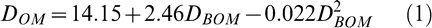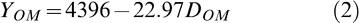Introduction
Peanut is indeterminate in growth habit and determining when the highest percentage of peanut pods is at optimum maturity is challenging. The decision to dig peanut can have a tremendous impact on pod yield and market grade characteristics (Jordan et al., 1998; Mozingo et al., 1991; Sholar et al., 1995). Although response can be variable and is dependant upon many factors, digging peanut as little as 1 wk prior to or 1 wk following optimum maturity can result in substantial reductions in pod yield (Jordan et al., 1998). Edaphic and environmental conditions combined with other management constraints at the farm level contribute to grower anxiety over when to dig. Poor plant condition and pod shedding associated with disease are also considered in digging decisions (Sholar et al., 1995).
The hull scrape method originally developed for runner market types has proven effective in helping growers predict optimum maturity (Williams and Drexler, 1981). Recently, a peanut maturity profile chart was developed for virginia market types grown in the Virginia-Carolina production region (Jordan et al., 2005). In addition to providing estimates of optimum digging date based on mesocarp color, this new chart provides recommendations on how to consider the role of disease incidence, freeze potential, and practicality of heat unit accumulation in digging decisions (Jordan et al., 2005).
Disease management is critical in optimizing yield and quality of peanut. Peanut growers in the United States use a range of tactics to control disease, including host-plant resistance and application of fungicides (Sherwood et al., 1995). Early leaf spot (caused by Cercospora arachidicola Hori), late leaf spot [caused by Cercosporidium personatum (Berk. & M.A. Curtis) Deighton syn. Phaeoisariopsis personata von Arx], and web blotch (caused by Phoma arachidicola Marasas, Pauer, and Boerema) are the most prevalent foliar diseases in peanut in North Carolina (Shew, 2007). Biweekly fungicide applications or applications according to a weather-based advisory are effective in controlling these diseases.
Yield loss from foliar diseases is associated with defoliation and subsequent shedding of pods (Aquino et al., 1992; Backman and Crawford, 1984; Johnson et al., 1986; Nutter and Littrell, 1996; Pixley et al., 1990). Disease control with fungicides is critical in maintaining sufficient foliage to allow continued pod development, maturation, and peg strength. In spite of regular fungicide applications, some fields may experience high levels of defoliation in September and October. Defoliation from foliar diseases can be a particular problem in wet years when growers may be unable to make timely fungicide applications or when fungicides are washed from leaves after heavy rains. Consequently, growers must balance the decision of whether to delay digging to optimize maturity with concerns that defoliation will result in pod shedding if the crop remains in the field. Although considerable research has been conducted to determine the relationships among digging date, pod yield, and market grade when disease is controlled, research addressing interactions of digging date and canopy defoliation is limited (Nutter and Littrell, 1995).
Varying fungicide rates and application intervals have been used as techniques to determine relationships between canopy defoliation and pod yield of runner market type peanut (Bowen et al., 1997). Backman and Crawford (1984) found that for every 1% increase in canopy defoliation within 2 to 3 weeks of optimum digging, there was yield loss of approximately 57 kg/ha when optimum yield was approximately 4400 kg/ha. Yield loss has been related to canopy reflectance at 800 nm in several studies (Aquino et al., 1992; Nutter et al., 1990; Nutter and Littrell, 1996; Pixley et al., 1990), but the exact relationship varied among fields. Yield loss relationships have been less frequently characterized for virginia market-type cultivars. Johnson and Beute (1986) reported increasing yield and value for the cultivars Florigiant and NC 5 with increasing rates of chlorothalonil application, but did not explicitly develop yield loss equations. Yield potential of fungicide-treated peanut probably has increased since the 1980's due to the greater efficacy of modern fungicides. Thus, yield loss relationships in modern virginia market-type cultivars need better definition under current production conditions.
The level of canopy defoliation at which yield loss from pod shedding would offset expected yield gains from digging at optimum maturity was not addressed in earlier studies. Determining if digging prior to optimum maturity offsets pod loss due to defoliation and disease is critical for peanut growers and their advisors. Therefore, the objectives of this research were to determine relationships among canopy defoliation, yield, and crop maturity in peanut, and to determine whether peanut should be dug prior to optimum pod maturity when high levels of defoliation are present.
Materials and Methods
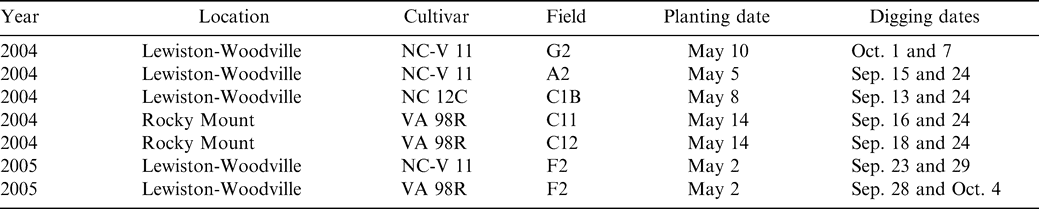
Experiments were conducted in North Carolina from 2004–2005 at the Peanut Belt Research Station located near Lewiston-Woodville and at the Upper Costal Plain Research Station located near Rocky Mount (Table 1). Soil at Lewiston-Woodville was a Norfolk sandy loam (fine-loamy, kaolinitic, thermic Typic Kandiudults); soil at Rocky Mount was a Goldsboro fine, sandy loam (fine-loamy, siliceous, subactive, thermic Aquic Paleudults). Plot size was two rows (91-cm spacing) by 9 m in length. Peanut was seeded in conventionally prepared seedbeds at a rate designed to achieve a final in-row plant population of 13 plants/m. Two non-treated rows separated each plot. With the exception of fungicide treatments and digging dates, all other production and pest management practices were held constant over the entire experiment and were based on Cooperative Extension recommendations appropriate for the region (Brandenburg, 2007; Jordan, 2007a, 2007b; Shew, 2007).
A prediction of optimum maturity was determined based on pod mesocarp color (Williams and Drexler, 1981) by collecting a composite of approximately 150 pods from treatments receiving bi-weekly sprays approximately 2 wks before optimum maturity was anticipated (Jordan et al., 2005). Sampling date was determined based on days after peanut emergence and heat unit accumulation for each cultivar in a given field.
Seven experiments, five at Lewiston-Woodville [Fields G2, C1B, C2B, and A2 (NC-V 11 and VA 98R)] and two at Rocky Mount [Fields C11 and C12 (VA 98R)] each consisted of three fungicide programs and two digging dates. Fungicide programs included no fungicide, two fungicide applications in July, and five applications throughout the season spaced approximately two weeks apart beginning in early July through early September. Two digging dates were included for each fungicide program and consisted of digging dates at optimum maturity for the bi-weekly fungicide program and digging 6 to 12 days prior to optimum maturity (Table 1).
The cultivars NC-V 11 and VA 98R were planted in three experiments each and one experiment was planted with NC 12C (Table 1). The cultivar NC-V 11 is considered intermediate in maturity among virginia market-type cultivars while the cultivar VA 98R is considered early maturing ( Jordan, 2007). The cultivar NC 12C matures intermediately between VA 98R and NC-V 11 ( Jordan, 2007).
Fungicides included chlorothalonil (Bravo Weather Stik, Syngenta Crop Protection, Greensboro, NC) applied at 1260 g ai/ha as the first spray; tebuconazole (Folicur, Bayer CropScience, Research Triangle Park, NC) at 227 g ai/ha for two consecutive sprays; pyraclostrobin (Headline, BASF Corporation, Research Triangle Park, NC) at 160 g ai/ha as the fourth spray; and finally chlorothalonil at 1260 g/ha as the final spray. Fungicides were applied in 187 L/ha aqueous spray solution at 159 kPa using a CO2–pressurized backpack sprayer equipped with hollow-cone spray nozzles (TXVS-8 Conejet hollow-cone spray tips, Spraying Systems Company, Wheaton, IL).
Treatments were replicated four times and were arranged in a randomized complete block design. The percentage of canopy defoliation was estimated visually in all experiments within 2 days prior to digging using a scale of 0 (no defoliation) to 100 (complete defoliation). The combined effects of early leaf spot, late leaf spot, and web blotch caused canopy defoliation. However, web blotch and late leaf spot were the predominant diseases. Although southern stem rot (caused by Sclerotium rolfsii Sacc.) may have contributed to response to fungicides, no attempt was made to quantify this disease. Additionally, fields at Lewiston-Woodville were fumigated with metam sodium to minimize Cylindrocladium black rot (caused by Cylindrocladium parasiticum Crous, M.J. Wingfield and Alfenas). Although spotted wilt caused by Tomato spotted wilt virus was present at these locations, few plants exhibited visible symptoms of this disease. Peanut plants were dug in late September or in October and plants were allowed to dry in the field for 4 to 7 days prior to threshing. Pod yield was adjusted to 8% moisture.
Data for peanut canopy defoliation and pod yield were subjected to analysis of variance appropriate for the factorial arrangement of treatments (experiments, fungicide programs, digging dates). Means of significant main effects and interactions were separated using Fisher's Protected LSD at P ≤ 0.05. Additionally, experiments were considered replications and data were subjected to analysis of variance using the factorial treatment arrangement.
Regression procedures were used to determine linear relationships of the percent of maximum yield 6–12 days prior to optimum maturity or at optimum maturity versus percent canopy defoliation 6–12 days before optimum maturity (BOM) for each experiment. Percent of maximum yield was calculated using the mean yield of bi-weekly fungicide treatments as the maximum value for each experiment.
Polynomial regressions were developed to determine the relationship between percent defoliation BOM and percent defoliation at optimum maturity for the data set pooled over all seven location-years. Criteria used to accept regression models were: significance of model and model parameters (P ≤ 0.05), tests for lack-of-fit not significant (P ≤ 0.05), and residual plots without obvious patterns.
Results and Discussion
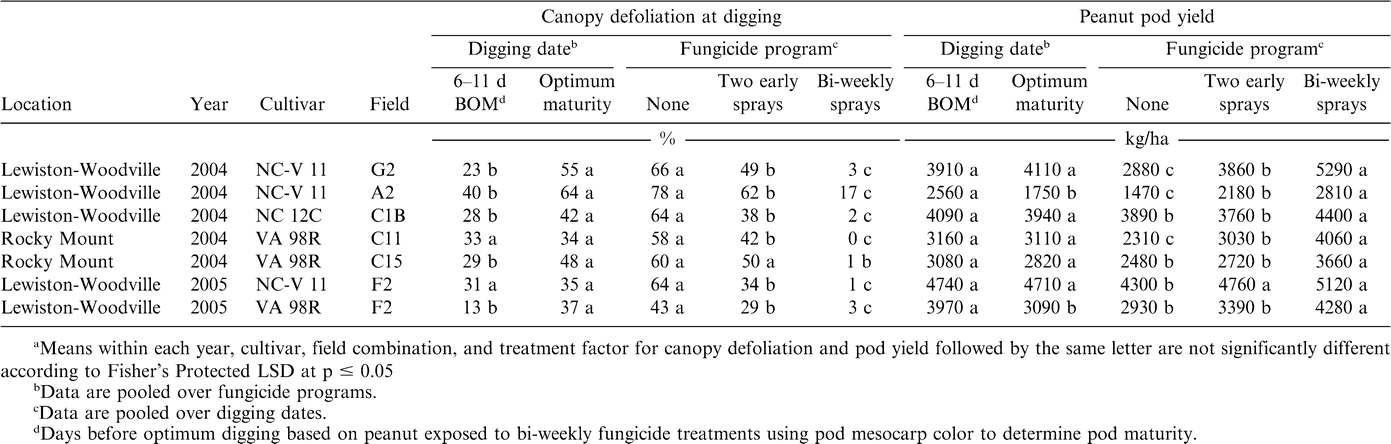
The interaction of fungicide program × digging date was not significant for pod yield, but experiment × digging date and experiment × fungicide program significantly influenced pod yield. Although the interaction of experiment × digging date × fungicide program was not significant for canopy defoliation, the means of experiment × digging date and experiment × fungicide program are presented with yields for comparison (Table 2). Pod yield reflected differences in defoliation in some but not all cases. Generally, fewer differences in pod yield were noted compared with differences noted for defoliation. Defoliation was significantly higher at optimal maturity than 6–12 days earlier in five of seven experiments. Yield was significantly lower at optimal maturity than 6–12 days earlier in two of seven experiments; no other differences were found (Table 2). Likewise, differences in canopy defoliation were noted for all three fungicide treatments in six of seven experiments. Yields were significantly lower with two early sprays than with bi-weekly sprays in six of seven experiments, whereas yield was higher for two early sprays compared to non-sprayed treatments in only three of seven experiments (Table 2). Increasing the number of fungicide sprays decreased defoliation and increased pod yield in all experiments (Table 2).
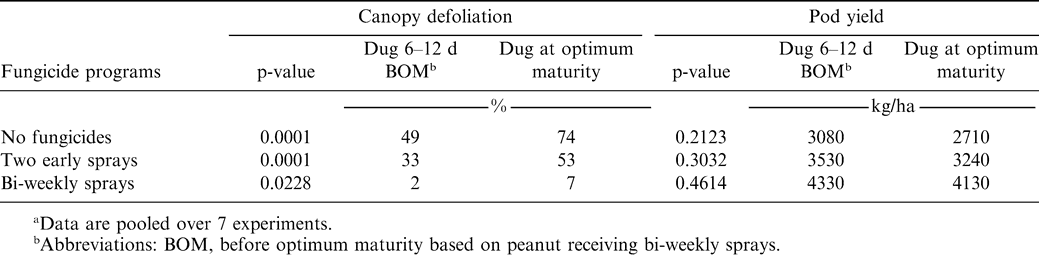
When experiments were considered replications, canopy defoliation increased as digging was delayed for each fungicide treatment, although the magnitude of the difference was small for bi-weekly sprays (Table 3). However, significant decreases in yield were not observed between digging dates within a fungicide program (Table 3).
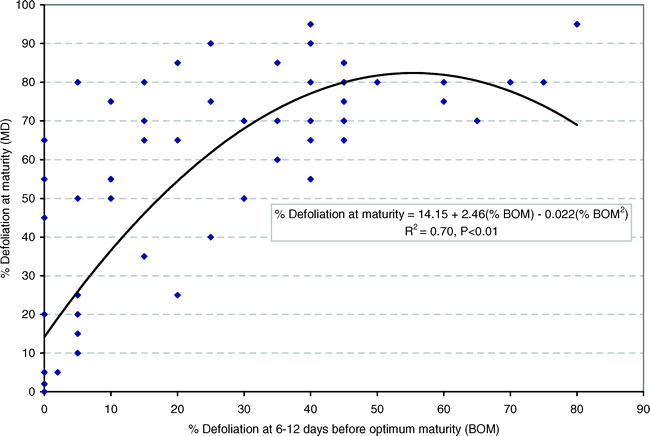
When all data were pooled, the following quadratic relationship (Fig. 1) was found between percent defoliation 6–12 days before optimal maturity (DBOM) and at optimal maturity (DOM).
The regression model had an R2 = 0.70 and all parameters were highly significant.
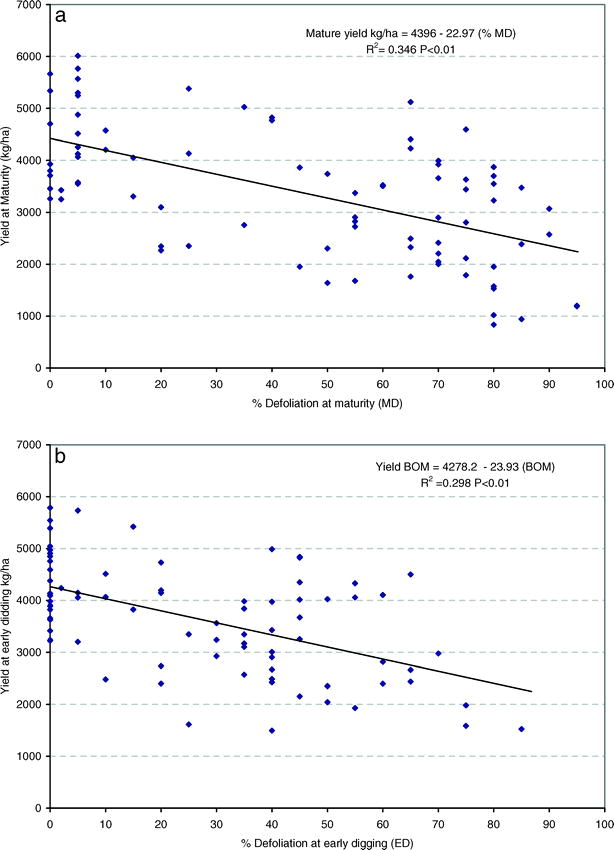
Regression analyis showed that yield before optimum maturity (YBOM) and at optimum maturity (YOM) decreased as percent defoliation increased. The models (eqns. 2 and 3) had R2 values of 0.35 and 0.30, respectively and are shown in Figures 2a and 2b.
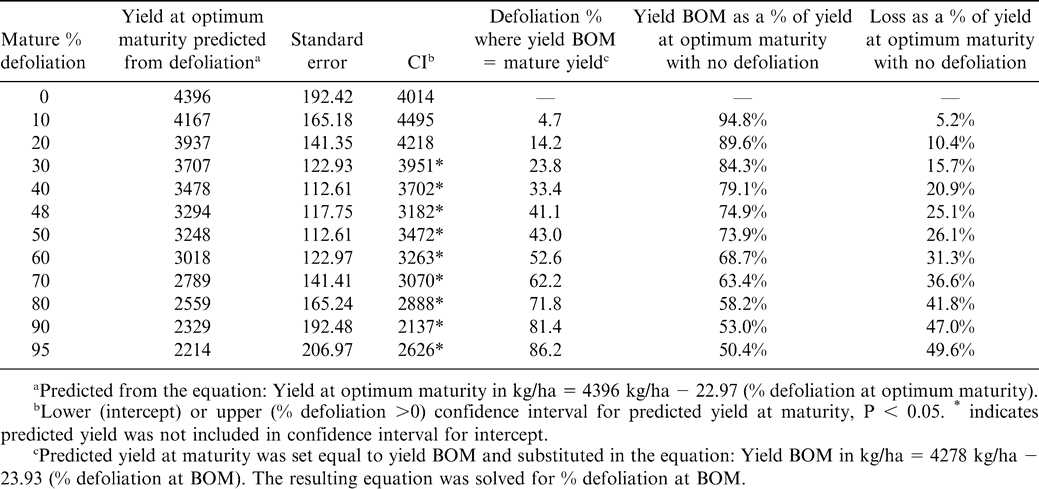
Yield at optimum harvest was calculated by substituting fixed values of percent defoliation using equation 2. That yield was assumed to be the same as the yield before optimum harvest and substituted into equation 3. Equation 3 was then solved for the level of defoliation at 6–12 days BOM that resulted in yield BOM equal to that at optimum maturity with the corresponding higher defoliation at maturity. Confidence intervals were calculated to compare predicted yields with the intercept of equation 1. Predicted BOM yields were calculated as a percentage of yield at optimum maturity and these values were subtracted from 100% to express yield loss for the fixed range of defoliation values.
Because yield decreased linearly with defoliation in both models, a threshold level of defoliation below which no yield loss occurred could not be assumed. However, when predicted yields for fixed levels of defoliation at optimal maturity were compared to the intercept of model 2, the yield corresponding to approximately 30% defoliation at maturity (3951 kg/ha) fell outside the confidence interval for the respective predicted values, indicating a measurable yield loss at 30% defoliation (Table 4). The corresponding defoliation before optimum maturity giving the same yield 6–12 days BOM was about 24% (Table 4). This yield represented a loss of approximately 16% compared to the intercept of 4396 kg/ha. Similarly, 25% yield loss was found with 48% defoliation at optimum maturity or 41% defoliation 6–12 BOM, and approximately 50% yield loss was found with 95% defoliation at optimum maturity, which was equivalent to yield loss for 86% defoliation 6–12 BOM.
Conclusions
As described by Backman and Crawford (1984) and Nutter and Littrell (1996), we found linear relationships between defoliation and yield; this implied that all increases in defoliation decreased yield. The regression models for both digging times were similar, predicting between 24 and 23 kg/ha in yield reductions for every 1% increase in defoliation as assessed at 6–12 days BOM or at optimum maturity, respectively. The yield loss per defoliation unit was about half the 57 kg/ha found by Backman and Crawford (1984), perhaps indicating greater resistance or tolerance in modern virginia market-type cultivars compared to Florunner. Maximum yields (intercepts) of around 4400 kg/ha were strikingly similar to those reported by Backman and Crawford (1984). Predicted yield loss at 95% defoliation was about 50% at optimum maturity, agreeing with statements commonly found in the literature (Nutter and Shokes,1995).
In the bi-weekly (five spray) fungicide program, two applications of tebuconazole and one application of pyraclostrobin were made. Thus, some of the unexplained variability in yield probably should be attributed differences in stem rot control. We consistently find moderate (e.g., r = −0.40 to −0.55) negative correlations between stem rot and yield in fungicide evaluation trials (B. Shew, unpublished). However, in multiple regression of fungicide trial data from Lewiston-Woodville in 2003 and 2004, stem rot incidence accounted for only 1.4% of variability in yield whereas leaf spot, defoliation, and web blotch accounted for 17% and CBR incidence accounted for 15% of yield variability (B. Shew, unpublished).
Yield loss predicted from the regression equations also illustrated the difficulty in selecting a trigger for early digging. Generally, the differences in percent defoliation needed to give equivalent yields at early or mature digging were small; for example 24% defoliation BOM was found to give yield equivalent to 30% defoliation at optimum maturity (Table 4). Confidence intervals for values predicted from the regression equations indicated that this level of defoliation would cause detectable yield loss. Because we found little to no (and sometimes negative) gain in yield in the individual trials in the period from 6–12 days BOM to optimum maturity (Table 2), perhaps it would be advisable to dig if defoliation predicts measurable yield loss, regardless of days to projected maturity.
Results from analyses within fungicide programs with varying levels of canopy defoliation demonstrate the complexity of determining whether early digging is advisable. Inconsistent response most likely reflects the difficulty in clearly defining the balance between pod shed and increased pod maturation that result in higher yield and improved market grades. Complicating the results of our research was added variability associated with different cultivars, two locations with experiments conducted in different fields, and a range of edaphic and environmental conditions. Additionally, the experimental constraint of digging at specific intervals meant that soil conditions were not always optimum, and invariably soil contained more moisture than desirable in some instances. In other cases, digging was delayed or peanut was dug sooner to avoid poor digging conditions. The range of days between early digging and digging at optimum maturity varied by 6 days, and during late September and early October one week can greatly influence pod maturation and pod shed. Variation in the causal agent of defoliation in experiments, namely early leaf spot, late leaf spot, or web blotch, also may have contributed to variation and difficulty in establishing clear relationships. A clear recommendation was not developed from this research to assist growers in deciding whether digging prior to optimum maturity, which minimizes pod shed, is more advantageous than leaving peanut in the field until optimum maturity although greater risk of pod shed exists. Additionally, the impact of disease on the maturation of individual pods that remain on the plant is poorly understood, and this unknown may contribute to variation in responses observed in these experiments. Further research is also needed to quantify incidence of these and other diseases with respect to benefits of digging peanut prior to optimum pod maturity. These data reinforce value of controlling early leaf spot, late leaf spot, and web blotch with timely fungicide applications and the importance of digging at optimum pod maturation.
Acknowledgements
The North Carolina Peanut Growers Associated and The North Carolina Biotechnology Center grant 2002-CFG-8012, and the Peanut CRSP through the United States Agency for International Development grant LAG-G-00-96-0013-00 supported this research financially. Appreciation is expressed to staff at the Peanut Belt Research Station and the Upper Coastal Plain Research Station and to Dewayne Johnson and Carl Murphey for technical support.
Literature Cited
Aquino V. M. , Shokes F. M. , Berger R. D. , Gorbet D. W. , and Kucharek T. A. 1992 Relationships among late leafspot, healthy leaf area duration, reflectance and pod yield of peanut. Phytopathology 82 : 546 – 52 .
Backman P. A. and Crawford M. A. 1984 Relationship between yield loss and severity of early and late leafspot diseases of peanut (Arachis hypogaea). Phytopathology 74 : 1101 – 1103 .
Bowen K. L. , Hagan A. K. , and Weeks J. R. 1997 Number of tebuconazole applications for maximizing disease control and yield of peanut in grower's fields in Alabama. Plant Disease 81 : 927 – 931 .
Brandenburg R. L. 2007 Peanut insect management. 75 – 93 In 2007 Peanut Information North Carolina Coop. Ext. Serv. Ser. AG-331 132 .
Johnson C. S. , Phipps P. M. , and Beute M. K. 1986 Cercospora leafspot management decisions: an economic analysis of a weather-based strategy for timing fungicide applications. Peanut Sci 13 : 82 – 85 .
Jordan D. L. 2007a Peanut production practices. 23 – 46 In 2007 Peanut Information, North Carolina Coop. Ext. Ser. AG-331. 132 pp.
Jordan D. L. 2007b Peanut weed management. 47 – 74 In 2007 Peanut Information North Carolina Coop. Ext. Ser. AG-331. 132 pp.
Jordan D. , Johnson D. , Spears J. , Penny B. , Shew B. , Brandenburg R. , Faircloth J. , Phipps P. , Herbert A. , Coker D. , and Chapin J. 2005 Determining peanut pod maturity and estimating the optimal digging date: using pod mesocarp color for digging virginia market type peanut. North Carolina Cooperative Extension Service Publication AG-633
Jordan D. L. , Spears J. F. , and Sullivan G. A. 1998 Influence of digging date on yield and gross return of virginia-type peanut cultivars in North Carolina. Peanut Sci 25 : 45 – 50 .
Mozingo R. W. , Coffelt T. A. , and Wright F. S. 1991 The influence of planting and digging dates on yield, and grade of four virginia-type peanut cultivars. Peanut Sci 18 : 55 – 62 .
Nutter F. W. and Littrell R. H. 1996 Relationships between defoliation, canopy reflectance and pod yield in the peanut-late leafspot pathosystem. Crop Prot 15 : 135 – 142 .
Nutter F. W. , Littrell R. H. , and Brenneman T. B. 1990 Utilization of a multispectral radiometer to evaluate fungicide efficacy to control late leaf spot in peanut. Phytopathology 80 : 102 – 108 .
Nutter F. W. and Shokes F. M. 1995 Management of foliar diseases caused by fungi. 65 – 73 In Melouk H. A. and Shokes F. M. Peanut Health Management The Am. Phytopathological Soc., St. Paul, MN. 117 pp.
Pixley K. V. , Boote K. J. , Shokes F. M. , and Gorbet D. W. 1990 Growth and partitioning characteristics of four peanut genotypes differing in resistance to late leaf spot. Crop Sci 30 : 796 – 804 .
Sherwood J. L.
,
Buete M. K.
,
Dickson D. W.
,
Elliott V. J.
,
Nelson R. S.
,
Opperman C. H.
, and
Shew B. B.
1995
Biological and biotechnological control in Arachis diseases.
160
–
206
In
Patteee H. E.
and
Stalker H. T.
Advances in Peanut Science,
Am. Peanut Res. and Educ. Soc., Inc., Stillwater, OK. 614 pp.
Shew B. B. 2007 Peanut disease management. 94 – 118 In 2007 Peanut Information, North Carolina Coop. Ext. Ser. AG-331. 132 pp.
Sholar J. R.
,
Mozingo R. W.
, and
Beasley J. P.
1995
Peanut cultural practices.
354
–
382
In
Pattee H. E.
and
Stalker H. T.
Advances in Peanut Science,
Amer. Peanut Res. Educ. Soc., Inc., Stillwater, OK. 614 pp.
Williams E. J. and Drexler J. S. 1981 A non-destructive method for determining peanut pod maturity. Peanut Sci 8 : 134 – 141 .
Notes
1 Former Graduate Assistant and Professor, Department of Crop Science, North Carolina State University, Box 7620, Raleigh, NC 27695; Research Assistant Professor and Professor, Department of Plant Pathology, Box 7616, North Carolina State University, Raleigh, NC 27695; Research Scientist, Syngenta Crop Protection, Inc., P.O. Box 18300, Greensboro, NC 27419; Professor, Department of Plant Pathology, Box 7405, North Carolina State University, Raleigh, NC 27695; and Professor, Department of Entomology, Box 7613, Raleigh, NC 27695.
*Corresponding author's e-mail: david_jordan@ncsu.edu.