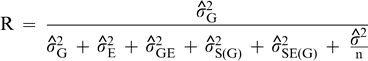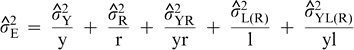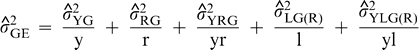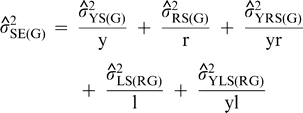Introduction
Flavor and texture are the aspects of sensory quality most often mentioned as important by processors of peanut (Arachis hypogaea L.) in the USA. Manufacturers of peanut products desire to deliver products with consistent flavor and texture. Composition influences the flavor and texture of roasted peanuts and peanut products (Ahmed and Young, 1982; Ahmed and Ali, 1986). Therefore, development of peanut cultivars whose seed composition profiles do not vary greatly from those of existing cultivars is desirable. However, peanut composition is influenced by several groups of factors including environmental factors (associated with years, production regions, locations within regions, and interactions of region and location with year), genetic factors (cultivars or breeding lines), and interaction between environmental and genetic factors (genotype-by-environment or “GxE” interaction) (Tai and Young, 1975; Layrisse et al., 1980; Yadava et al., 1980; Ali and Prasada Rao, 1982; Sykes and Michaels, 1986; Raheja et al., 1987; Branch et al., 1990; Grosso et al., 1994; Harch et al., 1995; Hammond et al., 1997; Ku et al., 1998; Upadhyaya and Nigam, 1999; Dwivedi et al., 2000; Andersen and Gorbet, 2002). In order to ascertain reasonable goals with respect to consistency of seed composition of peanuts, one must know the relative contributions of genotype, environment, and GxE interaction to seed composition.
In 2002, the USDA, Agricultural Research Service, Market Quality and Handling Research Unit in Raleigh, NC (USDA, ARS, MQHRU) implemented a program to evaluate sensory quality and composition of peanuts grown in the Uniform Peanut Performance Test (UPPT) (Branch et al., 2006). The UPPT is a collaborative program in which advanced breeding lines developed by the several public-sector peanut breeding programs are evaluated for agronomic performance and grade across the three US peanut-producing regions. Beginning in 2002, USDA-ARS personnel applied standard composition evaluation procedures to samples of UPPT entries from each test site, measuring oil content, fatty acid profile of seed oil, and contents of various tocopherols and sugars. The UPPT data set provides a unique opportunity to examine the contributions of genotype, environment, and GxE interaction to variation in seed composition. Each year's UPPT includes 13 to 16 breeding lines and cultivars evaluated at each of 9 locations across 7 states. UPPT breeding line entries generally do not remain in the test for more than two years, but the standard cultivars Florunner and NC 7 are included in all years, providing some across-year orthogonality. Within years, there is perfect orthogonality. The objective of this study was to use published UPPT results to estimate variance components associated with environmental, genotypic, and GxE effects on seed composition traits of peanuts.
Materials and Methods
Methodology used by USDA, ARS, MQHRU has been published in annual reports available online (USDA, 2003). The data from the UPPT include seed composition traits measured on bulk samples from the replicate plots grown at each of nine test locations (Suffolk, VA and Lewiston, NC in the Virginia-Carolina production area; Tifton, GA, Marianna, FL, and Headland, AL in the Southeastern production area; and Denver City, TX, Pearsall, TX, Stephenville, TX, and Fort Cobb, OK in the Southwestern production area). Growing conditions at the specific locations are described in the annual reports of yield and grade of UPPT entries (Branch et al., 2003, 2004, 2005, 2006). Processing of the shelled peanuts at the USDA, ARS, National Peanut Research Laboratory resulted in samples separated into medium and jumbo grade size fractions (“sizes”) for runner market types. Whenever possible, both fractions were subjected to analysis of composition. For virginia market type entries, only the extra large kernel fraction was analyzed. During the period of the study, a total of 40 breeding lines and cultivars were evaluated in the UPPT, of which 16 exhibited the high oleic fatty acid trait (Norden et al., 1987; Moore and Knauft, 1989; Knauft, et al., 1993).
Data from the 2002 through 2005 crop years were used in the analysis. The mixed models procedure (PROC MIXED) of the SAS statistical software package (SAS Inst., Cary, NC) was used to apply restricted maximum likelihood (REML) estimation of variance components associated with year, production region, year-by-region interaction, location within regions, year-by-location interaction in region, genotype, year-by-genotype interaction, region-by-genotype interaction, year-by-region-by-genotype interaction, location-by-genotype interaction in region, year-by-location-by-genotype interaction in region, kernel grade (jumbo or medium) within genotype, year-by-grade interaction in genotype, region-by-grade interaction in genotype, year-by-region-by-grade interaction in genotype, location-by-grade interaction in region and genotype. All of these effects were considered random in order to estimate a variance component. All other effects were pooled as a residual effect.
Repeatability coefficients were estimated as indicators of the magnitude of the genetic component of variance relative to the variance among genotype means estimated from one or two years in the UPPT trials. The estimates were obtained as
where
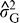 is the variance attributable to genotypes,
is the variance attributable to genotypes,
s the estimate of variance attributable to environments,
is the estimate of variance attributable to GxE interaction,
is the estimate of variance attributable to interaction between environments and grade sizes, and
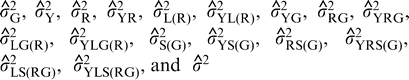 are the estimates of variances attributable to genotypes; years; regions; year-by-region interaction; locations within regions; interaction of years with locations within regions; year-by-genotype interaction; region-by-genotype interaction; year-by-region-by-genotype interaction; interaction between genotypes and locations within regions; interaction among years, genotypes, and locations within regions; seed grades within genotypes; interaction of years with seed grades within genotypes; interaction of regions with seed grades within genotypes; interaction among years, regions, and seed grades within genotypes; interaction of locations within regions with seed grades within genotypes; and interaction among years, locations within regions, and seed grades within genotypes; and residual effects (experimental error). For purposes of estimation, the number of years (y) was either 1 or 2, the number of regions (r) was 3, the total number of locations (l) was 9, and the number of kernel grades measured per genotype was 1.
are the estimates of variances attributable to genotypes; years; regions; year-by-region interaction; locations within regions; interaction of years with locations within regions; year-by-genotype interaction; region-by-genotype interaction; year-by-region-by-genotype interaction; interaction between genotypes and locations within regions; interaction among years, genotypes, and locations within regions; seed grades within genotypes; interaction of years with seed grades within genotypes; interaction of regions with seed grades within genotypes; interaction among years, regions, and seed grades within genotypes; interaction of locations within regions with seed grades within genotypes; and interaction among years, locations within regions, and seed grades within genotypes; and residual effects (experimental error). For purposes of estimation, the number of years (y) was either 1 or 2, the number of regions (r) was 3, the total number of locations (l) was 9, and the number of kernel grades measured per genotype was 1.
Results and Discussion
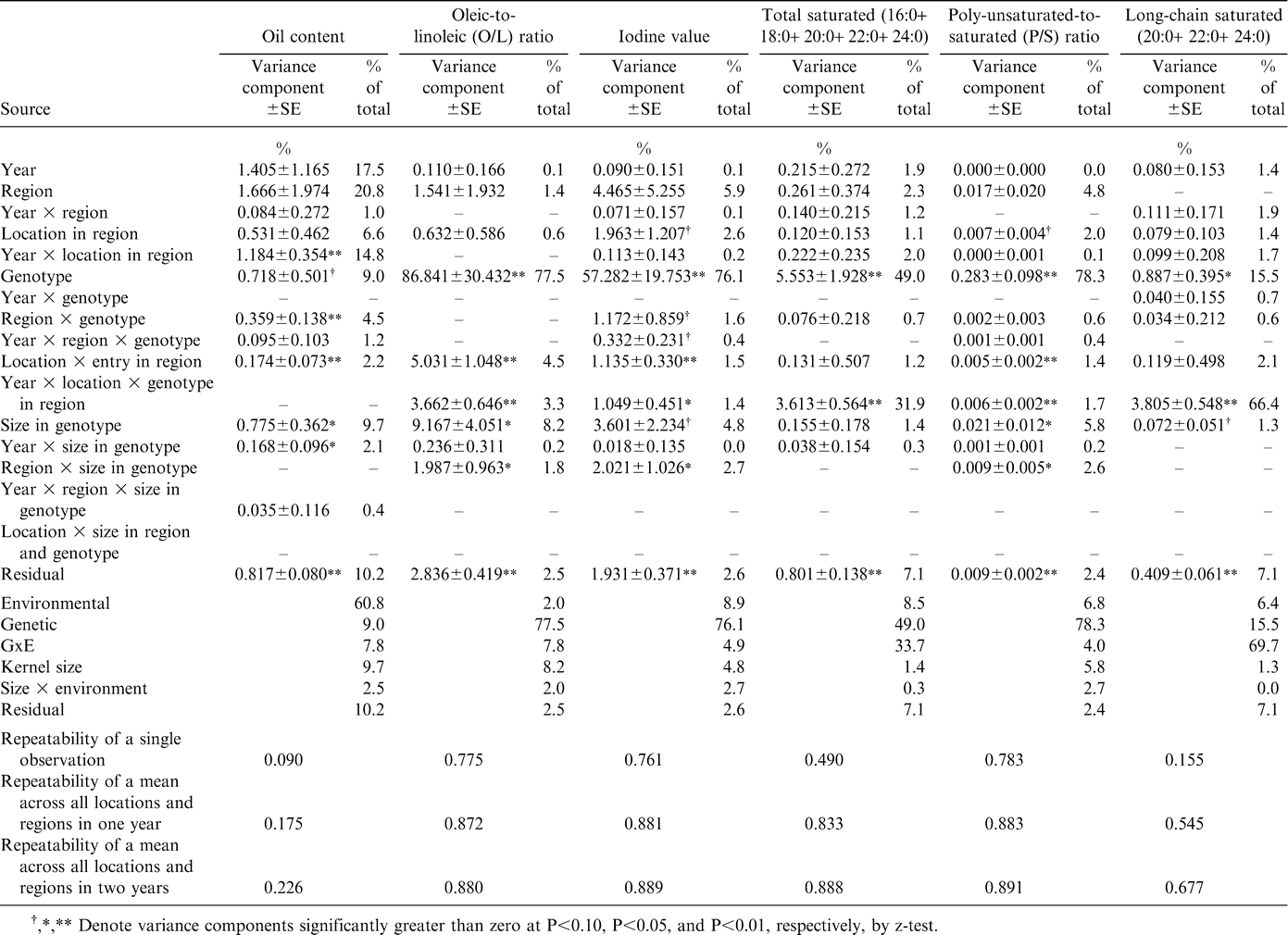
Because of their influence on processing quality and shelf life, oil content and fatty acid concentrations are among the most important seed composition traits (Ahmed and Young, 1982; Sanders et al., 1995). Very little genetic variation in measured oil content was noted among the samples, approximately 9% of the total variation (Table 1). In the current study, UPPT entries were either runner or virginia market types, perhaps constraining genetic variance, while the environmental representation was wide. Greater genetic variation might have been observed had there been UPPT entries of the spanish or valencia market types. Environmental effects accounted for 61% of the total variation for oil content, GxE effects only 8%, and seed grade (size) within genotype 10%, slightly more than the amount due to genotypes. Almost all of the variation in size was the result of runner-type breeding lines producing appreciable amounts of jumbo and medium kernels. Jumbo runner kernels generally had higher oil content than medium runner kernels.
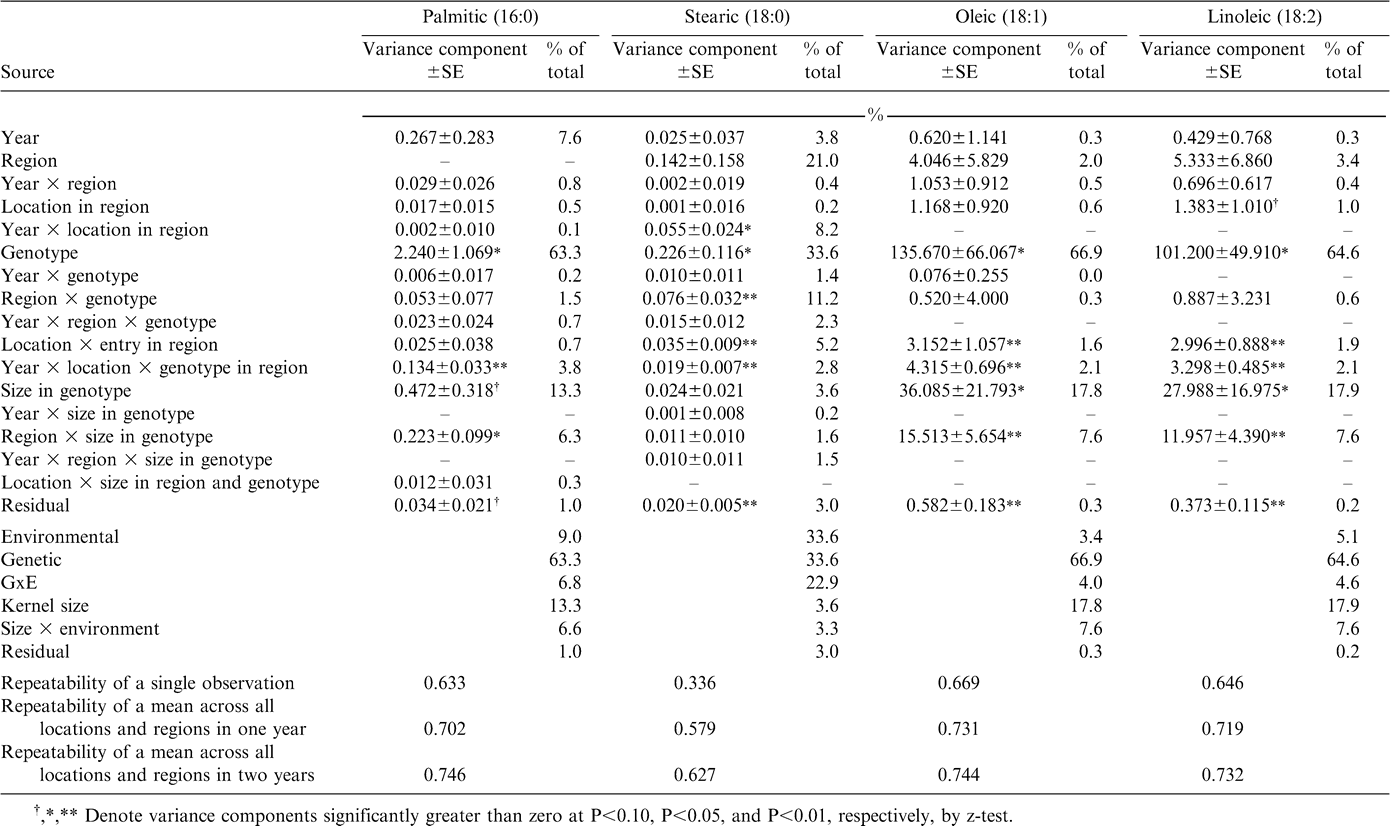
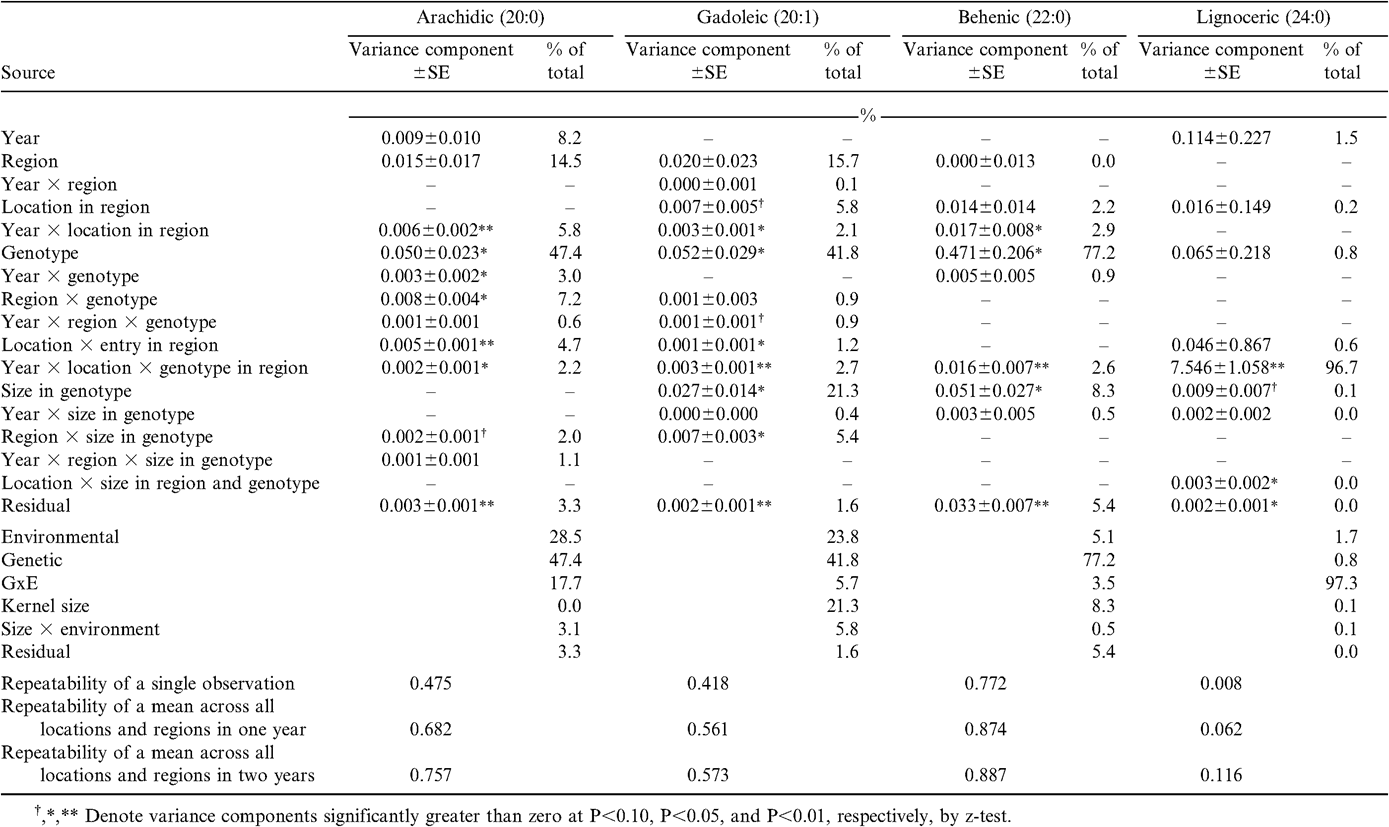
In contrast to oil content, most measures of oil quality were strongly influenced by genotype (Table 1), especially the traits that reflected the difference in oleic and linoleic fatty acids conditioned by the high-oleic seed oil trait identified by researchers at the Univ. of Florida (Norden et al., 1987; Moore and Knauft, 1989, Knauft et al., 1993). The high-oleic trait influenced not only the contents of oleic and linoleic fatty acids (67% and 65% genotypic relative to total variation, respectively), but also palmitic (63%) and stearic (34%) acids (Table 2) as well as long-chain species arachidic (48%), gadoleic (42%), and behenic (77%) fatty acids (Table 3). These effects on specific fatty acids resulted in similarly large genotypic influence in oleic-to-linoleic ratio (78%), iodine value (76%), total saturates (49%), and ratio of polyunsaturates to saturates (78%) (Table 1). Similar genotypic responses of oil quality indicators were not unexpected; the pleiotropic effects of the genes that cause the high-oleic trait in peanut have been reported previously (Isleib et al., 2006). The only fatty acid that was not strongly influenced by genetics was lignoceric acid for which 97% of total variation was attributed to GxE interaction arising from specific combinations of genotypes, years, and individual locations within regions (Table 3). Lack of genetic variation in this fatty acid is also reflected in the limited genetic variation value of long-chain saturated fatty acids (15%) (Table 1).
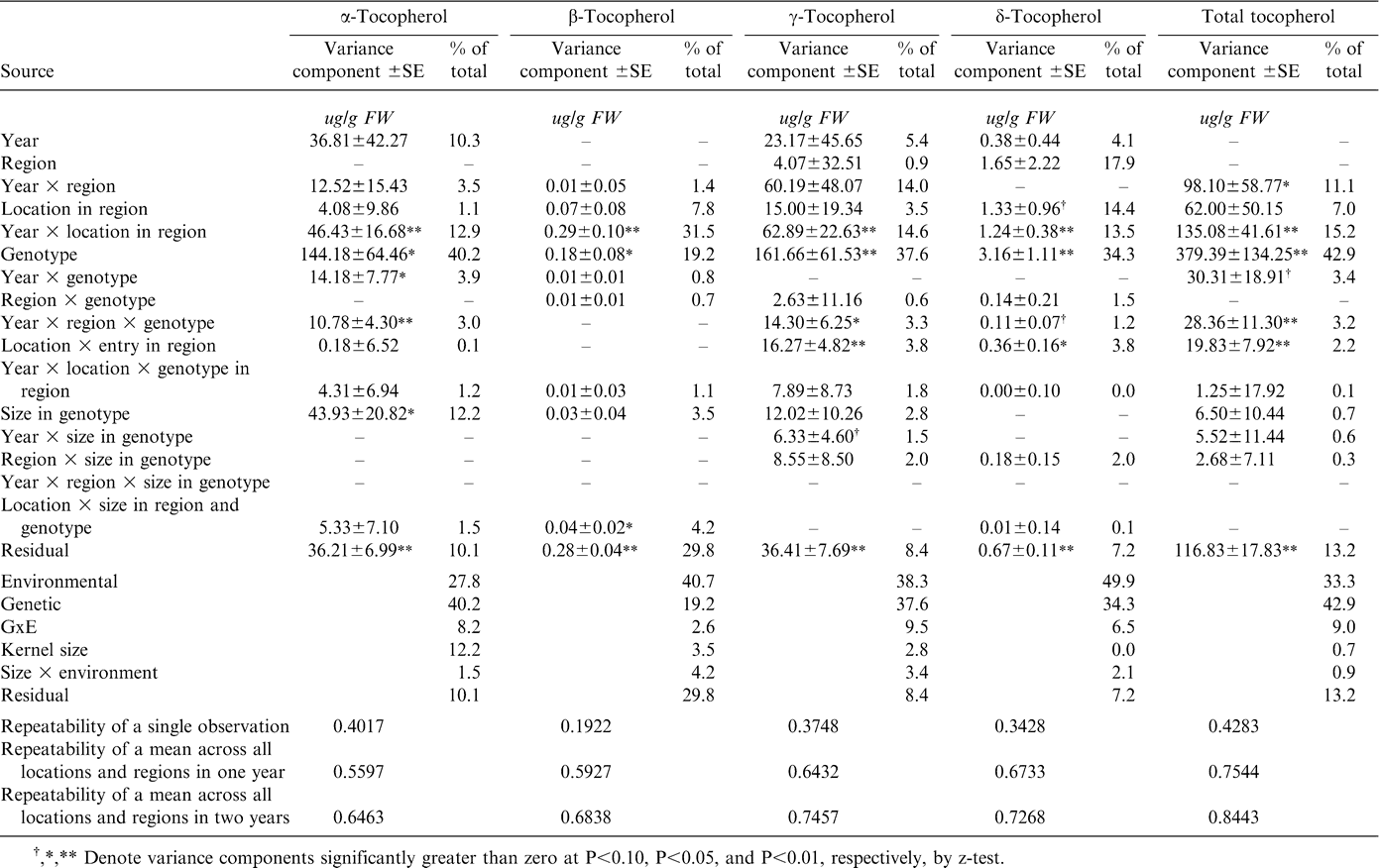
Tocopherols were generally less influenced by genetics than were fatty acids as exemplified by the repeatability of a single observation which ranged from 19% to 42% (Table 4). However, compared with pod yield (data not shown), this range of repeatability would be considered high. Environmental variation in concentration of the four tocopherols and for total tocopherol was greater than variation attributable to GxE. The relative contents of the tocopherol forms were associated with the high-oleic acid trait. High-oleic lines contained less α- (93.6 vs. 99.5 ug/gFW, P<0.01) and β-tocopherols (3.3 vs. 3.5 ug/gFW, P<0.01) and more γ- (92.6 vs. 85.2 ug/gFW, P<0.01) and δ-tocopherol (6.0 vs. 5.8 ug/gFW, P<0.10), although the total tocopherol content was not different (191.2 vs. 195.7 ug/gFW, NS)
In contrast to oil and tocopherol contents and fatty acid concentrations, genetic variation for sugar concentrations was low (Table 5). There was a preponderance of environmental variation for concentrations of sucrose (69% of total variation), raffinose (37%), stachyose (64%), and total sugars (68%). Sucrose comprises the majority of the total sugars in peanut seeds, explaining the similar findings for sucrose and total sugars. Interaction between years and locations within production regions was the largest single environmental factor influencing sucrose and total sugar concentrations. Although they make up only a small part of total sugars, genotypic variability of raffinose and stachyose was 11.2% and 11.9% of total variation, respectively. Variation due to GxE effects was the largest part of total variation for inositol (52% of total variation) and was mostly associated with specific combinations of year, location, and genotype within production regions. The largest contributor to variation for glucose and fructose concentrations was residual error associated with specific samples.
The magnitude of genetic influence on oil content and fatty acid concentrations suggests that these traits are amenable to genetic improvement. Development of cultivars with the high-oleic seed oil trait is already a common feature of all U.S.-based peanut breeding programs. With the recent public interest in biofuels, genetic improvement of oil content may also become a common breeding objective. Alteration of tocopherol profiles also appears to be feasible based on the magnitude of genetic relative to total variation. Low genetic variation of total sugar content suggests that its modification would be challenging. However, modification of raffinose and/or stachyose may be feasible. As was the case for sensory quality (Isleib et al., 2008), environmental influence on total sugar content of peanut begs the question of which specific environmental factors produce the variation. The importance of specific combinations of locations, years, and production regions suggests highly localized factors.
Literature Cited
Ahmed E. M. and Young C. T. 1982 Composition, quality, and flavor of peanuts. 655 – 688 In Pattee H. E. and Young C. T. Peanut Science and Technology Amer. Peanut Res. Educ. Soc Stillwater, OK .
Ahmed E. M. and Ali T. 1986 Textural quality of peanut butter as influenced by peanut seed and oil contents. Peanut Sci 13 : 18 – 20 .
Ali M. I. M. and Prasada Rao R. D. V. J. 1982 Effect of tomato spotted wilt virus on the oil content of groundnut seeds. Madras Agric. J 69 : 269 – 270 .
Andersen P. C. and Gorbet D. W. 2002 Influence of year and planting date on fatty acid chemistry of high oleic acid and normal peanut genotypes. J. Agric. Food Chem 50 : 1298 – 1305 .
Branch W. D. , Coker D. L. , Isleib T. G. , Chapin J. W. , Bostick J. P. , Gorbet D. W. , Tillman B. L. , Simpson C. E. , Burow M. D. , Baring M. L. , and Greenhagen B. 2006 Uniform Peanut Performance Tests, 2005. Coastal Plain Exp. Sta. Res. Prog. Rep. No. 4-06 25 pp .
Branch W. D. , Mozingo R. W. , Isleib T. G. , Bostick J. P. , Gorbet D. W. , Simpson C. E. , Burow M. D. , Baring M. , and Dashiell K. E. 2003 Uniform Peanut Performance Tests, 2002. Univ. Georgia Coastal Plain Exp. Stn. Prog. Rep. No. 4-03 24 pp .
Branch W. D. , Mozingo R. W. , Isleib T. G. , Bostick J. P. , Gorbet D. W. , Simpson C. E. , Burow M. D. , Baring M. , and Dashiell K. E. 2004 Uniform Peanut Performance Tests, 2003. Univ. Georgia Coastal Plain Exp. Stn. Prog. Rep. No. 4-04 25 pp .
Branch W. D. , Mozingo R. W. , Coker D. L. , Isleib T. G. , Bostick J. P. , Gorbet D. W. , Tillman B. L. , Simpson C. E. , Burow M. D. , Baring M. , Dashiell K. E. , and Greenhagen B. 2005 Uniform Peanut Performance Tests, 2004. Univ. Georgia Coastal Plain Exp. Stn. Prog. Rep. No. 4-05 24 pp .
Branch W. D. , Nakayama T. , and Chinnan M. S. 1990 Fatty acid variation among U.S. runner-type peanut cultivars. J. Amer. Oil Chem. Soc 67 : 591 – 593 .
Dwivedi S. L. , Nigam S. N. , and Nageswara Rao R. C. 2000 Photoperiod effects on seed quality traits in peanut. Crop Sci 40 : 1223 – 1227 .
Grosso N. R. , Lamarque A. , Maestri D. M. , Zygadlo J. A. , and Guzman C. A. 1994 Fatty acid variation of runner peanut (Arachis hypogaea L.) among geographic localities from Cordoba (Argentina). J. Amer. Oil Chem. Soc 71 : 541 – 542 .
Hammond E. G. , Duvick D. , Wang T. , Dodo H. , and Pittman R. N. 1997 Survey of the fatty acid composition of peanut (Arachis hypogaea) germplasm and characterization of their epoxy and eicosenoic acids. J. Amer. Oil Chem. Soc 74 : 1235 – 1239 .
Harch B. D. , Basford K. E. , DeLacy I. H. , Lawrence P. K. , and Cruickshank A. 1995 Patterns of diversity in fatty acid composition in the Australian groundnut germplasm collection. Genet. Resources Crop E 42 : 243 – 256 .
Isleib T. G. , Tillman B. L. , Pattee H. E. , Sanders T. H. , Hendrix K. W. , and Dean L. O. 2008 Genotype-by-environment interactions for flavor attributes of breeding lines in the Uniform Peanut Performance Test. Peanut Sci 35 : 54 – 59 .
Isleib T. G. , Wilson R. F. , and Novitzky W. P. 2006 Partial dominance, pleiotropism, and epistasis in the inheritance of the high-oleate trait in peanut. Crop Sci 46 : 1331 – 1335 .
Knauft D. A. , Moore K. M. , and Gorbet D. W. 1993 Further studies on the inheritance of fatty acid composition in peanut. Peanut Sci 20 : 74 – 76 .
Ku K. L. , Lee R. S. , Young C. T. , and Chiou R. Y. Y. 1998 Roasted peanut flavor and related compositional characteristics of peanut kernels of spring and fall crops grown in Taiwan. J. Agric. Food Chem 46 : 3220 – 3224 .
Layrisse A. , Wynne J. C. , and Isleib T. G. 1980 Combining ability for yield, protein and oil of peanut lines from South American centers of diversity. Euphytica 29 : 561 – 570 .
Moore K. M. and Knauft D. A. 1989 The inheritance of high oleic acid in peanut. J. Hered 80 : 252 – 253 .
Norden A. J. , Gorbet D. W. , Knauft D. A. , and Young C. T. 1987 Variability in oil quality among peanut genotypes in the Florida breeding program. Peanut Sci 14 : 7 – 11 .
Raheja R. K. , Batta S. K. , Ahuja K. L. , Labana K. S. , and Singh M. 1987 Comparison of oil content and fatty acid composition of peanut genotypes differing in growth habit. Qualitas plantarum [Plant Foods Human Nutrition] 37 : 103 – 108 .
Sanders T. H. , Pattee H. E. , Vercellotti J. R. , and Bett K. R. 1995 Advances in peanut flavor quality. 528 – 553 In Pattee H. E. and Stalker H. T. Advances in Peanut Science Amer. Peanut Res. Educ. Soc Stillwater, OK .
Sykes E. E. and Michaels T. E. 1986 Combining ability of Ontario-grown peanuts (Arachis hypogaea L.) for oil, fatty acids, and taxonomic characters. Peanut Sci 13 : 93 – 97 .
Tai Y. P. and Young C. T. 1975 Genetic studies of peanut proteins and oils. J. Amer. Oil Chem. Soc 52 : 377 – 385 .
Upadhyaya H. D. and Nigam S. N. 1999 Detection of epistasis for protein and oil contents and oil quality parameters in peanut. Crop Sci 39 : 115 – 118 .
USDA 2003 Uniform Peanut Performance Tests, Chemical, Sensory and Shelf-life Properties. Data Presented by Location. USDA-ARS-SAA Market Quality and Handling Research Unit, Raleigh, NC, pp. 4–7. (http://152.1.118.33/, last accessed 21 Feb 2007) .
Yadava T. P. , Kumar P. , and Yadav A. K. 1980 Stability analysis for oil content and yield components in groundnut (Arachis hypogaea L.). J. Res. Haryana Agric. Univ 10 : 560 – 563 .
Notes
- First and second authors: Res. Agron. , USDA-ARS and Agric. Res. Statistician; Coastal Plain Exp. Sta., Tifton, GA 31793-0748. Third author: President and CEO, Hebert Green AgroEcology; Asheville, NC 28801. [^]
- T.G. Isleib, Dep. of Crop Sci., Box 7629, N.C. State Univ., Raleigh, NC 27695-7629 [^]
- B.L. Tillman, Univ. of Fla. N. Fla. Res. & Ext. Ctr., 3925 Hwy. 71, Marianna, FL 32446-8091 [^]
- H.E. Pattee, Dep. of Biol. & Agric. Engineering, Box 7625, N.C. State Univ., Raleigh, NC 27695-7625 [^]
- T.H. Sanders, K.W. Hendricks, and L.O. Dean, USDA-ARS Market Quality and Handling Res. Unit, Raleigh, NC 27695-7624 [^] *Corresponding author. E-mail: tom_isleib@ncsu.edu.
Author Affiliations