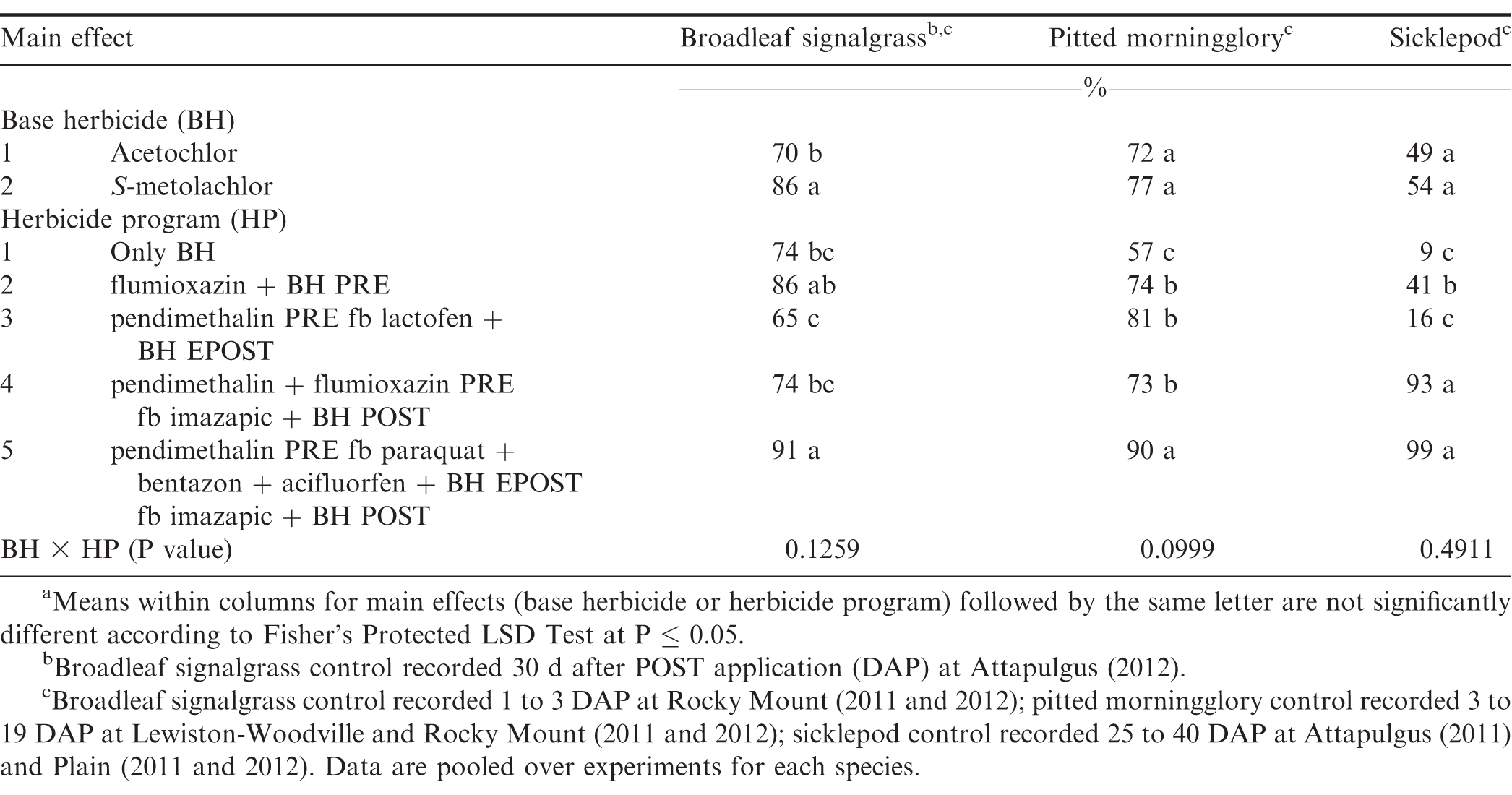
S-metolachlor controlled 86% broadleaf signalgrass which was greater than control by acetochlor (Table 8). Broadleaf signalgrass can rapidly emerge under warm conditions and throughout the growing season in high numbers (Burke et al., 2003). Therefore, multiple herbicide applications are generally needed for season-long control. The herbicide program including pendimethalin PRE fb paraquat plus bentazon plus acifluorfen plus either acetochlor or S-metolachlor EPOST fb imazapic plus acetochlor or S-metolachlor POST controlled broadleaf signalgrass 91%. Clewis et al. (2007) reported that imazapic plus 2,4-DB POST followed by PRE and EPOST herbicide application controlled broadleaf signalgrass at least 96%.
Pitted morningglory
There was no difference between acetochlor and S-metolachlor for pitted morningglory control (Table 8). The most effective herbicide program for pitted morningglory was pendimethalin PRE fb paraquat plus bentazon plus acifluorfen plus either acetochlor or S-metolachlor EPOST fb imazapic plus either acetochlor or S-metolachlor POST which provided 90% control.
Sicklepod
There was no difference between acetochlor and S-metolachlor for sicklepod control (Table 8). Herbicide program including pendimethalin plus flumioxazin PRE fb imazapic plus either acetochlor or S-metolachlor POST and pendimethalin PRE fb paraquat plus bentazon plus acifluorfen plus either acetochlor or S-metolachlor EPOST fb imazapic plus either acetochlor or S-metolachlor POST controlled sicklepod at least 93%, while all other herbicide programs provided ± 41% control. Previous studies have documented effective control of sicklepod by imazapic POST or flumioxazin PRE followed by paraquat plus bentazon EPOST (Grey and Wehtje, 2005; Webster et al., 1997; Wilcut et al., 1996).
Texas millet
Acetochlor and S-metolachlor alone provided 53% control of Texas millet (Table 9). Previous research (Wilcut et al., 1995) indicated that the racemic form of metolachlor provides little or no Texas millet control; however, the dinitroaniline herbicides provide excellent control of annual grasses (Wilcut et al., 1994). Imazapic controls Texas millet and broadleaf signalgrass (Grichar et al., 2005; Wilcut et al., 1995). Herbicide programs including pendimethalin plus flumioxazin PRE fb imazapic plus either acetochlor or S-metolachlor POST and pendimethalin PRE fb paraquat plus bentazon plus acifluorfen plus either acetochlor or S-metolachlor EPOST fb imazapic plus acetochlor or S-metolachlor POST controlled Texas millet at least 87%, while all other herbicide programs provided ± 77% control. Grichar et al. (2005) reported that S-metolachlor PRE in combination with imazapic POST provided 94% control of Texas millet which was better than S-metolachlor alone.
Peanut injury and yield
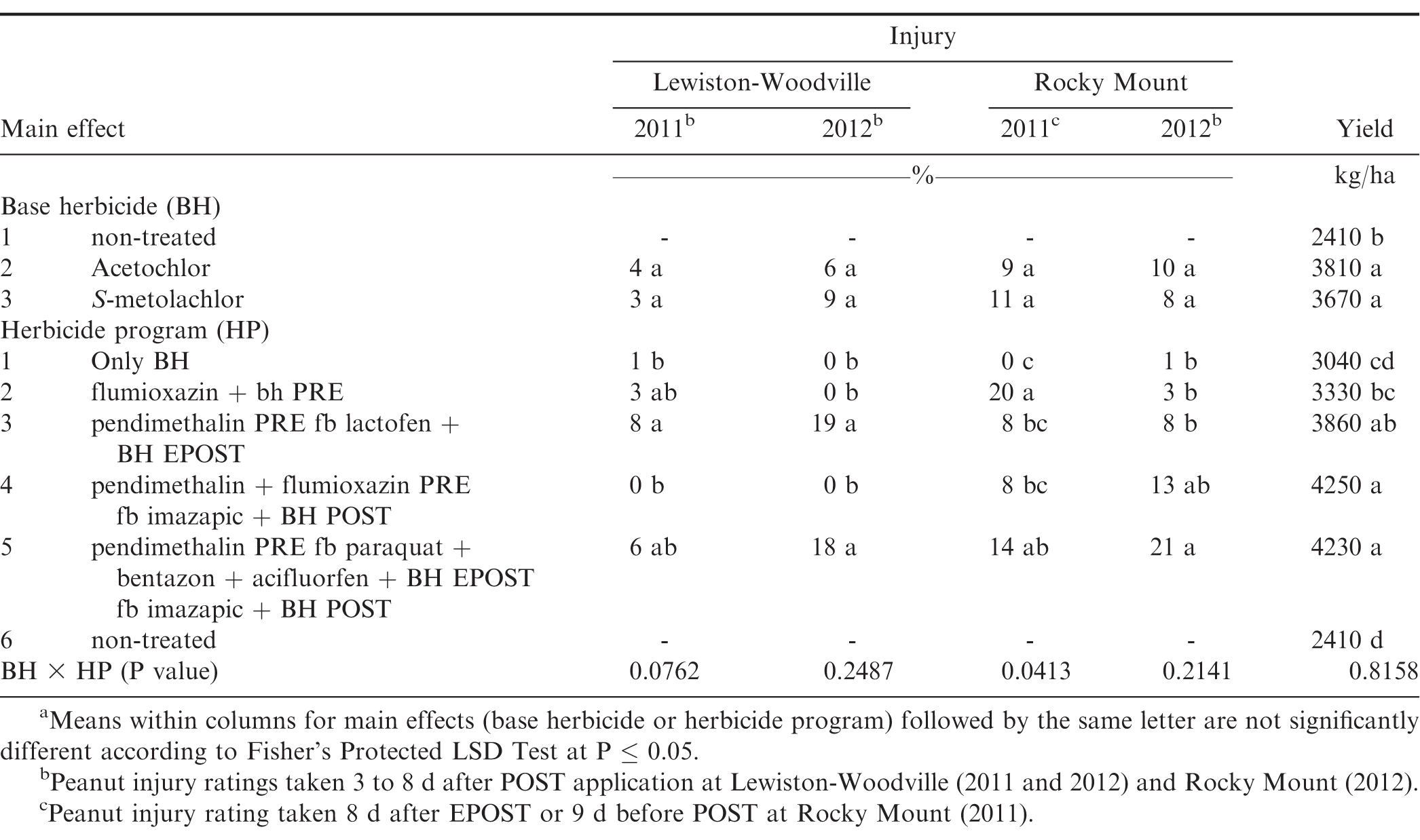
The interaction of experiment by base herbicide by herbicide program was not significant for peanut yield for North Carolina (P=0.90); therefore data were combined over experiments. However, this interaction was significant for peanut injury for Georgia (P=0.04) and North Carolina (P=0.03); therefore, data are presented by experiments. Further analysis indicated that the interaction of base herbicide by herbicide program was not significant for peanut injury and yield. Therefore, data for the main effects were combined.
Peanut injury was similar from acetochlor and S-metolachlor at all the locations at Georgia and North Carolina except Attapulgus 2012 and Plains 2011, where greater injury was observed with S-metolachlor as compared to acetochlor (Tables 10 and 11). Peanut injury with respect to herbicide programs was inconsistent, and maximum injury (21%) was reported from pendimethalin PRE fb paraquat plus bentazon plus acifluorfen plus either acetochlor or S-metolachlor EPOST fb imazapic plus either acetochlor or S-metolachlor POST at Rocky Mount 2012 (Table 11).
There was no difference in peanut yield between acetochlor and S-metolachlor and peanut treated with either herbicide yielded more than the non-treated control (Table 11). Herbicide programs including pendimethalin PRE fb lactofen plus either acetochlor or S-metolachlor EPOST, pendimethalin plus flumioxazin PRE fb imazapic plus either acetochlor or S-metolachlor POST, pendimethalin PRE fb paraquat plus bentazon plus acifluorfen plus either acetochlor or S-metolachlor EPOST fb imazapic plus either acetochlor or S-metolachlor POST resulted in greater yields as compared to non-treated control. The greater yield from these herbicide programs, which contain EPOST and/or POST treatments, may be attributed to effective weed management throughout the peanut growing season. Increased peanut yield has been reported in other studies by using combinations of PPI, PRE, EPOST, or POST herbicide applications (Clewis et al., 2007; Grichar and Dotray, 2012; Jordan et al., 2009).
Conclusions
Results from this research demonstrated that acetochlor exhibited similar phytotoxicity to peanut as S-metolachlor, a herbicide currently used in peanut production. This herbicide will provide growers with another option in their battle against hard-to-control weeds. In most instances, acetochlor or S-metolachlor are not stand-alone herbicides and should be included in a systems approach for the most effective weed control. The addition of herbicides with multiple sites of action in weed management program not only provided increased control of weeds in peanut but may also provide optimum resistance management (Heap, 2017).
Acknowledgements
Monsanto Co. provided partial funding for this research.
Literature Cited
Anonymous 2017 a Dual Magnum label SCP 816A-L1W 0715 Greensboro, NC: Syngenta Crop Protection, 50 pages.
Anonymous 2017 b Warrant herbicide label, preplant, at-planting, preemergence and postemergence applications in peanut. Monsanto Corp., St. Louis, MO, 2 pages.
Anonymous 2017 c Peanut Update Cooperative Extension Service Series CSS-17-0118 ed. W.S. Monfort Athens, GA: University of Georgia. 56 pages.
K.J. Boote, (1982). Growth Stages of Peanut (Arachis hypogaea L.). Peanut Sci 9: 35- 40.
W.D. Branch, (2007). Registration of 'Georgia-06G' peanut. J. Plant Reg 1: 120.
G.A., Buchanan, E.W. Hauser, W.J. Ethredge, and S.R. Cecil (1976). Competition of Florida beggarweed and sicklepod with peanuts II. Effects of cultivation, weeds, and SADH. Weed Sci 24: 29- 39.
I.C., Burke, M. Schroeder, W.E. Thomas, and J.W. Wilcut (2007). Palmer amaranth interference and seed production in peanut. Weed Technol 21: 367- 371.
I.C., Burke, W.E. Thomas, J.F. Spears, and J.W. Wilcut (2003). Influence of environmental factors on broadleaf signalgrass (Brachiaria platyphylla) germination. Weed Sci 51: 683- 689.
C.W., Cahoon, A.C. York, D.L. Jordan, W.J. Everman, R.W. Seagroves, L.R. Braswell, and K.M. Jennings (2015). Weed control in cotton by combinations of microencapsulated acetochlor and various residual herbicides applied preemergence. Weed Technol 29: 740- 750.
J. Cardina, and B.J. Brecke (1991). Florida beggarweed (Desmodium tortuosum) growth and development in peanuts (Arachis hypogaea). Weed Technol 5: 147- 153.
J. Cardina, and C.W. Swann (1988). Metolachlor effects on peanut growth and development. Peanut Sci 15: 57- 60.
D.S., Carley, D.L. Jordan, R.L. Brandenburg, and L.C. Dharmasri (2009). Factors influencing response of Virginia market type peanut (Arachis hypogaea) to paraquat under weed-free conditions. Peanut Sci 36: 180- 189.
S.B., Clewis, S.D. Askew, and J.W. Wilcut (2001). Common ragweed interference in peanut. Weed Sci 49: 768- 772.
S.B., Clewis, W.J. Everman, D.L. Jordan, and J.W. Wilcut (2007). Weed management in North Carolina peanuts (Arachis hypogaea) with S-metolachlor, diclosulam, flumioxazin, and sulfentrazone systems. Weed Technol 21: 629- 635.
W.J., Everman, S.B. Clewis, W.E. Thomas, I.C. Burke, and J.W. Wilcut (2008). Critical period of weed interference in peanut. Weed Technol 22: 63- 67.
P.W., Geier, P.W. Stahlman, D.L. Regehr, and B.L. Olson (2009). Preemergence herbicide efficacy and phytotoxicity in grain sorghum. Weed Technol 23: 197- 201
T.L. Grey, and G.R. Wehtje (2005). Residual herbicide weed control systems in peanut. Weed Technol 19: 560- 567.
T.L., Grey, D.C. Bridges, and E.P. Prostko (2003). Residual weed control with imazapic, diclosulam, and flumioxazin in southeastern peanut (Arachis hypogaea). Peanut Sci 30: 22- 27.
W.J., Grichar, B.A. Besler, R.G. Lemon, and K.D. Brewer (2005). Weed management and net returns using soil-applied and postemergence herbicide programs in peanut (Arachis hypogaea L.). Peanut Sci 32: 25- 31.
W.J. Grichar, and P.A. Dotray (2012). Peanut cultivar response to S-metolachlor and paraquat alone and in combination. Peanut Sci 39: 15- 21.
W.J., Grichar, B.A. Besler, P.A. Dotray, W.C. Johnsonand E.P. Prostko (2004). Interaction of flumioxazin with dimethenamid or metolachlor in peanut (Arachis hypogaea L.). Peanut Sci 31: 12- 16.
W.J., Grichar, P.A. Dotray, and L.M. Etheredge (2015). Weed control and peanut (Arachis hypogaea L.) cultivar response to encapsulated acetochlor. Peanut Sci 42: 100- 108.
W.J., Grichar, P.A. Dotray, and T.A. Baughman (2008). Yellow nutsedge (Cyperus esculentus) control and peanut tolerance to S-metolachlor and diclosulam combinations. Weed Technol 22: 442- 447.
Heap, I. 2017 The International Survey of Herbicide Resistant Weeds Online. Internet March 26, 2017. Available http://www.weedscience.org/summary/MOA.aspx?MOAID=12
T.G., Isleib, S.R. Milla-Lewis, H.E. Pattee, S.C. Copeland, M.C. Zuleta, B.B. Shew, J.E. Hollowell, T.H. Sanders, L.O. Dean, K.W. Hendrix, M. Balota, and J.W. Chapin (2011). Registration of 'Bailey' peanut. J. Plant Reg 5: 27- 39.
T.G., Isleib, P.W. Rice, R.W. Mozingo S.C. Copeland, J.B. Graeber, H.E. Pattee, T.H. Sanders, R.W. Mozingo, and D.L. Coker (2006). Registration of 'Phillips' peanut. Crop Sci 46: 2308- 2309.
Jordan, D.L. 2016 Peanut weed management Pages 47- 80 in Jordan, D.L., R.L. Brandenburg, A.B. Brown, S.G. Bullen, G.T. Roberson, and B. Shew (eds.) Peanut Information North Carolina Cooperative Extension Pub. AG-331, College of Agriculture and Life Sciences, North Carolina State University, Raleigh, NC.
Jordan, D.L., R.L. Brandenburg, A.B. Brown, S.G. Bullen, G.T. Roberson, and B. Shew (eds.) 2016 Peanut Information North Carolina Cooperative Extension Pub. AG-331, College of Agriculture and Life Sciences, North Carolina State University, Raleigh, NC.
D.L., Jordan, S.H. Lancaster, J.E. Lanier, B.R. Lassiter, and P.D. Johnson (2009). Weed management in peanut with herbicide combinations containing imazapic and other pesticides. Weed Technol 23: 6- 10.
R.W., Mozingo, T.A. Coffelt, P.M. Phipps, and D.L. Coker (2006). Registration of 'CHAMPS' peanut. Crop Sci 46: 2711- 2712.
D.C., Parker, F.W. Simmons, and L.M. Wax (2005). Fall and early preplant application timing effects on persistence and efficacy of acetamide herbicides. Weed Technol 19: 6- 13.
H.B., Scher, M. Rodson, and K. Lee (1998). Microencapsulation of pesticides by interfacial polymerization utilizing isocyanate of aminoplast chemistry. Pest Manag. Sci 54: 394- 400.
Shaner, D.L. 2014 Herbicide Handbook. 10th ed Lawrence, KS: Weed Science Society of America Pages 513.
L.E., Steckel, C.L. Sprague, and A.G. Hager (2002). Common waterhemp (Amaranthus rudis) control in corn (Zea mays) with single preemergence and sequential applications of residual herbicides. Weed Technol 16: 755- 761.
[USDA] U.S. Department of Agriculture 2016 a Crop values 2015 summary, 12 Sept. 2016. http://usda.mannlib.cornell.edu/usda/current/CropValuSu/CropValuSu-02-24-2016.pdf.
[USDA] U.S. Department of Agriculture 2016 b Crop production 2015 summary, 12 Sept. 2016. http://www.usda.gov/nass/PUBS/TODAYRPT/cropan16.pdf.
R.H., Walker, L.W. Wells, and J.A. McGuire (1989). Bristly starbur (Acanthospermum hispidium) interference in peanuts (Arachis hypogaea). Weed Sci 37: 196- 200.
T.M., Webster, J.W. Wilcut, and H.D. Coble (1997). Influence of AC 263, 222 rate and application method on weed management in peanut (Arachis hypogaea). Weed Technol 11: 520- 526.
J.W., Wilcut, A.C. York, and G.R. Wehtje (1994). The control and interaction of weeds in peanut (Arachis hypogaea). Rev. Weed Sci 6: 177- 205.
Wilcut, J.W., A.C. York, W.J. Grichar, and G.R. Wehtje 1995 The biology and management of weeds in peanut (Arachis hypogaea) Pages 207- 244 In: H.E. Patteeand H.T. Stalker (eds.) Advances in Peanut Science Amer. Peanut Res. Educ. Soc., Stillwater, OK.
J.W., Wilcut, G.R. Wehtje, and R.H. Walker (1987). Economics of weed control in peanuts (Arachis hypogaea) with herbicides and cultivations. Weed Sci 35: 711- 715.
J.W., Wilcut, J.S. Richburgand G.L. Wiley (1996). Postemergence AC 263,222 systems for weed control in peanut (Arachis hypogaea). Weed Sci 44: 615- 621.
E.J., Williams, and J.S. Drexler (1981). A non-destructive method for determining peanut pod maturity, pericarp, mesocarp, color, morphology, and classification. Peanut Sci 8: 134- 141.
A.M., Wise, T.L. Grey, E.P. Prostko, W.K. Vencill, and T.M. Webster (2009). Establishing the geographic distribution level of acetolactate synthase resistance of Palmer amaranth (Amaranthus palmeri) accessions in Georgia. Weed Technol 23: 214- 220.
Notes
- Postdoctoral Research Scholar and Professor, Department of Crop and Soil Sciences, North Carolina State University, Raleigh, NC 27695 [^]
- Professor and Professor, respectively. Department of Crop and Soil Sciences, The University of Georgia, 2360 Rainwater Drive, Tifton, GA 31793. [^]
- Associate Professor, Department of Horticulture Science, North Carolina State University, Raleigh, NC 27695. [^] *Corresponding author Email: david_jordan@ncsu.edu
Author Affiliations