Introduction
Aflatoxins are the toxic secondary metabolites produced by Aspergillus flavus (Link) and A. parasiticus (Speare) contaminating staple crops like peanut (groundnut), maize, sorghum, pearl millet, chillies, pistachio, cassava, etc., and even found in milk from animals fed with the contaminated feed. Aflatoxin contamination effects quality of the produce, and food safety. It was reported that losses due to aflatoxin contamination in peanut was more than $26 million in the USA alone (Lamb and Sternitzke, 2001). Warm humid or drought conditions, insect/nematode pod damage in the field, over mature crops, rain at harvest, storing of improperly dried grains in the storage favor the proliferation of the fungi and subsequent aflatoxin production in peanut (Craufurd et al., 2006). Also, the existing technologies for peanut production, processing, and storage practices in most developing countries in the tropics and semi-arid tropics makes it difficult to totally eliminate aflatoxins, making them unavoidable contaminants. The problem of aflatoxin contamination is invisible and it is difficult to sort out the few contaminated grains from the commercial grain lots when it is present at low to moderate levels. In the absence of resistant varieties or technologies that eliminate the aflatoxin contamination in the food chain to mitigate hazardous effects on human and livestock health, it is essential to test the food products for aflatoxins before they are consumed (Waliyar et al., 2003). So, it is important to be able to detect and quantify aflatoxins in commodities to protect human and animal health.
Rationale for the development of immunological methods
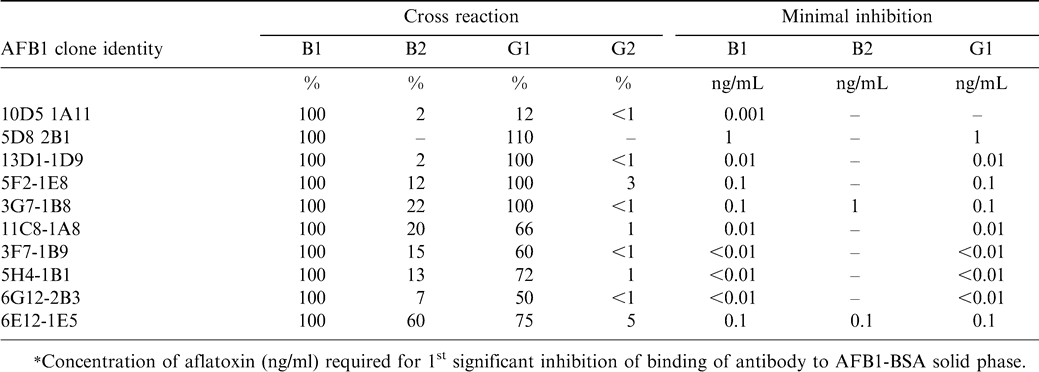
There are several chemical methods available for detection and estimation of aflatoxin, including high performance liquid chromatography (HPLC), thin layer chromatography (TLC), and mini-column methods. However, most of these methods are expensive, laborious, time consuming, and require extensive sample cleanup (Dell et al., 1990; Goto and Manabe, 1989). There has been increase in demand for monitoring aflatoxins in developing countries in South-East Asia and sub-Saharan Africa (SSA), where high incidence of liver and other cancers prevail. To assess the risk posed by aflatoxin contamination through food and feeds, also to develop the resistant varieties or testing procedures to minimize aflatoxin, there is need for simple cost effective technologies for aflatoxin detection and estimation. The aflatoxin analysis by physiochemical methods such as HPLC, HPTLC, and TLC is expensive, laborious, requires extensive sample clean up, and is time consuming. This demand led researchers at ICRISAT to develop low cost technologies for aflatoxin estimation using immunological methods. Polyclonal antibodies were produced to aflatoxin B1 (AFB1) and M1 (AFM1), and by using advances in hybridoma technology, monoclonal antibodies to aflatoxins also were produced for the detection of total aflatoxins (Devi et al., 1999; Thirumala-Devi et al., 2002). Ten hybridoma cell lines were selected that produce monoclonal antibodies with a range of specificities among the four major aflatoxins (Table 1). The monoclonal and polyclonal antibodies were used to develop a simple, sensitive, specific and inexpensive competitive enzyme-linked immunosorbent assay (cELISA) that has lower detection limits (1.0 µg/kg) and cost (about $1 per sample) less than other available methods. More than 100 samples can be analyzed in a day and the results obtained are comparable with HPLC analyses. The cELISA assay is simple, easy to perform, requires minimum laboratory facilities, and most of the chemicals are available locally in developing countries. Moreover sample extraction for ELISA test is simple and quick involving single step methanol extraction. Consequently, many types of immunoassays, including radioimmunoassay (RIA) and enzyme-linked immunosorbent assay (ELISA), as well as several novel immunochemical screening tests were developed.
Competitive ELISAs
The distinguishing feature of the competitive assay format is that the combination of an unknown amount of analyte introduced from the sample and the reference analyte compete for binding to a limited number of antibody binding site. This assay can be performed with either the analyte or the antibody adsorbed to the solid phase. Two types of cELISAs have been developed for the analysis of aflatoxins, and both types are heterogeneous assays which produce the uniform results. Direct ELISA involves the use of an aflatoxin-enzyme conjugate, whereas indirect ELISA involves protein-aflatoxin conjugate and a second antibody to which the enzyme has been conjugated (Ramakrishna and Mehan, 1993).
Indirect competitive ELISA
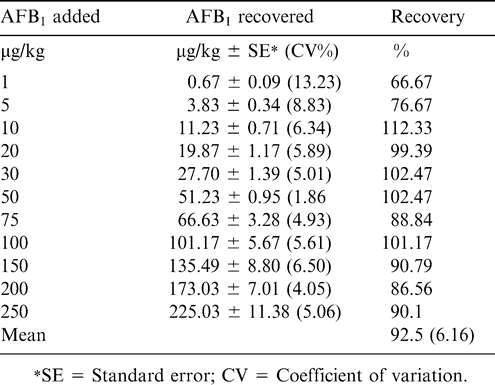
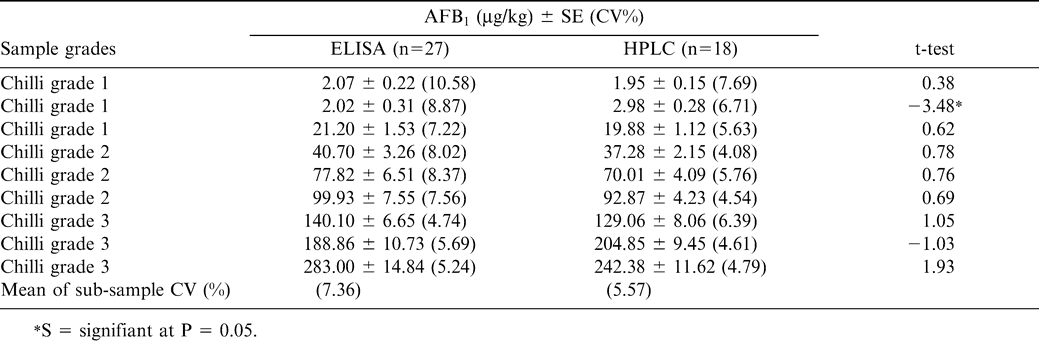
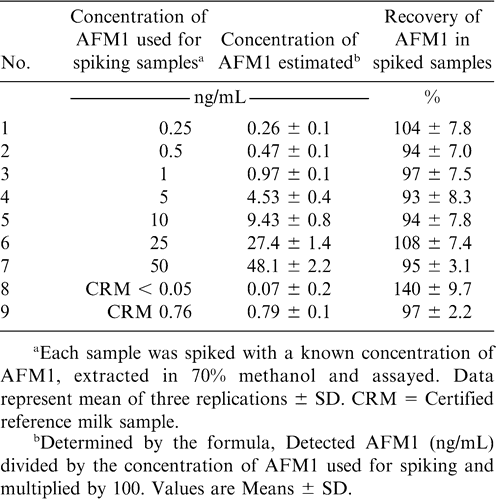
In the indirect competitive ELISA, commercially available AFB1-BSA conjugate was coated on to the wells of a microtiter plate. Later, aflatoxin standards, as well as samples with specific rabbit antibodies, were added to the plate before incubating it at 37 C for 1 hr. After washing the plate to determine the amount of antibody bound in the wells, goat anti-rabbit IgG labeled with alkaline phosphatase was added followed by addition of p-nitrophenyl phosphate substrate. Thus, toxin in the sample or standard and toxin bound on the well surface competes for the binding site on the specific antibody in the solution (Waliyar et al., 2005a). Aflatoxin recovery studies showed that mean recoveries of AFB1 from the peanut samples spiked with 1–250 µg/kg of toxin ranged from 67 to 112% (Table 2). Also, there was good correlation between ELISA and HPLC estimations (Table 3) in eight of the nine chilli samples tested in which the toxin ranged from 2–283 µg/kg (Reddy et al., 2001). Similarly, AFM1 recoveries ranged from 94–100% in artificially contaminated milk samples in the indirect competitive ELISA (Table 4). Indirect competitive ELISA requires less antiserum than direct competitive ELISA and does not require preparation of a toxin-enzyme conjugate, but it takes 1 hr of extra analytical time. To shorten the assay time for indirect ELISA, modifications can also be made by conjugating the enzyme to the antibody, which is then used in ELISA instead of using a second antibody-enzyme conjugate. A number of studies have been carried out to investigate the efficacy of direct and indirect immunoassays by comparing them with HPLC or TLC and there was good correlation among the methods (Ramakrishna and Mehan, 1993; Reddy et al., 2001).
Direct competitive ELISA
In the direct competitive assay, specific antibodies are first coated on to a high quality high binding microtiter plate. Then the sample solution or various concentrations of standard toxin are generally incubated simultaneously with the enzyme conjugate in the ELISA plate. After appropriate washings, the amount of enzyme bound to the plate is determined by incubation with suitable substrate (p-Nitrophenyl phosphate for the alkaline phosphatase enzyme). At ICRISAT, a different kind of conjugate was prepared using commercially available AFB1-BSA which was conjugated to enzyme alkaline phosphatase or penicillinase or horse radish peroxidase and used in the direct ELISA instead of AFB1-enzyme conjugate (Anjaiah et al., 1989; Waliyar et al., 2005a). Moreover, this procedure is simple and the conjugate is stable at 4 C for more than a year. In the direct ELISA, toxin in the sample and toxin-enzyme conjugate compete for the antibody binding coated on the solid plate surface.
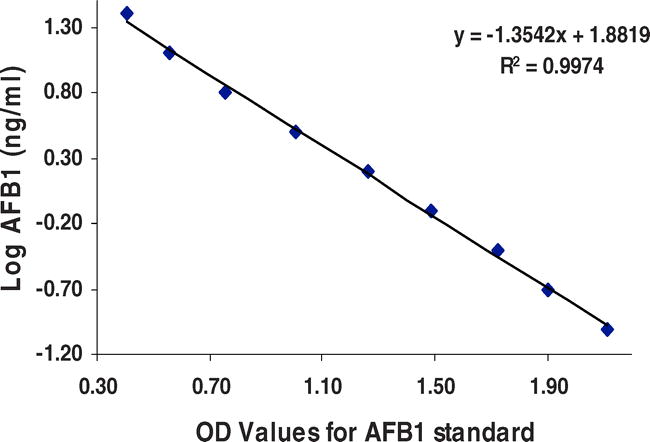
In both the cELISAs, after addition of the substrate, the resulting color development is then measured spectrophotometrically at 405 nm using an ELISA reader to record the optical density values for standards as well as unknown samples. To calculate the aflatoxin content in the unknown samples, a regression curve using AFB1 standard's optical density (OD) values was drawn, and based on the regression equation the AFB1 in the samples was determined (Fig. 1). Because the antibody and toxin-enzyme concentrations are constant, the color intensity as a function of enzyme reaction is inversely proportional to the toxin concentration in the testing samples or toxin standards. Less color indicates more toxin while more color indicates less/no toxin. In general, cELISA are approximately 10–100x more sensitive than radio immunoassay (RIA) when purified toxins are used. As little as 5 pg of pure aflatoxin can be measured. Since a clean-up step is generally not necessary, many samples can be analyzed within a relatively short period. The sensitivity of the cELISAs for the analysis of aflatoxins in foods and feeds is generally in the range of 0.1–250 µg/kg. Due to the use of better antibody and toxin-enzyme conjugates, the time required to run the cELISA has improved considerably. Thus, the entire ELISA procedure can be completed within 2 hr (Chu, 1995; Lee et al., 2004). The sensitivity of the ELISA is improved when the clean-up treatment is included in the assay protocols. Many mycotoxin detection kits based on immunoassays are available commercially; however, they are very expensive and some times non-specific reactions develop probably due to storage problems.
The application of mycotoxin immunoassays is not limited to foods and feeds; it has been used as a sensitive approach for monitoring of mycotoxins in body fluids and tissues and organs of humans and animals that have been exposed to the mycotoxins. Currently we are developing immunoassays to detect the aflatoxin in human and animal blood. This will not only be helpful in detecting aflatoxin in the human blood but also for monitoring aflatoxicosis in human beings and livestock (Anitha et al., 2007).
Matrix interference
One of the common challenges of immunoassay for food analysis is matrix interference causing false positives. Because there is a high probability of the presence of structurally related compounds in the sample that may react with the antibody, the sample matrix should be tested before the assay. This occurs when (a) the enzyme activity is inhibited by the presence of interferences in the sample extracts and/or, (b) the interaction between the antigen (AFB1) and the antibody is hindered in an immunoassay (Lee et al., 2004). The matrix interferences with the chilli sample extract were observed and were resolved by diluting the toxin standards in the toxin free extracts of chilli (Reddy et al., 2001). Moreover, these matrix interferences can be reduced by a number of methods such as dilution of sample extractor or removal of interferences by sample clean up procedures. The degree of matrix interference varied with different food samples, so individual validation and optimization of the extraction and estimation protocol would be necessary for each commodity sample type. In most of the immunoassays, sample cleanup is not necessary. Samples taken after extraction from solid matrix could be used directly in the assay after appropriate dilution in the assay buffer. Nevertheless, the sensitivity increased after appropriate clean-up treatment (Waliyar et al., 2005a).
Radioimmunoassay (RIA)
The principle of aflatoxin quantification is similar to cELISA. However, an enzyme label, radio isotope-labeled antibodies or aflatoxin standards are used as reporter molecules. The specific activity of the radioactive marker plays an important role in determining the sensitivity of the assay. Although 14C, 3H, and 125I labeled mycotoxins have been used in various RIAs, 3H labeled toxins were most commonly used. However, 125I labeled toxins have been shown to provide the highest sensitivity (Chu, 1995). The RIA procedure involves incubation of specific antibody simultaneously with a solution of unknown sample or known standard and a constant amount of labeled toxin. After separation of free and bound toxin, the radioactivity in those fractions is determined. The toxin concentration of the unknown sample is determined by comparing the results to the standard curve which is established by plotting the ratio of radio activities in the bound fraction and free fraction vs. log concentration of unlabeled standard toxin.
The RIAs have been used for analysis of aflatoxins in corn, wheat, peanut, milk, serum and eggs with a minimum detection limit ranging from 0.25–0.5 ng in each assay when titrated mycotoxins are used as the marker. However, because of sample matrix interference, the lower limit of mycotoxin detection in food or feed samples is about 2–5 µg/kg. The sensitivity of the RIA can be improved by a sample clean-up procedure after extraction. Although RIA provides sensitive and accurate mycotoxin analysis, use of radioactive legend poses difficulties in safe disposal. Thus, it has been primarily used in the laboratories permitted to use radioisotopes. As a consequence, it mainly remained as a research tool in well established laboratories.
Antibody based immuno-screening tests
By shortening the incubation time and adjusting antibody and toxin-enzyme conjugate concentrations in the direct or modified indirect competitive ELISA system, it is possible to do a quick screening test at certain toxin levels. Based on the principle of antigen-antibody interactions such as in ELISA, several other immuno-screening tests with sensitivity similar to ELISA have been developed. In this, the antibody is immobilized on a paper disk or other affinity membranes, which is used directly as a strip or mounted either on a plastic card or a cup. The reaction is carried out on the wetted membrane disk. Upon completion, the absence of color (or decrease in color that is generally blue) at the sample spot indicates the presence of toxin in the sample; and the entire test can be completed in less than 1 hr.
Another test is the immunoaffinity method, which is applicable to mycotoxins such as aflatoxins that have fluorescence. In this assay, aflatoxin extracted from the sample is first diluted with buffer at pH 7.0 and subjected to disposable affinity column containing anti-aflatoxin antibody coupled with Sepharose gel. Samples such as milk and urine can be applied to the column directly after adjusting the pH and dilution. After washing, aflatoxin is removed from the column with the methanol, subjected to treatment with iodine/bromine solution, and fluorescence is determined. The affinity column serves as a specific clean-up and concentration tool for further analysis by HPLC method (Chu, 1995).
Conclusions
The cELISA tests has provided a unique opportunity for ICRISAT and its partners to select breeding populations possessing resistance to aflatoxin contamination, and to evaluate food, feed and related commodities for aflatoxin contamination. This is contributing to stimulate appropriate interventions to enhance the food and human health safety and enhance trade and farmers' incomes. ICRISAT helped in establishing 17 aflatoxin monitoring laboratories in India, Mozambique, Kenya, Malawi and Mali that use our cELISA technologies. Training was provided to the local personnel to manage the facilities. The diagnostic reagents are widely distributed to partners in Asia and Sub-Sahara Africa. These laboratories are contributing to the quality certification of the farmers produce and enhancing the competitiveness of the produce in domestic and international markets. For instance, National Small Farmer Association of Malawi (NASFAM) and ICRISAT have established collaboration for testing the peanut produced for aflatoxin content. Based on the level of contamination, NASFAM graded peanut lots into permissible (<4 µg kg−1 or <20 µg kg−1) and non-permissible (>20 µg kg−1). Graded peanut lots found favorable market for regional and global export, benefiting the farmers (Waliyar et al., 2005b). Thus, the aflatoxin testing lab at Malawi contributed to the revival of peanut exports to Europe and South Africa. ICRISAT is planning to increase aflatoxin testing facilities to strengthen the local capacities for aflatoxin monitoring in Sub-Sahara Africa and Asia.
Literature Cited
Anitha S. , Waliyar F. , Reddy C. N. , and Lava-Kumar P. 2007 Top-Down strategy (TDS) to prevent the risk of human exposure to aflatoxins. In “2nd Asian Congress of Mygology and Plant Pathology”, 19−22 Dec. 2007, Osmania Univ., Hyderabad, Andhra Pradesh, India. (abstr.). 320 .
Anjaiah V. , Mehan V. K. , Jayanthi S. , Reddy D. V. R. , and McDonanld D. 1989 Enzyme-linked immunosorbent assay (ELISA) for aflatoxin B1 estimation in groundnut. ICRISAT(International Crops Research Institute for the Semi-Arid Tropics) 1989. 183 – 189 In Aflatoxin Contamination of Groundnuts: Proc. of the Internl. Workshop, 6-9 Oct. 1987, ICRISAT Center, India. Patancheru, A.P. 502 324, India.
Chu F. S.
1995
Mycotoxin analysis.
283
–
332
In
Jeon I. J.
and
Ikins W. G.
Analyzing Food for Nutrition Labeling and Hazardous Contaminants
Marcel Dekker, Inc
New York
.
Craufurd P. W. , Prasad P. V. V. , Waliyar F. , and Taheri A. 2006 Drought, pod yield, preharvest Aspergillus infection and aflatoxin contamination on peanut in Niger. Field Crops Res 98 : 20 – 29 .
Dell M. P. K. , Haswell S. J. , Roch O. G. , Coker R. D. , Medlock V. F. P. , and Tomlins K. 1990 Analytical methodology for the determination of aflatoxins in peanut butter: Comparison of high performance thin-layer chromatographic, enzyme-linked immunosorbent assay and high performance liquid chromatographic methods. Analyst 115 : 1435 – 1439 .
Devi K. T. , Mayo M. A. , Reddy K. L. N. , Delfosse P. , Reddy G. , Reddy S. V. , and Reddy D. V. R. 1999 Production and characterization of monoclonal antibodies for aflatoxin B1. Letters in Appl. Microbiol 29 : 284 – 288 .
Goto T. and Manabe M. 1989 Methods of analysis of aflatoxins in groundnut and other agricultural commodities. ICRISAT (International Crops Research Institute for the Semi-Arid Tropics) 1989. 173 – 182 In Aflatoxin Contamination of Groundnuts: Proc. of the Intern. Workshop, 6-9 Oct. 1987. ICRISAT Center, India. Patancheru, A.P. 502 324, India.
Lamb M. C. and Sternitzke D. A. 2001 Cost of aflatoxin to the farmer, buying point and sheller segments of the southeast U.S. peanut industries. Peanut Sci 28 : 59 – 63 .
Lee N. A. , Wang S. , Allan R. D. , and Kennedy I. R. 2004 Rapid aflatoxin B1 ELISA: Development and validation with reduced matrix effects for peanut, corn, pistachio, and soybeans. Jour. of Agric. Food Chemistry 52 : 2746 – 2755 .
Ramakrishna N. and Mehan V. K. 1993 Direct and indirect competitive monoclonal antibody-based ELISA of aflatoxin B1 in groundnut. Mycotoxin Research 9 : 53 – 63 .
Reddy S. V. , Kiran Mayi D. , Uma Reddy M. , Thirumala-Devi K. , and Reddy D. V. R. 2001 Aflatoxin B1 in different grades of chillies (Capsicum anum L.) in India as determined by indirect competitive ELISA. Food Additives and Contaminants 6 : 553 – 558 .
Thirumala-Devi K. , Mayo M. A. , Hall A. J. , Craufurd P. Q. , Wheeler T. R. , Waliyar F. , Subramanyam A. , and Reddy D. V. R. 2002 Development and application of an indirect-competitive enzyme-linked immunoassay for aflatoxin M1 in milk and milk-based confectionary. Jour. of Agric. Food Chem 50 : 933 – 937 .
Waliyar F. , Reddy S. V. , Subramanyam K. , Reddy T. Y. , Ramadevi K. , Craufurd P. Q. , and Wheeler T. R. 2003 Importance of mycotoxins in food and feed in India. Aspects of Appl. Biol 68 : 147 – 154 .
Waliyar F. , Reddy S. V. , and Kumar P. L. 2005a Estimation of Aspergillus flavus Infection and Aflatoxin Contamination in Seeds: Laboratory Manual. ICRISAT (International Crops Research Institute for the Semi-Arid Tropics) 2005, 26pp. Patancheru, A.P. 502 324, India.
Waliyar F. , Siambi M. , Jones R. , Reddy S. V. , Chibonga D. , Kumar P. L. , Denloye S. , and Chinoko Y. 2005b Institutionalization of aflatoxin testing facility in Africa. In Reducing Impact of Mycotoxins in Tropical Agriculture with Emphasis on Health and Trade in Africa. Accra, Ghana. International Institute of Tropical Agriculture and Myco-Globe. (abstr.). 38 .
Notes
Author Affiliations
1 Principal Scientist and Director, West and Central Africa, ICRISAT, BP 12404, Niamey, Niger.
2 Scientific Officer, ICRISAT, Patancheru 502 324, Andhra Pradesh, India.
3 Virologist, IITA, Oyo Road, PMB 5320, Ibadan, Nigeria.
*Corresponding author: Farid Waliyar (email: f.waliyar@cgiar. org)