Introduction
The National Peanut Council, which represents all segments of the U.S. peanut (Arachis hypogaea L.) industry, has identified preharvest aflatoxin contamination (PAC) as the most serious challenge facing the industry. Lamb and Sternitzke (2001) estimated that aflatoxin contamination costs over $20 million in losses to the southeast U.S. peanut industry. Progress in developing resistant cultivars would represent a major advance for the U.S. peanut industry.
There are two requirements for developing cultivars with resistance to preharvest aflatoxin contamination. First, there must be genetic variance for resistance in order to incorporate a gene or genes for resistance into cultivars. The second requirement is the availability and use of reliable and efficient screening techniques to identify plants containing genes for resistance. When this project was initiated, techniques were not available for large scale screening which is required for germplasm evaluations and/or plant breeding research. To be acceptable, the techniques must have a small number of escapes (uncontaminated susceptible samples) to avoid keeping susceptible types in the breeding program, and a relatively low coefficient of variation (C.V.) to accurately differentiate levels of resistance.
Development of Screening Techniques
Aflatoxin contamination in peanut is an extremely variable characteristic that primarily occurs under heat and drought stress (Wilson and Stansell, 1983; Cole et al., 1995). Holbrook et al. (1994) developed a large scale field system for screening peanut germplasm for resistance to aflatoxin contamination. Yuma, AZ was chosen as a screening site because it consistently has hot and dry conditions. This study demonstrated that aflatoxin contamination would occur in peanut subjected to a late summer drought stress and the extreme soil temperatures which occur at Yuma. Also concluded from this study was that the use of subsurface irrigation during periods of drought stress resulted in higher and more consistent contamination.
A moveable greenhouse system (Atlas Greenhouse Systems, Inc., Alapaha, GA) was developed to provide a screening site at Tifton, GA. Thirteen large (9.1 m wide x 25.5 m long) rainout shelters were constructed on skids. These structures can be moved in the field with tractors and are parked on the test plots for the 40 d immediately preceding harvest to provide the extended period of heat and drought stress necessary for consistent aflatoxin contamination of susceptible genotypes. They also can be used with two planting dates each season. This system is being successfully used to screen for resistance to preharvest aflatoxin contamination at Tifton.
Artificial inoculation is frequently used when screening germplasm for resistance. Artificial inoculation ensures uniform testing conditions which reduces the number of escapes and variation in the data which could mask genetic differences. The standard method for inoculating peanut with Aspergillus had been a spore suspension in water applied at midbloom. This provided a high initial fungal pressure; however, soil populations of Aspergillus rapidly declined shortly after inoculation. Will et al. (1994) developed a new inoculation method using cracked corn as a carrier and a food base for the fungus, thus resulting in more stable fungal inoculum on developing pods. This method resulted in significantly greater soil populations of Aspergillus at harvest than the use of water as a carrier. This inoculation technique should help reduce the inherent variability of preharvest aflatoxin contamination and is being used for our germplasm screening procedure.
Aflatoxin contamination is subject to a large amount of variability from the environmental effects of years and planting dates within years. To allow for comparisons across environments Relative toxin is calculated as follows:
Relative toxin = Entry mean PAC / Test mean PAC. Eq. 1
Relative yield is calculated as follows:
Relative yield = Entry mean yield / Test mean yield. Eq. 2
Genotypes that have relatively low toxin and/or relatively high yield are advanced in the breeding program.
Screening techniques for PAC in standard greenhouse facilities were developed by Anderson et al. (1996) by optimizing drought-stress and fungal infections. High amounts of preharvest aflatoxin accumulation were produced by completely isolating the pod zone and only providing restricted moisture to the root zone. These greenhouse methods have proven to be useful tools for identifying and studying sources of resistance to aflatoxin in peanut.
Screening for Sources of Resistance
Because of the large amount of field variation for PAC, very little was known about the amount of genetic variation in peanut before the 1990s. Genetic variation had been identified for resistance to seed colonization under laboratory conditions (Mixon and Rogers, 1973; Mehan et al., 1981; Mixon, 1986); however, there is not a strong correlation between this trait and resistance to aflatoxin contamination under drought and heat stress (Blankenship et al., 1985; Anderson et al., 1995).
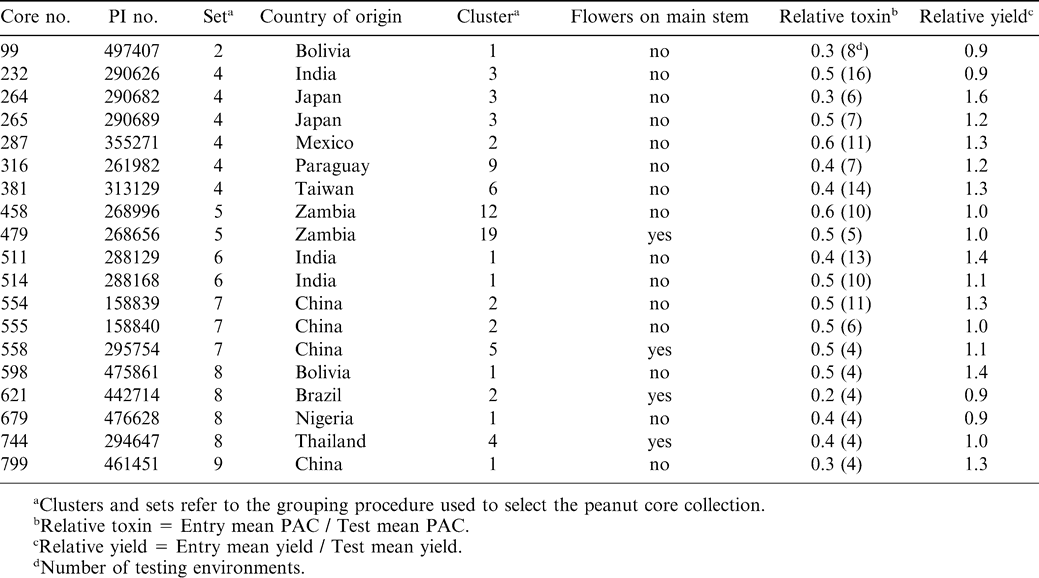
The USDA maintains an extensive working collection of A. hypogaea germplasm consisting of more than 9,000 entries (Holbrook, 2001). This germplasm collection has served as a valuable source of diversity for resistance to many pathogens of peanut (Holbrook and Isleib, 2001). However, it is not feasible to evaluate this collection, in its entirety, for resistance to PAC using currently available screening techniques. Thus, a core collection (a subsample of an entire germplasm collection which has been selected to represent the genetic variability of the entire collection) consisting of 831 accessions was selected from the U.S. germplasm collection of peanut (Holbrook et al., 1993). All accessions in the peanut core collection were first examined for PAC in preliminary screens using five replications in one environment. Genotypes which had low contamination levels were then examined for a 2nd year using 10 replications in two environments. Nineteen core accessions were identified with low levels of aflatoxin contamination in multiple environments (Table 1). Some of these accessions also exhibited high relative yield.
The theory behind core collections is that the multivariate clustering procedure groups accessions that are genetically similar. We previously demonstrated that this clustering procedure was effective in clustering material that had resistance to late leaf spot [Cercosporidium personatum (Berk. & M.A. Curtis)] (Holbrook and Anderson, 1995) and the peanut root-knot nematode [Meloidogyne arenaria (Neal) Chitwood race 1] (Holbrook et al., 2000b). Set, country, and cluster information for the 19 accessions with resistance to PAC is included in Table 1. These clusters should be promising places to search for additional sources of resistance to PAC. The three clusters that were represented by two resistant accessions (Cluster 3 from Japan set 4; Cluster 1 from India set 6; and Cluster 2 from China set 7) are particularly noteworthy and should be examined in their entirety for additional sources of resistance.
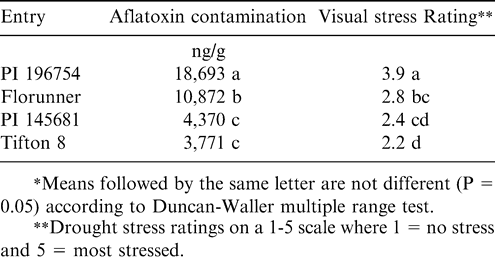
Aflatoxin contamination is an expensive characteristic to measure and is subject to extreme variability. The development of resistant cultivars could be accelerated if an effective characteristic for indirect selection was found. Holbrook et al. (2000a) evaluated the resistance to preharvest aflatoxin contamination in a set of genotypes that had been documented with varying levels of drought tolerance (Rucker et al., 1995) and determined the correlation of drought tolerance characteristics with aflatoxin contamination. Genotypes that exhibited drought tolerance also exhibited reduced aflatoxin contamination in Tifton. PI 196754, a drought-intolerant genotype had greater PAC than the check cultivar Florunner (Table 2). Two drought tolerant genotypes (PI 145681 and Tifton 8) had less PAC than Florunner. Other genotypes that had drought tolerance and relatively low PAC included PI 259639, PI 269106, PI 295722, PI 298836, and PI 315628 (Holbrook et al., 2000a). Significant positive correlations were observed between aflatoxin contamination and leaf temperature, and between aflatoxin contamination and visual stress ratings. A significant negative correlation was also observed between aflatoxin contamination and yield under drought-stress conditions. Leaf temperature, visual stress ratings, and yield are all less variable and cheaper to measure than aflatoxin concentration. These characteristics may be useful as indirect selection tools for selecting lines with reduced aflatoxin contamination.
Aflatoxin contamination in a drought-tolerant, a drought-intolerant, and two check genotypes grown in two tests at Tifton, GA in 1997* (Holbrook et al., 2000a).
Guo et al. (2006) identified a novel PLD gene in peanut, encoding a putative phospholipase D (a main enzyme responsible for the drought-induced degradation of membrane phospholipids in plants). PLD expression was induced faster by drought stress in the drought sensitive lines than in the drought tolerant lines, suggesting that peanut PLD may be involved in drought sensitivity responses, which could be useful as a tool in germplasm screening for drought tolerance. Liang et al. (2005, 2006) investigated the possible association of storage proteins with resistance to aflatoxin contamination and used total protein profiles to identify possible proteins as resistance “markers”. Identification of authentic resistance related protein markers and/or genes could lead to the enhancement of antifungal activities in peanut seeds through marker-assisted selection, but this methodology has not been successful to date.
Epidermal conductance was also evaluated as a potential drought tolerance trait (Cantonwine et al., 2006). Unfortunately, the genetic variation in epidermal conductance does not appear to be large enough to be useful in a breeding program. Use of the SPAD chlorophyll meter reading (SCMR) as a possible selection criteria (Dong et al., 2002) did not result in significant correlations between SCMR and visual drought stress ratings or between SCMR and aflatoxin contamination. More promising results were observed in the use of ground-based remote sensing of canopy reflectance as a selection criteria for drought- and aflatoxin-resistant peanut genotypes (Sullivan and Holbrook, 2007).
Breeding Peanut for Resistance to PAC
Sources of resistance to PAC and/or drought have been crossed with high yielding cultivars and breeding lines that also have resistance to tomato spotted wilt virus (TSWV). These populations are advanced to the F4 generations using single seed descent. The seed from individual F4 plants are harvested and F4:5 progeny are grown in small plots and subjected to selection pressure for yield and resistance to TSWV. The selections are tested the following year under our drought shelters in Tifton using five replications. Progeny that exhibit high relative yield and/or low relative PAC are retested using 10 replications. This procedure has been used to develop late generation breeding lines with resistance to drought. Several of these lines also exhibited reduced aflatoxin contamination in multiple environments.
Breeding for resistance to the peanut root-knot nematode may also result in reduced aflatoxin contamination of peanut. Timper et al. (2004) demonstrated that infection of peanut by M. arenaria can lead to an increase in aflatoxin contamination of peanut seeds when the plants are subjected to drought stress during pod maturation. Holbrook et al. (2008) recently released Tifguard, a nematode-resistant cultivar with excellent resistance to TSWV. Research is ongoing to determine if this cultivar can be used as a tool to reduce aflatoxin contamination in the southeastern U.S.
Literature Cited
Anderson W. F. , Holbrook C. C. , and Wilson D. M. 1996 Development of greenhouse screening for resistance to Aspergillus parasiticus infection and preharvest aflatoxin contamination in peanut. Mycopathologica 135 : 115 – 118 .
Anderson W. F. , Holbrook C. C. , Wilson D. M. , and Matheron M. E. 1995 Evaluation of preharvest aflatoxin contamination in some potentially resistant peanut genotypes. Peanut Sci 22 : 29 – 32 .
Blankenship P. D. , Cole R. J. , and Sanders T. H. 1985 Comparative susceptibility of four experimental peanut lines and the cultivar Florunner to preharvest aflatoxin contamination. Peanut Sci 12 : 70 – 72 .
Cantonwine E. , Maddie S. , Buchannon B. , Holbrook C. C. , and Kvien C. K. 2006 Evaluation of epidermal conductance as a potential drought tolerant trait of peanut. Proc. Aflatoxin Elimination Workshop 72 .
Cole R. J. , Dorner J. W. , and Holbrook C. C. 1995 Advances in mycotoxin elimination and resistance. 456 – 474 In Pattee H. E. and Stalker H. T. Advances in Peanut Science. Amer. Peanut Res. and Educ. Soc Stillwater, OK.
Dong W. , Holbrook C. C. , Guo B. , Luo M. , Wilson D. M. , Kvien C. K. , and Rowland D. L. 2002 Evaluation of physiological measurements of drought stress to indirectly select for reduced aflatoxin contamination in peanut. Proc. Aflatoxin Elimination Workshop 145 .
Guo B. Z. , Xu G. , Cao Y. G. , Holbrook C. C. , and Lynch R. E. 2006 Identification and characterization of phospholipase D and its association with drought susceptibilities in peanut (Arachis hypogaea). Planta 223 : 512 – 520 .
Holbrook C. C. 2001 Status of the Arachis germplasm collection in the United States. Peanut Sci 28 : 84 – 89 .
Holbrook C. C. and Anderson W. F. 1995 Evaluation of a core collection to identify resistance to late leafspot in peanut. Crop Sci 35 : 1700 – 1702 .
Holbrook C. C. , Anderson W. F. , and Pittman R. N. 1993 Selection of a core collection from the U.S. germplasm collection of peanut. Crop Sci 33 : 859 – 861 .
Holbrook C. C. and Isleib T. G. 2001 Geographical distribution of genetic diversity in Arachis hypogaea. Peanut Sci 28 : 81 – 85 .
Holbrook C. C. , Kvien C. K. , Ruckers K. S. , Wilson D. M. , and Hook J. E. 2000a Preharvest aflatoxin contamination in drought tolerant and intolerant peanut genotypes. Peanut Sci 27 : 45 – 48 .
Holbrook C. C. , Matheron M. E. , Wilson D. W. , Anderson W. F. , Will M. E. , and Norden A. J. 1994 Development of a large-scale field screening system for resistance to pre-harvest aflatoxin contamination. Peanut Sci 21 : 20 – 22 .
Holbrook C. C. , Timper P. , Culbreath A. K. , and Kvien C. K. 2008 Registration of ‘Tifguard’ peanut. J. Plant Registrations 2 : 92 – 94 .
Holbrook C. C. , Timper P. , and Xue H. Q. 2000b Evaluation of the core collection approach for identifying resistance to Meloidogyne arenaria in peanut. Crop Sci 40 : 1172 – 1175 .
Lamb M. C. and Sternitzke D. A. 2001 Cost of aflatoxin contamination to the farmer, buying point, and sheller segments of the southeast United States peanut industry. Peanut Sci 28 : 59 – 63 .
Liang X. , Holbrook C. C. , Lynch R. E. , and Guo B. Z. 2005 Beta-1,3-glucanase activity in peanut seed (Arachis hypogaea) is induced by inoculation with Aspergillus flavus and copurifies with a conglutin-like protein. Phytopathology 95 : 506 – 511 .
Liang X. Q. , Luo M. , Holbrook C. C. , and Guo B. Z. 2006 Storage protein profiles in Spanish and runner market type peanuts and potential markers. BMC Plant Biology 6 : 24 .
Mehan V. K. , McDonald D. , Nigam S. N. , and Lalitha B. 1981 Groundnut cultivars with seed resistant to invasion by Aspergillus flavus. Oleagineux 36 : 501 – 507 .
Mixon A. C. 1986 Reducing Aspergillus species infection of peanut seed using resistant genotypes. J. Environ. Qual 15 : 101 – 103 .
Mixon A. C. and Rogers K. M. 1973 Peanut accessions resistant to seed infection by Aspergillus flavus. Agron. J 65 : 560 – 562 .
Rucker K. S. , Kvien C. K. , Holbrook C. C. , and Hook J. E. 1995 Identification of peanut genotypes with improved drought avoidance traits. Peanut Sci 21 : 14 – 18 .
Sullivan D. G. and Holbrook C. C. 2007 Using ground-based reflectance measurements as selection criteria for drought- and aflatoxin-resistant peanut genotypes. Crop Sci 47 : 1040 – 1050 .
Timper P. , Wilson D. M. , Holbrook C. C. , and Maw B. W. 2004 Relationship between Meloidogyne arenaria and aflatoxin contamination in peanut. J. of Nematol 36 : 167 – 170 .
Will M. E. , Holbrook C. C. , and Wilson D. M. 1994 Evaluation of field inoculation techniques for screening of peanut genotypes for reaction to preharvest A. flavus group infection andaflatoxin contamination. Peanut Sci 21 : 122 – 125 .
Wilson D. M. and Stansell J. R. 1983 Effect of irrigation regimes on aflatoxin contamination of peanut pods. Peanut Sci 10 : 54 – 56 .
Notes
Author Affiliations
1 Research Geneticists, Crop Genetics and Breeding Res. Unit, USDA-ARS, Tifton, GA 31793.
2 Research Plant Pathologist, Crop Protection and Management Res. Unit, USDA-ARS, Tifton, GA 31793.
3 Dept. of Plant Path., Univ. of Georgia (Retired), Tifton, GA 31793.
4 Research Plant Pathologist, Crop Protection and Management Res. Unit, USDA-ARS, Tifton, GA 31793.
*Corresponding author: Email: Corley.Holbrook@ars.usda.gov