Introduction
Peanut (Arachis hypogaea L.) is the major oilseed crop of India, cultivated on 6.22 million ha in 2008-09 yielding 1180 Kg ha−1. India is the second largest peanut producer after China. Besides income for farmers, peanut provides an inexpensive source of high quality nutrition. Peanut seed contain 44 to 56% oil and 22 to 30% protein on a dry seed basis (Savage and Keenan, 1994). Although India is world's largest producer of edible oils, it imports large quantities (∼1.2 million tons per annum). One reason for India's large edible oil deficit is poor yields which may result from wrong selection of peanut genotypes and/or poor agronomic practices. Therefore, it would be pertinent to assess the potential of peanut genotypes under in vitro conditions and new combination of plant growth regulators (PGRs) for regeneration and biochemical manipulation to improve the shooting potential per plant (vegetative growth) which will further increase crop yield. M-13 (spreading; Mungala et al., 2008) and PBS24030 (semi-spreading; Samdur et al., 2000) peanut cultivars are the most commonly grown in the state of Rajasthan.
In recent years nitric oxide (NO), a biologically active gas effective in nanomolar concentration (1.0 nmol L−1), has been shown to be ubiquitous in plants and to regulate various physiological and developmental processes. Nitric oxide is involved in germination and induction of lateral roots (Creus et al., 2005; Tewari et al., 2008). Moreover, applications of NO donors (sodium nitroprusside; SNP) enhance plant tolerance to specific stresses such as salt stress (Molassiotis et al., 2010), osmotic stress (Zhang et al., 2005), and heavy metal stress (Rodríguez-Serrano et al., 2009) in many plants. Peanut is a genotype specific crop so the micropropagation of peanut cultivars requires specific protocols for each type. This approach in the present study is preferred so as to minimize the occurrence of chimeric plants. Although innumerable works have confirmed the potential of plant hormones to synergistically improve crop performance under normal conditions, very little information on the influence of NO donor under in in vitro conditions exists. The objective of this research was to analyze how a NO donor (SNP) is involved in the micropropagation and alterations of biochemical characteristics in peanut so that this approach can be used in future for multiplication and production of in vitro genetically transformed peanut plants.
Material and Methods
Peanut genotypes, M-13 and PBS24030 varieties were procured from Agricultural Research Station (ARS), Durgapura, Jaipur, India. The seeds were washed with tap water (10 to 15 min) followed by immersion in liquid detergent solution labolene (5 min). After washing with distilled water, seeds were immersed in 70% ethanol (3 to 5 min) and then rinsed with distilled water (3 to 4 times). Then seeds were brought to the inoculation chamber and surface sterilized with 0.1% HgCl2 for 8 min and rinsed with sterile distilled water for 3 to 4 times. Four to six surface sterilized seeds were germinated aseptically in a 250 mL wide mouthed conical flask (Riviera, India) containing sterilized wet cotton bed with water in dark. Based on previous research, cotyledonary nodes (CN) were used as explants. After 10 to 12 d of growth, primary leaves and the epicotyls were detached from the seedling using a sterile surgical scalpel blade. Then the seedling radicle was excised, leaving approximately 1 to 2 cm long hypocotyls intact. Explants consisting of cotyledon and axillary meristem regions with hypocotyls were then inoculated vertically on MS medium (Murashige and Skoog, 1962) containing 3% (w/v) sucrose and varying concentrations of i.e. 5 µM (SNP1), 50 µM (SNP2), 100 µM (SNP3) and 500 µM (SNP4) of SNP, alone and in combination with BA (3 mg L−1). Cultures were incubated at 24±2°C under a 16/8-h (light/dark) photoperiod with a light intensity of 80 µEm−2s−1 intensity provided by fluorescent tubes for 4 to 5 weeks. A control set was devoid of any PGR. Shooting potential (multiple shoots, axillary branches and shoot buds) was recorded after 40 days of inoculation.
Biochemical and Enzymatic Estimations
Estimation of biochemical parameters and enzyme activities were carried out in in vitro grown 25to 30 d old leaves from lateral branches. All the chemicals and reagents used for estimations were of analytical grade and purchased from Merck Chemical Co., India. Total chlorophyll content was estimated by the method of Coombs et al. (1985). The chlorophyll was extracted in 80% acetone. The absorption of the extracts at wavelengths of 663nm (D663) and 645nm (D645) were measured with a 117- UV-VIS spectrophotometer (Systronics, India). The concentration of total chlorophyll was then calculated using the equations as proposed by Coombs et al. (1985).
For estimation of antioxidative enzyme activities one gram of in vitro grown 25 to 30 d old leaves were homogenized in 10 mL of the extraction buffer composed of 50 mM potassium phosphate buffer (pH 7.0), 10 g L−1 PVP, 0.2 mM EDTA and 10 mM Triton X-100. The resulting homogenate was centrifuged at 12,000 g for 20 min at 4°C and the supernatant was used for the determination of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), peroxidase (POX) and polyphenol oxidase (PPX) activities. SOD activity was estimated by the modified method of Beauchamp and Fridovich (1971). Enzyme corresponding to 50% inhibition of reaction was considered as one enzyme unit. CAT activity was determined by following the initial rate of disappearance of H2O2 at 240 nm (Aebi, 1984). APX activity was measured by the amount required to decompose 1 µmol ascorbic acid oxidized min−1 (Zhu et al., 2004). POX and PPX activity was assayed adopting the method of Kar and Mishra (1976). One unit (U) of enzyme activity was defined as the amount of enzyme that caused an increase in absorbance at 420 nm of 0.01 min−1. Lipid peroxidation was determined as the content of malondialdehyde (MDA) using thiobarbituric acid reaction as described by Moshaty et al. (1993). Total soluble sugars were estimated following the method of Clegg (1956). To the ethanolic extract of fresh leaves, anthrone reagent and concentrated H2SO4 were added. The color intensity of the greenish color thus developed was read at 620 nm. The quantity of total soluble sugars was calculated from the standard plot of D-glucose (0-50 µg ml−1). Total soluble proteins were estimated by Lowry et al. (1951) method. Total protein was extracted from fresh leaves and freshly prepared alkaline copper sulfate reagent was added followed by 1N Folin-Ciocalteau reagent.. The absorbance of the resulting blue color was recorded at 660 nm against the blank. The amount of soluble proteins was calculated in mg g−1 FW with the help of standard plot of BSA (0–150 µg).
Each growth experiment was conducted thrice taking three replicas of each treatment, under in vitro conditions. The data from all experiments was combined for analysis of variance (ANOVA) with genotypes, SNP treatments and their interactions included as the possible sources of variation using statistical analysis system (SAS) (SAS, 2000).
Results and Discussion
SNP Induces Multiple Shoot Induction
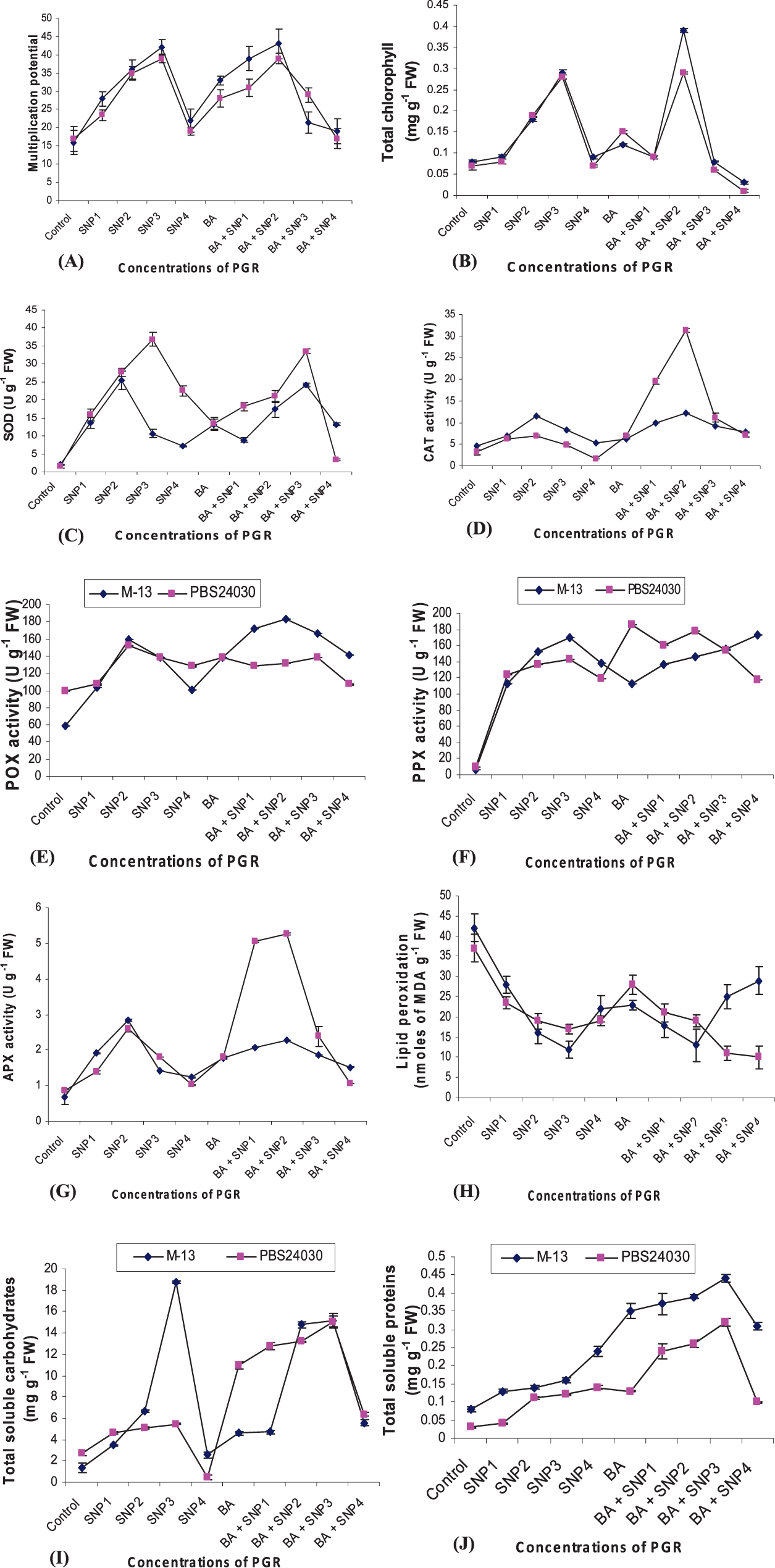
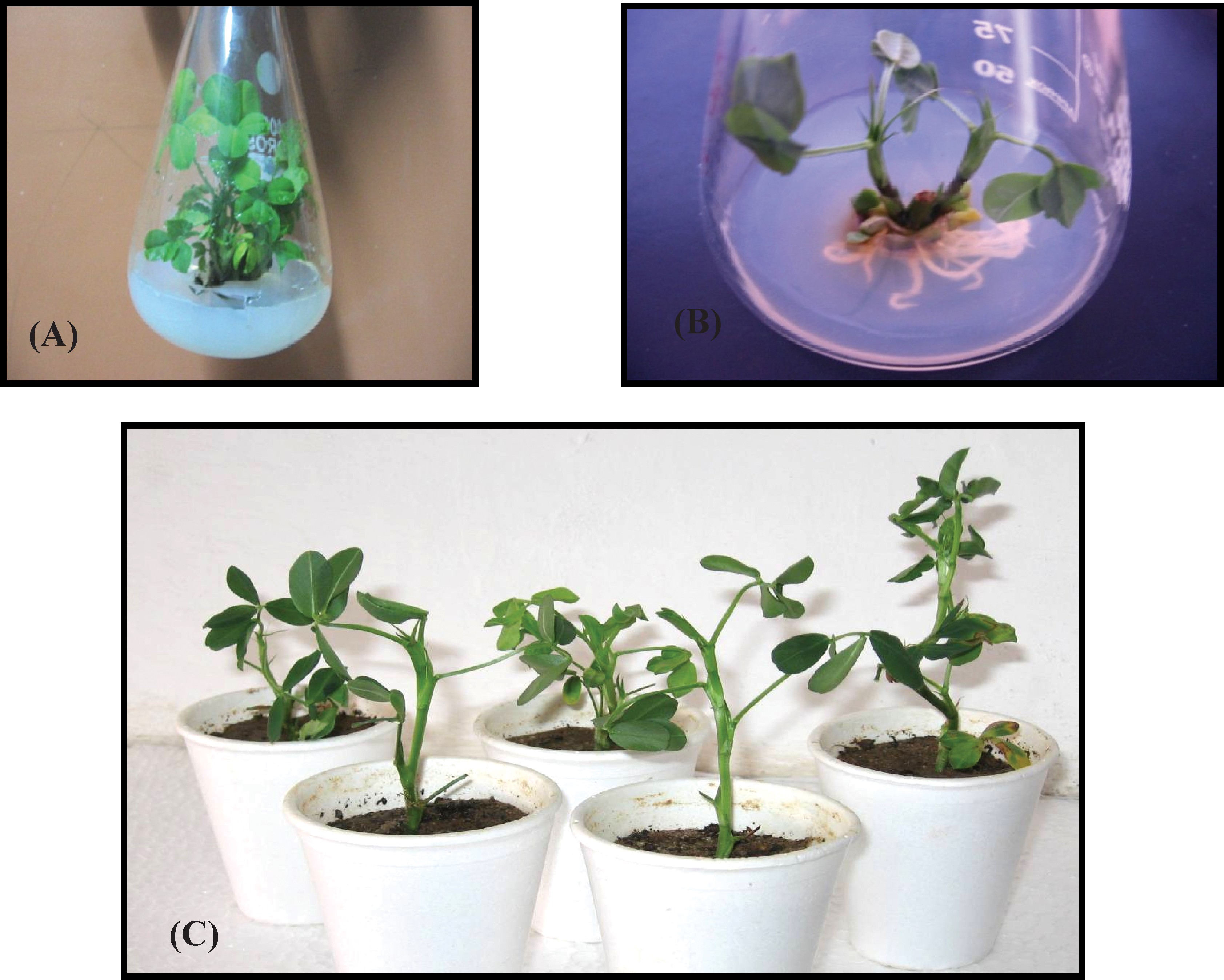
Investigations of the effect of different concentrations of SNP on shoot multiplication revealed that SNP alone, and in combination with BA, induced regeneration of multiple shoots from CN explant. Analysis indicated significant differences between cultivars (Table 1). The mean number of shoots per explant enhanced with increasing concentrations of SNP alone from SNP1 to SNP3 for M-13 (Fig. 1A) and PBS24030. This may be attributed to the genetic variations between the cultivars. However, at SNP4, multiplication of plantlets declined and leaves turned brown and necrotized in both cultivars. Thus, with regard to induction of shoot multiplication, the optimum concentration of SNP alone was SNP3 but in combination with BA it was SNP2 (Fig. 2A) for both cultivars. Moreover, medium containing SNP alone produced roots directly from the shoot base and the response was markedly high (Fig. 2B). However, combination of SNP with BA proved to be inhibitory for rooting in both cultivars. Number of adventitious and lateral roots gradually increased with increasing SNP concentrations for both genotypes. Well rooted plantlets of 6 to 8 cm in length were hardened (Fig. 2C) and transferred to the field, where they flowered and set viable seeds (Data not presented).
Effect of sodium nitroprusside (SNP, NO donor) at SNP1-SNP4 with or without 6-Benzyl Adenine (BA; 3 mg L−1) on in vitro; (A) shoot multiplication, (B) total chlorophyll content, (C) superoxide dismutase (SOD), (D) catalase (CAT), (E) peroxidase (POX), (F) polyphenol oxidase (PPX), (G) ascorbate peroxidase (APX) activity, (H) lipid peroxidation, (I) total soluble carbohydrates and (J) protein content in M-13 and PBS24030 genotypes of peanut. Bars represent the SE (n = 3).
(A) Shooting potential (multiple shoots, axillary branches and shoot buds) of M-13 on MS medium supplemented with SNP2 and 3 mg L−1 6-Benzyl Adenine (BA) after 30 d of inoculation; (B) Rhizogenesis in M-13 on MS medium supplemented with SNP2 alone; (C) Hardening of in vitro grown well rooted plantlets in thermocol pots of M-13.
Exogenously supplied NO donor significantly promoted shoot differentiation in many plants (Kalra and Babbar, 2010; Han et al., 2008; Xu et al., 2009). However, the mechanism by which SNP improves the micropropagation efficiency is still not clear. Several studies have proved that NO interact with different plant hormones (auxins, cytokinin, ethylene etc.) at different steps of signaling cascades to evoke various responses and finally increase the yield (Garcia-Mata and Lamattina, 2002). Carimi et al. (2005) found that cytokinin (BA) induced NO accumulation in cell suspension cultures. Adventitious rooting involves the development of a meristematic tissue after removal of the primary root system. NO has also been reported to play a central role in the lateral root formation (Creus et al., 2005). Any deleterious effect of NO on plant growth has not been reported till date.
SNP Increases Total Chlorophyll Content
Chlorophyll content markedly increased (P<0.01; Table 1) with increasing concentrations of SNP in both cultivars (Fig. 1B). An increase of 28% in M-13 and 25% in PBS24030 in chlorophyll content was noted at SNP3. However, a sharp decline in the total chlorophyll content was observed at SNP4 in both the cultivars. Unlike SNP alone, SNP in combination with BA exhibited a variable effect on total chlorophyll content depending upon its concentration. SNP2 along with BA (3 mg L−1) had a stimulatory effect whereas at SNP3 and SNP4 caused marked decrease in chlorophyll content compared to the values recorded in the presence of SNP alone in both the cultivars.
For this research, total chlorophyll content was increased by SNP when applied at lower concentrations. The increase in chlorophyll content after NO treatment has also been reported by various researchers (Cevahir et al., 2005; Pahwa et al., 2009). Earlier, NO was shown to protect chlorophyll against losses due to pathogenesis (Laxalt et al., 1997), to increase leaf chlorophyll content by de-etiolation and to be involved in light-mediated greening of seedlings (Zhang et al., 2006).
Effect of NO Donor (SNP) on Antioxidant Enzymes
Superoxide dismutase (SOD) as indicated by the data was increased in SNP-treated cultures. However, supplementation of BA alone and BA plus SNP was unable to enhance SOD activity (Fig. 1C). The Fe-containing antioxidative enzymes (CAT, APX, POX and PPX) were also evaluated. The CAT activity was highly significant at P<0.01 (Table 1). In M-13, CAT activity increased in the presence of SNP alone as well as in combination with BA at SNP1 and SNP2 and thereafter a steep decline was observed (Fig. 1D). Similar results were obtained in the other genotype i.e. PBS24030. Therefore, treatment with both SNP and BA was more effective in enhancing the CAT activity as compared to SNP alone. An increase in SOD activity was observed in many SNP-treated plants (Zhou et al. 2005; Uchida et al. 2002). The increase in CAT activity by SNP might help in removal of toxic ROS generated in the cell. NO has been reported to activate CAT by several researchers (Tewari et al., 2007). On the contrary, NO has been shown to inhibit or repress ROS-scavenging enzymes, APX and CAT (Clarke et al., 2000). It appears that the effects of NO might be dose dependent as observed during the present study. This statement gains support from the results of Xu et al. (2009) in Dioscorea opposita.
The data obtained of POX activity was highly significant at P<0.01 (Table 1). It was observed that NO donor enhanced the activity of POX at SNP2 over the control in both the genotypes as shown in Fig. 1E. The combined effect of SNP and BA on POX activity was more stimulatory in M-13 as compared to PBS24030 genotype. PPX activity was maximum at SNP3 in both the cultivars (Fig. 1F). With further increase in concentration, a varied response was observed among the cultivars which might be due to their different genetic makeup. When SNP was applied in combination with BA, the maximum activity was observed at SNP4 in M-13 and SNP2 in PBS24030. Clarke et al. (2000) showed that NO inhibited POX activity at higher concentrations. The increase in the activity of PPX in response to SNP was also reported by Tewari et al. (2008) in Panax ginseng. The enhancement of PPX activity by SNP indicated an activation of the antioxidative metabolism by NO which in turn would help in protecting the plants against the oxidative stress. NO most likely acted as an antioxidant by reacting directly with ROS particularly with H2O2, causing an increase in PPX activity (Tewari et al., 2007).
Like other antioxidant enzymes, APX activity in the leaves of M-13 cultivar, increased initially reaching a maximum at SNP2, although the activity was still higher than the untreated control. The effect of BA alone was stimulatory over the untreated control, but it was not able to raise the APX activity as performed by SNP alone (Fig. 1G). On treatment with BA +SNP, PBS24030 showed tremendous increase in the APX activity. The accumulation of MDA, an index of lipid peroxidation, was higher in the control than that in the presence of at all the concentrations of SNP alone as well as in combination with BA in both the cultivars (Fig. 1H). The results pertaining to the effect of SNP on the activity of APX revealed that SNP enhanced the APX activity at lower concentrations but was inhibitory at higher concentrations. These observations indicated an antioxidative effect of NO as suggested earlier (Beligni and Lamattina, 1999). Contrary to our results, an inhibitory effect of NO on APX activity was reported which was attributed to the direct binding of NO to the heme group of the enzyme (Clarke et al., 2000). The inconsistency between this observation and our data might be ascribed to the use of different NO generators viz. S-nitroso-N acetyl-DL-penicillinamine (SNAP) and S-nitroso-L-glutathione (GSNO) which produced NO at least 10 to 15 times higher than SNP. The high concentration of NO produced from the NO donors might be responsible for inhibition of the APX activity. The decline in MDA content at lower concentrations but increase at higher concentrations of SNP has been reported in wheat leaves (Tu et al., 2003) and Hydrilla verticillata (Wang et al., 2010).
The results of the present study revealed that NO enhanced the activities of the antioxidant enzymes at lower concentrations but inhibited at higher concentrations. The earlier reports showed an NO-mediated activation of antioxidant enzymes (Dobashi et al., 1997) and expression of genes encoding the enzymes. It could be inferred from these findings that NO stimulated the antioxidative metabolism by increasing the activities of the antioxidative enzymes thereby protecting the cells against the harmful effects of ROS.
SNP increases total Soluble Carbohydrate and Protein Content
An increase in total soluble carbohydrate content (P<0.01) was observed in the in vitro grown leaves of peanut (Fig. 1I). When SNP was applied alone, the accumulation of soluble carbohydrates was maximum at SNP3 in both the genotypes. However, M-13 showed marked increase as compared to PBS24030. The content of total soluble carbohydrates enhanced up to BA+SNP3 in both the cultivars. It was evident from the data that the accumulation of carbohydrates in M-13 was more in the presence of SNP alone whereas, in PBS24030, combination with BA showed significant rise. The soluble protein content in the leaves was found to increase (P<0.01) under the influence of SNP alone as well as in combination with BA (Table 1; Fig. 1J). Both the cultivars showed continuous rise in total protein content in the presence of SNP alone as well as in combination with BA except at BA+SNP4. However, content of total soluble protein in M-13 was more as compared to PBS24030. An increase in soluble carbohydrates and protein content was likely to result in better growth. An increase in soluble sugars upon exogenous application of NO was also reported in spinach leaves (Jin et al., 2009). Ganjewala et al. (2010) found that treatment of lemongrass tillers with 1mM SNP increased whereas 2mM SNP decreased the protein content indicating that the effect was concentration dependent.
Conclusions
Previous research has noted that NO had protective effects against various stressful conditions (Xu et al., 2009) and participates in plant growth and signal transduction pathways. This research has demonstrated that SNP as an NO donor, can be effectively used for the direct organogenesis from the CN explant for both cultivars. The enzymes activities in SNP-treated cultures were kept to higher levels than the controls, however, there are some differences in the responsive behaviors of the two peanut cultivars in the presence of SNP which may be attributed to the genetic differences or due to varying endogenous level of PGRs. These results indicate that SNP (NO donor) is a potent plant growth regulator in various aspects.
Literature Cited
Aebi H 1984 Catalase in vitro , In: Packer L (ed.) Methods in Enzymology Vol. 105 New York : Academic Press . pp. 121 – 126 .
Beauchamp C and Fridovich I 1971 Superoxide dismutase: improved assays and assays applicable to acrylamide gels Anal. Biochem 44 : 276 – 287 .
Beligni M.V and Lamattina L 1999 Nitric oxide protects against cellular damage produced by methylviologen herbicides in potato plants Archives of Biochem. and Biophys 3 : 199 – 208 .
Carimi F Zottini M Costa A Cattelan I De Michele R Terzi M and Lo Schiavo F 2005 NO signalling in cytokinin-induced programmed cell death Plant Cell Environ 28 : 171 – 178 .
Cevahir G Yentür S Aytamka E Eryılmaz F and Yılmazer N 2005 The effect of nitric oxide, salicylic acid and hydrogen peroxide on the pigment content in excised cotyledons of red cabbage (Brassica oleraceae L.) Fresenius Environmental Bulletin 14 : 591 – 598 .
Clarke D Durner J Navarre D.A and Klessig D.F 2000 Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase Mol. Plant-Microbe Interact 13 : 1380 – 1384 .
Clegg K.M 1956 Application of anthrone reagent to the estimation of starch in cereals J. Sci. Food Agric 7 : 40 – 44 .
Coombs J Hall D.O Long S.P and Scurlock J.M.O 1985 Techniques in Bioproductivity and Photosynthesis 2nd ed Oxford: Pergamon International , pp. 223 – 234 .
Creus C.M Graziano M Casanovas E.M Pereyra M.A Simontacchi M Puntarulo S et al 2005 Nitric oxide is involved in the Azospirillum brasilense-induced lateral root formation in tomato Planta 221 : 297 – 303 .
Dobashi K Pahan K Chahal A and Singh I 1997 Modulation of endogenous antioxidant enzymes by nitric oxide in rat C6 glial cells Journal of Neurochemistry 68 : 1896 – 903 .
Ganjewala D Nagaraja C Nayak M.R and Devi S.A 2010 Effects of sodium nitroprusside on activity of acid and alkaline invertases and alkaline phosphatase in lemongrass (Cymbopogon flexuosus Steud) Wats Int. J. Plant Biol 1 : 9 – 12 .
Garcia-Mata C and Lamattina 2002 Nitric and abscisic acid cross talk in guard cells Plant Physiol 128 : 790 – 792 .
Han X.J Yang H.Q Duan K.X Zhang X.R Zhao H.Z You S.Z and Jiang Q.Q 2008 Sodium nitroprusside promotes multiplication and regeneration of Malus hupehensis in vitro plantlets Plant Cell Tiss. Organ Cult 96 : 29 – 34 .
He Y.K Tang R.H Yi H Stevens R.D Cook C.W and Ahn S.M 2004 Nitric oxide represses the Arabidopsis floral transition Science 305 : 1968 – 1971 .
Jin X Yin H Wang W Mi Q and Liu X 2009 Effects of sodium nitroprusside on callus induction and shoot regeneration in micropropagated Dioscorea opposite Plant Growth Regul 59 : 279 – 285 .
Kalra C and Babbar S.B 2010 Nitric oxide promotes in vitro organogenesis in Linum usitatissimum L. Plant Cell Tiss Organ Cult 103 : 353 – 359 .
Kar M and Mishra D 1976 Catalase, peroxidase and polyphenol oxidase activities during rice leaf senescence Plant Physiol 57 : 315 – 319 .
Laxalt A.M Beligni M.V and Lamattina L 1997 Nitric oxide preserves the level of chlorophyll in potato leaves infected by Phytophthora infestans Eur. J. Plant Pathol 103 : 643 – 651 .
Lowry O.H Rosebrough N.J Farr A.L and Randall R.J 1951 Protein measurement with Folin-Phenol reagent J. Biol. Chem 193 : 265 – 275 .
Molassiotis A Tanou G and Diamantidis G 2010 NO says more than ‘YES’ to salt tolerance: Salt priming and systemic nitric oxide signaling in plants Plant Signal Behav 5 : 209 – 212 .
Moshaty E.F.I.B Pike S.M Novacky A.J and Sehgal O.P 1993 Lipid peroxidation and superoxide production in cowpea (Vigna unguiculata) leaves infected with tobacco ringspot virus or southern bean mosaic virus Physiol. Mol. Plant Pathol 43 : 109 – 119 .
Mungala A.J Radhakrishnan T and Jayanti R.D 2008 In vitro screening of 123 Indian peanut cultivars for sodium chloride induced salinity tolerance World J. of Agri. Sci 4 ( 5 ): 574 – 582 .
Murashige T and Skoog F 1962 A revised medium for rapid growth and bioassays with tobacco tissue cultures Plant Physiol 15 : 473 – 497 .
Pahwa S Setia R.C and Setia N 2009 Effect of exogenous nitric oxide on chlorophyll content and Hill reaction activity in leaves of Brassica napus L. Environ Ecol 27 : 278 – 280 .
Rodríguez-Serrano M Romero-Puertas M.C Pazmiño D.M Testillano P.S Risueño M.C Del Río L.A and Sandalio L.M 2009 Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium Plant Physiol 150 : 229 – 243 .
Samdur M.Y Singh A.L Mathur R.K Manivel P Chikani B.M and Khan M.A 2000 Field evaluation of chlorophyll meter for screening groundnut (Arachis hypogaea L.) genotypes tolerant to iron-deficiency chlorosis Current Science 79 ( 2 ): 211 – 214 .
Statistical Analysis System (SAS) 2000 SAS Online Doc. Version 8 Cary, NC : SAS Institute Inc .
Savage G.P and Keenan J.I 1994 The Composition and Nutritive value of Groundnut Kernels In: The Groundnut Crop - A Scientific Basis for Improvement ( Smartt J (ed.) Chapman and Hall , London . pp. 173 – 213 .
Tewari R.K Lee S.Y Hahn E.J and Paek K.Y 2007 Temporal changes in the growth, saponin content and antioxidant defense in the adventitious roots of Panax ginseng subjected to nitric oxide elicitation Plant Biotechnol. Rep 1 : 227 – 235 .
Tewari R.K Lee S.Y Hahn E.J and Paek K.Y 2008 Involvement of nitric oxide-induced NADPH oxidase in adventitious root growth and antioxidant defense in Panax ginseng Plant Biotechnol. Rep 2 : 113 – 122 .
Tu J Shen W.B and Xu L.L 2003 Regulation of nitric oxide on the aging process of wheat leaves Acta Botanica Sinica 45 : 1055 – 1062 .
Uchida A Jagendorf A.T Hibino T Takabe T and Takabe T 2002 Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice Plant Sci 163 : 515 – 523 .
Wang H Zhang S Zhang W Wei C and Wang P 2010 Effects of nitric oxide on the growth and antioxidant response of submerged plants Hydrilla verticillata (L.f.) Royle African J. of Biotechnol 9 ( 44 ): 7470 – 7476 .
Xu J Yin H Wang W Mi Q and Liu X 2009 Effects of sodium nitroprusside on callus induction and shoot regeneration in micropropagated Dioscorea opposite Plant Growth Regul 59 : 279 – 285 .
Zhang H Jiang Y He Z and Ma M 2005 Cadmium accumulation and oxidative burst in garlic (Allium sativum) J. Plant Physiol 162 : 977 – 984 .
Zhang Y Wang L Liu Y Zhang Q Wei Q and Zhang W 2006 Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump and Na+/H+ antiport in the tonoplast Planta 224 : 545 – 555 .
Zhou B Guo Z Xing J and Huang B 2005 Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis J. of Experim. Bot 56 : 3223 – 3228 .
Zhu Y.Z Huang S.H Tan B.K.H Sun J Whiteman M and Zhu Y.C 2004 Antioxidants in Chinese herbal medicines: a biochemical perspective National Production Rep 21 : 478 – 489 .
Notes
- Assistant Professor and Professor, School of Life Sciences, Jaipur National University, Jaipur-302025, India; and Professor, Department of Biochemistry, Kurukshetra University, Kurukshetra-136119, India, respectively * Corresponding author's Email: verma.aman1980@gmail.com
Author Affiliations