Introduction
Tomato spotted wilt virus (TSWV), the tospovirus that causes tomato spotted wilt of peanut (Arachis hypogaea L.) (Todd et al., 1990; Culbreath et al., 2003) and a contributing factor to the occurrence of peanut yellowing death (PYD) (Mitchell, 1996), is a limiting factor in peanut production in the southeastern U.S. The virus is known to infect peanut in all commercial production areas in North Carolina (Lyerly et al., 2002) and in 2005, TSWV infection was verified in nearly 50% of all peanut plants in experiment test plots (Brandenburg, 2006).
Tomato spotted wilt virus is transmissible in symplastic tissue and is transmitted in a persistent-propagative manner (Ullman et al., 1993; Wijkamp and Peters, 1993; Ullman, 1996; Wetering et al., 1996) solely by thrips in the family Thripidae (German et al., 1992). Frankliniella fusca (Hinds), tobacco thrips (TT) is the primary TSWV vector found on peanut in North Carolina (Barbour and Brandenburg, 1994; Cho et al., 1995; Eckel et al., 1996; Groves, 2001; Groves et al., 2003). Frankliniella occidentalis (Pergande), western flower thrips also occurs in peanut producing areas in North Carolina, but are rarely found on peanut foliage (Eckel et al., 1996; Groves, 2001; Groves et al., 2003). F. occidentalis is known to reproduce poorly on peanut, while peanut serves as a good host for reproduction of TT (Chamberlin et al., 1992; 1993; Todd et al., 1995). Only adult thrips and late instar larvae (Wijkamp and Peters, 1993; Wijkamp et al., 1995; Wetering et al., 1996) that acquired the virus as first instars can transmit TSWV to plants.
Tomato spotted wilt virus can cause a wide range of symptoms in peanut including chlorotic spots, ringspots, mottling, silvering, chlorotic streaks, vein streaking, chlorosis, distortion of foliage, defoliation, stunting, wilting, bud necrosis, and local lesions (Culbreath et al., 1992a; Lyerly et al., 2002; Shew, 2006). However, not all infected plants show symptoms. Tomato spotted wilt virus is commonly found in the roots of asymptomatic peanuts plants (Mandal et al., 2001). The proportion of TSWV infected peanut plants that remain asymptomatic can be two to three times greater than the proportion of plants that become symptomatic (Culbreath et al., 1992b; Murakami et al. 2006). Infected plants that remain asymptomatic can still experience reduced photosynthesis and reduced water efficiency (Rowland et al., 2005).
In addition to spotted wilt of peanut, TSWV has been linked as a causal agent of late-season foliar chlorosis (Culbreath et al., 1991) or peanut yellowing death (PYD) (Mitchell, 1996). Peanut yellowing death occurs in older plants often after a precipitation event (Mitchell, 1996). Symptoms are expressed in the form of foliar chlorosis and root necrosis (Culbreath et al., 1991) that leads to water stress, severe wilting, and rapid death of the plant (Mitchell, 1996).
Cultivar selection has repeatedly been shown to have the greatest and most consistent impact on reducing the incidence of spotted wilt and losses to TSWV (Brown et al., 1996; Marois and Wright, 2003; Tillman et al., 2006). For example, Perry is highly susceptible to TSWV, while Gregory exhibits moderate field resistance (Brown et al., 2005; Hurt et al., 2005; Brandenburg, 2006). Public breeding programs develop cultivars with a range of characteristics designed to increase yield and market grade components. Additionally, breeding lines are evaluated for pest reaction. Assessing these lines for tolerance of TSWV is important when determining suitability of lines for release as cultivars. Our objectives were to a) determine the relationship of thrips populations to TSWV incidence and disease impact; b) determine if peanut genotype influences thrips density, thrips injury, TSWV incidence, or disease impact; c) to characterize any interactions among genotype, thrips density, thrips damage, TSWV incidence, and disease impact; d) to determine if there is a relationship between TSWV infection and PYD incidence.
Materials and Methods
Experiments were conducted in North Carolina during 2004 and 2005 at the Peanut Belt Research Station (PBRS) near Lewiston-Woodville. Twenty-four breeding lines were evaluated, including commercially-grown cultivars Gregory and Perry (Table 1). Peanut was planted in conventional-raised beds. Plot size was two rows (91 cm spacing) by 7 m. In-row seed spacing was 51 cm. Peanut was planted on 15 May 2004 and 11 May 2005. No insecticide was applied either year. All other production and pest management practices were based on Cooperative Extension recommendations for the region (Brandenburg, 2006; Jordan, 2006; Shew, 2006). The experimental design was a randomized complete block with three replicates.
Thrips Injury and Impact Rating
Thrips damage was assessed on 2 and 17 June 2004 (18 and 33 DAP) and 5, 14, and 27 June 2005 (25, 34, and 47 DAP). The percentage of thrips-injured plants was determined by randomly examining 10 plants within each plot for evidence of thrips feeding on a recently-emerged leaf. A plant was considered thrips-injured if the leaf had any scarring from thrips feeding.
A thrips “impact” rating was also conducted in conjunction with the injury assessment described above. The impact rating system was designed to assess the general effect of thrips feeding on a specific peanut line. Plots were assessed using a visual rating system that included three impact levels; where 1) represented minor plant stunting, 2) moderate plant stunting, and 3) severe plant stunting attributed to thrips injury.
Thrips Density
To evaluate thrips density, samples were collected on 2 and 17 June 2004 (18 and 33 DAP), and on 5 and 27 June (25 and 47 DAP) in 2005. Samples consisted of ten random, non-opened leaves that were collected from each plot and placed immediately into 20 ml vials of 70% ethanol and refrigerated until processing. A complete count of adult tobacco thrips and larval thrips (Mound, 1996) was conducted using a stereo microscope. Thrips found in either the leaf tissue or liquid of the sample were counted.
Tswv Incidence and Disease Impact
In 2004, visual assessment of foliar symptoms of spotted wilt were recorded 29 June, 5, 13, 21, 27 July, 4, 10, 19, 31 Aug., and 9 Sep. (45, 51, 59, 67, 73, 81, 87, 96, 108, and 117 DAP). In 2005, assessments occurred 7, 20 July, 2, 16, 27 Aug., 11, and 23 Sep. (56, 69, 82, 96, 107, 122, and 134 DAP). A plant was designated as symptomatic if one or more leaflets exhibited any symptoms of spotted wilt. Any plant that was symptomatic was marked with a survey flag and immediately labeled and scored.
Scoring consisted of rating the “disease impact” of tomato spotted wilt from 1 to 3; where 1) was defined as plants with only mild and localized symptoms, 2) was plants with widespread symptoms but no, or limited stunting; and 3) as plants that were severely stunted or malformed. Once a plant was flagged, disease impact was reevaluated on each subsequent sampling date to examine any changes in symptom severity over time.
For each date, after all plots were surveyed, foliar samples were collected from each newly-flagged plant. Samples consisted of at least three symptomatic leaflets pulled from the symptomatic plant and placed into a plastic bag labeled identically as the survey flag marking the plant. Samples collected in this manner were processed the day of collection using the ImmunoStrip® assay from Agdia Inc. (STX 39300, ACC 00996, ACC 00925, Elkhart, IN). Flagged plants that did not test positive for TSWV infection during their initial assay were retested at 2 weeks intervals until either infection was confirmed, the plant died, or the plant was dug at harvest. Only plants that tested positive for TSWV under ImmunoStrip® assay were considered to be infected.
In September, visual scouting was expanded to include plants exhibiting severe foliar chlorosis or peanut yellowing death (PYD) (Mitchell 1996). On 14 and 23 Sep. 2004 (122 and 131 DAP), and in 2005 on 2 Oct. (143 DAP) the chlorotic plants in each plot were dug by hand. Taproot samples were collected from chlorotic plants by cutting the root off at the crown and again at least 2.5 cm below the crown and placing the taproot into a plastic bag labeled with the plot number. Taproot samples were processed the same day as collection using ImmunoStrip® assay.
To prepare the field for asymptomatic plant sampling, all plants testing positive for TSWV were manually removed from the field on 23 Sep. 2004 and 2 Oct. 2005 (131 and 143 DAP, respectively). Plants were dug and inverted mechanically using a commercial peanut digger, and stand counts were taken on 5 Oct. and 6 Oct. (141 and 147 DAP) for 2004 and 2005, respectively. Stand was determined by counting the number of taproots per plot and adding to that the number of plants that were previously removed. After digging, root samples were collected, as previously described, from 10 randomly selected plants per plot on 7 Oct. and 13 Oct. (143 and 154 DAP) for 2004 and 2005, respectively. All samples were refrigerated and processed using ImmunoStrip® assay within 72 h of collection. As this sampling method was destructive, yield data could not be collected.
Statistical Analyses
Data were pooled across years for all variables except thrips impact ratings for which data were drawn only from 2004. Thrips data were separated and analyzed based on sampling date. Early season thrips infestation (early-June thrips) consisted of thrips collected on 2 June 2004 and 5 June 2005 and later thrips infestation (late-June thrips) of thrips collected on 17 June 2004 and 27 June 2005. Tomato Spotted Wilt Virus incidence data were drawn from the final sampling dates. The data were subjected to ANOVA (α = 0.05) using the SAS 9.1.3 (SAS Institute Inc., Cary, NC) general linear model (GLM) procedure after appropriate transformations. Thrips densities were transformed to square-roots. Virus incidences were transformed into proportion infected and then subjected to angular transformation. All results are reported in the original scale. Thrips-injured plants and thrips damage ratings did not require transformation. Fisher's protected least significant difference (LSD) values were calculated for comparison of genotypes (SAS 1999).
Results and Discussion
The main effect of year was significant for density of adult thrips (F = 145.40; df = 1 and 92; P < 0.0001), larval thrips density (F = 15; df = 1 and 92; P = 0.0002) and asymptomatic TSWV incidence (F = 116.0; df = 1 and 92 ; P < 0.0001). Plants in 2005 had approximately 2.1× greater adult thrips density than in 2004; plants in 2004 had approximately 1.5× greater larval thrips density than in 2005. Plants in 2005 had 3.7× higher asymptomatic TSWV incidence than 2004 (data not shown). The interaction of year with genotype was not significant for any of the parameters.
Thrips Injury, Impact Rating, and Density
No differences across genotype were detected for thrips injury or thrips impact ratings. Across all lines and both years, a total of 22,284 F. fusca were collected and sorted based on life stage. The majority (92%) of thrips were collected the first week of June (2 June 2004 and 5 June 2005), while the remaining 8% were collected on the subsequent sampling dates. The higher counts in early June are in agreement with Groves et al. (2003), who found tobacco thrips at higher density in peanut producing areas in North Carolina in late May and early June than later in the season. Of the thrips collected 14.4% were adults and the remainder immatures. When analyzed separately, the effect of genotype on late June thrips counts was not significant for either adult thrips density or larval thrips density, although the early June thrips counts were significantly different among genotypes for adult density (F = 3.06; df = 23 and 92 ;P < 0.0001).
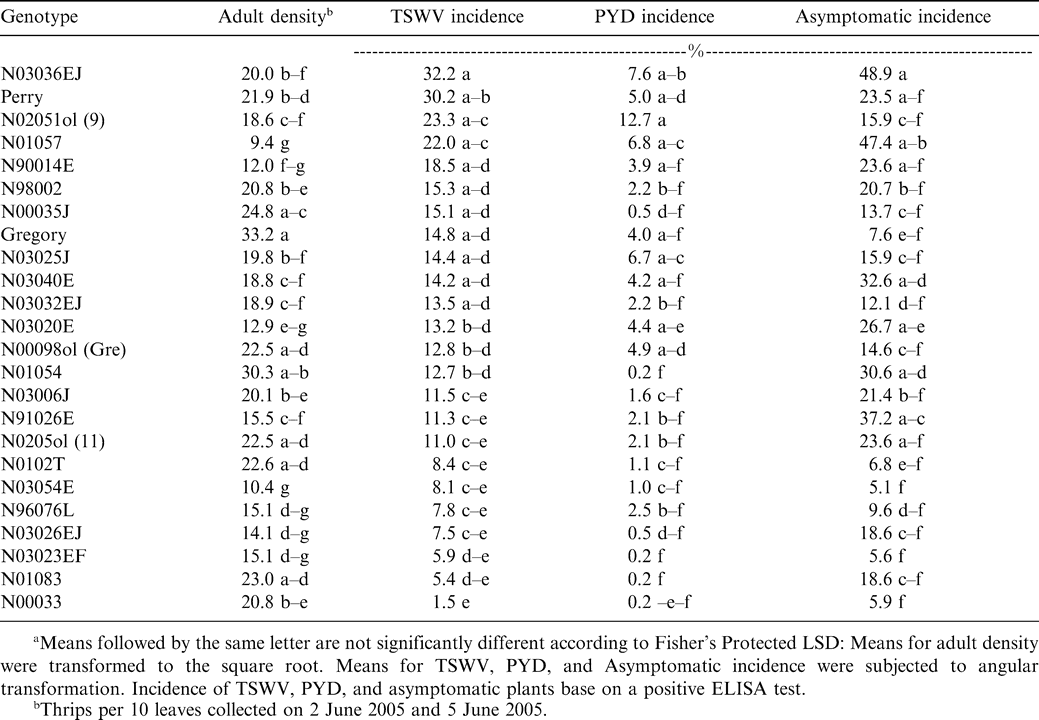
The commercially-grown cultivar Gregory had the highest density of adult thrips, while genotypes N01057 and N03054E had the lowest density (Table 1). The strong genotype effect suggests that adult F. fusca may exhibit host preference in North Carolina during the stage when peanut is most susceptible to TSWV infection (Brown et al., 2005). Although Perry is considered highly susceptible to TSWV (Brandenburg, 2006; Shew, 2006), fewer thrips and less feeding damage were noted with Perry than TSWV-tolerant selections (Hurt, 2003). Conversely, Gregory is considered tolerant of TSWV (Brandenburg, 2006; Shew, 2006), yet had the highest adult thrips density of any of the genotypes tested in these experiments and significantly more adult thrips than Perry (Table 1). No significant correlation was detected between adult density (P = 0.20), thrips injury (P = 0.20), or thrips damage ratings (P = 0.94) when related to TSWV incidence. These results suggest that TSWV incidence cannot be accurately predicted across a wide range of genotypes using adult thrips density or thrips feeding injury. These results corroborate previous reports (Culbreath et al. 1996, 1997).
TSWV Incidence and Disease Impact
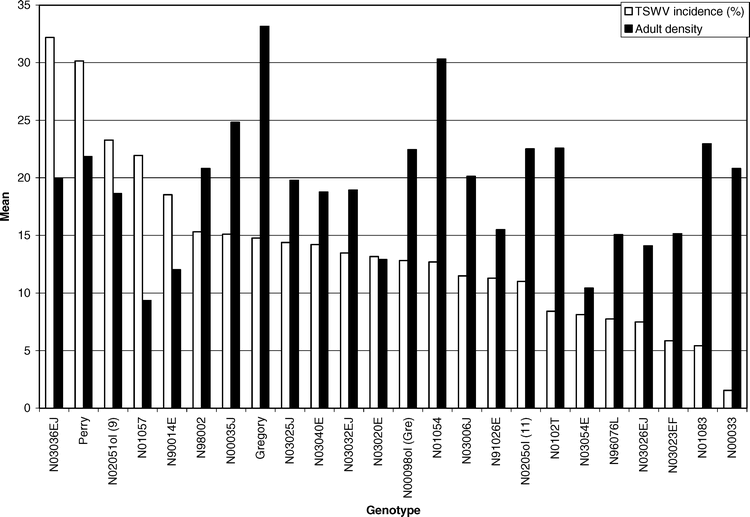
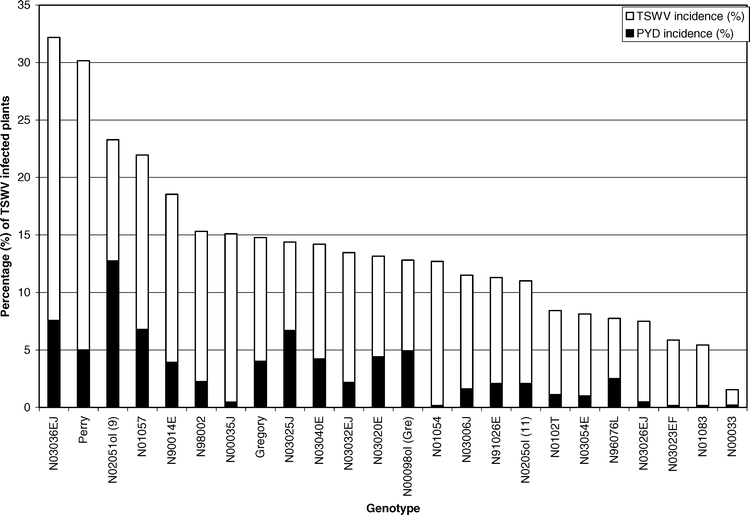
Across years and genotypes, a total of 525 (94%) TSWV infected plants were confirmed of the 559 symptomatic plants that were flagged. Only 369 (70%) of the plants that exhibited visual symptoms of infection were diagnosed as suffering from spotted wilt of peanut; the remaining 156 (30%) plants were diagnosed as suffering from PYD (Mitchell 1996). The main effect of genotype was significant for TSWV incidence (F = 1.74; df = 23 and 92; P = 0.03). Genotype N03036EJ had the highest TSWV incidence. Perry had the second highest TSWV incidence, and genotype N00033 had the lowest TSWV incidence (Table 1). Perry had greater TSWV incidence than Gregory. This is in agreement with past research conducted in North Carolina (Hurt, 2003) and risk assessment values assigned to those two varieties in the southeastern U.S. (Brown et al. 2005). Although Gregory had the highest adult thrips density and a significantly higher adult density than Perry, Perry had a greater TSWV incidence (Table 1). These data suggest that higher adult thrips densities do not account for higher incidence of TSWV (Figure 1). Across all genotypes only line N00033 had a significantly lower TSWV incidence than Gregory (Table 1). Line N00033 was found to have an adult density comparable to Perry, but these two lines with similar adult densities had greatly varying incidences of TSWV.
In 2004, 79 (92%) of the PYD symptomatic plants tested positive for TSWV infection, while in 2005, 77 (97%) of the PYD symptomatic plants tested positive for TSWV. Comparatively, only 10% and 40% of asymptomatic plants were found to be infected in 2004 and 2005, respectively. Analysis of the proportions confirmed that PYD was associated with TSWV infection for both years (P < 0.0001). This is in agreement with past research that found PYD highly associated with TSWV infection (Culbreath et al., 1991). The main effect of genotype was significant for PYD incidence (F = 2.06; df = 23 and 92; P = 0.0082). Genotype N02051ol had greater PYD incidence than other genotypes, while genotypes N01083, N03023EF, and N01054 had the least, closely followed by line N00033 (Table 1). Although genotype N03036EJ and Perry had the highest TSWV incidences, they did not have the greatest PYD incidence (Figure 2). However, genotype N00033 had significantly less TSWV incidence than all other genotypes and also had one of the lower PYD incidences. Perry was not significantly different than Gregory in PYD incidence (Table 1). These data suggest that genotypes have varying incidence of PYD, and that genotypes resistant to TSWV will have lower PYD incidence than genotypes that are not resistant (Figure 2).
The etiology of PYD has not been clearly elucidated. Peanut plants that appear healthy become chlorotic and rapidly wilt into necrosis. A relationship is apparent between PYD and TSWV infection; however we have yet to determine the cause of the association, or the manner in which TSWV resistance can influence PYD incidence. Further research will be required to fully understand the factors that cause PYD in peanut and how to manage the disease.
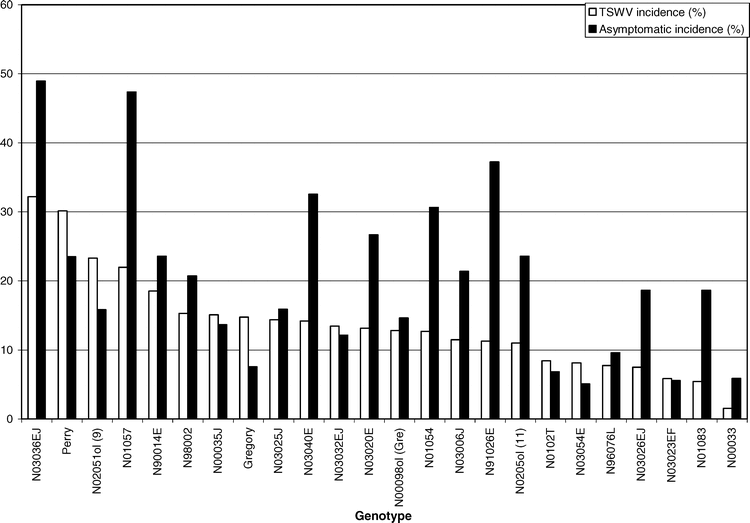
The main effect of genotype was significant for asymptomatic incidence (F = 2.19; df = 23 and 92; P = 0.0047). Genotype N03036EJ had the greatest number of asymptomatic infected plants, followed closely by line N01057. Genotypes N03054E, N03023EF, and N00033 had a lower asymptomatic incidence than all other genotypes (Table 1). These data suggest it is common for a high proportion of TSWV-infected plants to never show symptoms within a growing season. The number of infected plants that fail to express symptoms may be higher than the number of infected plants that actually become symptomatic (Figure 3). This is in agreement with past research in other states (Culbreath et al., 1992b; Mandel et al., 2001).
Proportion of symptomatic and asymptomatic plants testing positive for Tomato spotted wilt virus infection by ELISA. aTomato spotted wilt virus incidence from June through October as the percentage of total plants at digging exhibiting TSWV symptoms at some point throughout the growing season that were also confirmed to be infected by ImmunoStrip® assay.
A moderate, yet significant correlation was detected between expressed TSWV incidence and asymptomatic incidence (r = 0.43, P = 0.0001). These data indicate that genotypes that have a greater number of plants expressing visual symptoms of TSWV infection also have a greater number of plants that become infected with TSWV but remain asymptomatic. These results suggest that lines susceptible to TSWV are actually more likely to become infected, rather than just more likely to express symptoms if infected. Further research is needed to better identify the factors affecting symptom expression and tolerance to TSWV infection within peanut.
The main effect of genotype did not have a significant influence on the disease impact of TSWV infection. The number of plants that were severely stunted or malformed or became necrotic (scored 3) did not differ among genotypes (F = 1.82; df = 23 and 92; P = 0.24). Less severe infections, plants that scored between 1 and 2, also did not differ among genotypes (F = 0.97; df = 23 and 92; P = 0.51 and F = 0.54; df = 23 and 92; P = 0.95, respectively); plants exhibiting PYD symptoms were not accounted for within this impact scale. Other field evaluations have shown disease severity can be influenced by cultivar selection (Culbreath et al., 1997). Past research has shown that any plant infected with TSWV is impacted to some degree (Rowland et al., 2005). Thus it remains important to continue to identify genotypes with mildly symptomatic plants.
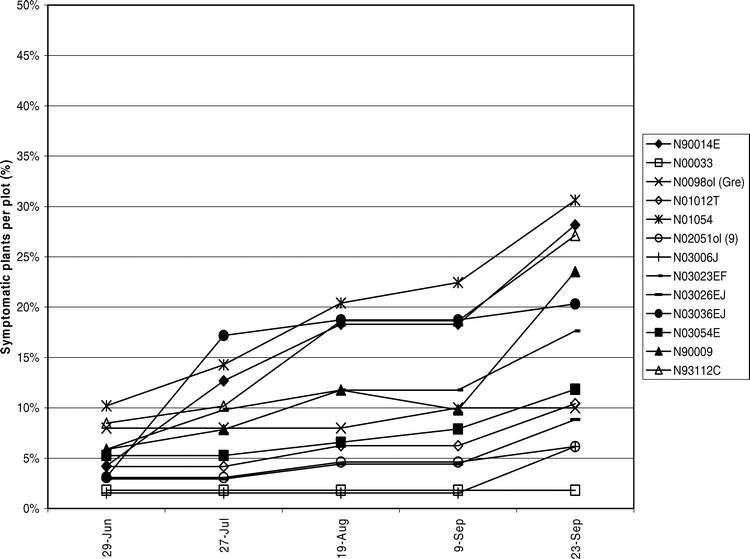
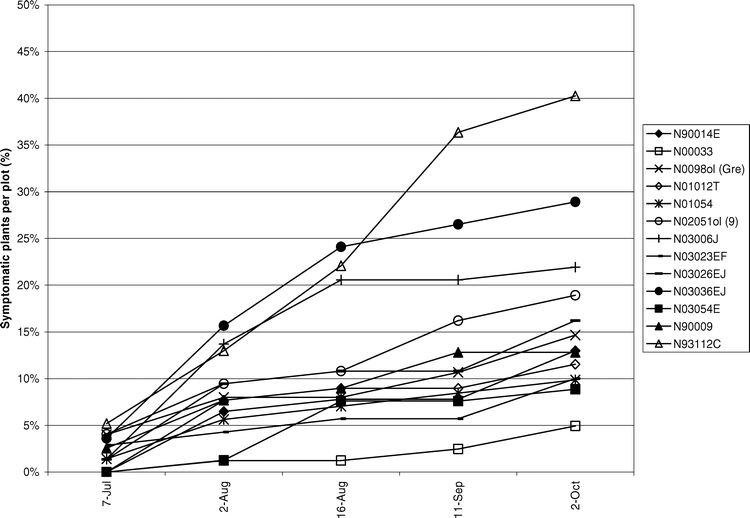
Tomato spotted wilt virus incidence was assessed over time to elucidate disease progression in the field. During 2004 (Figure 4) and 2005 (Figure 5) TSWV incidence increased gradually until the middle of Aug. Another subsequent increase in incidence seen in Sep. was attributed to a high proportion of PYD symptomatic plants testing positive for TSWV.
Information on the linage of the more resistant genoypes could prove useful. Genotype N00033 is descended from genotypes N90010E and N92020. Genotypes N01083 is descended from parentages N91054E and VA 9210162. Genotype N03023EF is descended from parents derived from crossing VA 98R with X98007, and NC 12C with N95003C.
Summary and Conclusions
Our studies indicate that TSWV incidence is significantly affected by genotype selection. Typically the cultivar Perry has one of the highest incidences of infection, while Gregory has a lower incidence. In these experiments, Perry had one of the highest incidences of infection but did not differ significantly from Gregory in regards to TSWV incidence. Line N00033 was found to have a lower TSWV incidence than Perry and was the only line found to have a lower TSWV incidence than Gregory, suggesting that Gregory remains a cultivar that should be useful for management of tomato spotted wilt. Late-season foliar chlorosis was identified as PYD and found to be very highly associated with TSWV infection. A large proportion of asymptomatic peanut plants were found to be infected with TSWV. In specific lines, the amount of asymptomatic infection was more than twice that of symptomatic TSWV infection. These findings suggest that overall incidence of TSWV infection in peanut throughout North Carolina is greater than visual estimates alone would imply. A moderate degree of correlation was detected between symptomatic TSWV incidence and asymptomatic incidence. This suggests that lines that are more susceptible to TSWV are more likely to become infected, rather than just more likely to express symptoms if infected.
Greater numbers of adult thrips were collected from Gregory than any other line. Although Gregory had a greater adult density at the time of sampling than Perry, neither cultivar differed in any other of the other parameters measurements. No correlation was detected between thrips density or thrips impact and any incidence of TSWV infection. This suggests that thrips density does not serve as a good indicator of resistance to TSWV.
References Cited
Barbour J. D. and Brandenburg R. L. 1994 Vernal infusion of thrips into North Carolina peanut fields. J. Econ. Entomol 87 : 446 – 451 .
Brandenburg R. L. 2006 Peanut Insect and Mite Management. 61 – 77 In 2006 Peanut Information, North Carolina Coop. Ext. Ser. Series AG-331.
Brown S. , Gorbet D. W. , Culbreath A. K. , Baldwin J. A. , Todd J. W. , and Beasley J. P. 2005 Development of a Method of Risk Assessment to Facilitate Integrated Management of Spotted Wilt of Peanut. Plant Dis 89 : 348 – 352 .
Brown S. L. , Todd J. W. , and Culbreath A. K. 1996 Effect of selected cultural practices on incidence of Tomato spotted wilt virus and populations of thrips vectors in peanuts. Acta Hortic 431 : 491 – 498 .
Chamberlin J. R. , Culbreath A. K. , Todd J. W. , and Demski J. W. 1993 Detection of Tomato spotted wilt virus in tobacco thrips (Thysanoptera: Thripidae) overwintering in harvested peanut fields. J. Econ. Entomol 86 : 40 – 45 .
Chamberlin J. R. , Todd J. W. , Beshear R. J. , Culbreath A. K. , and Demski J. W. 1992 Overwintering hosts and wingform of thrips, frankliniella spp., in Georgia (Thysanoptera: Thripidae) implications for management of spotted wilt disease. Eviron. Entomol 21 : 121 – 128 .
Cho K. , Eckel C. S. , Walgenbach J. F. , and Kennedy G. G. 1995 Overwintering of thrips (Thysanoptera: Thripidae) in North Carolina. Environ. Entomol 24 : 58 – 67 .
Culbreath A. K. , Todd J. W. , and Brown S. L. 2003 Epidemiology and management of tomato spotted wilt in peanut. Annu. Rev. Pytopathol 41 : 53 – 75.
Culbreath A. K. , Todd J. W. , Gorbet D. W. , Shokes F. M. , and Pappu H. R. 1997 Field Response of New Peanut Cultivar UF 91108 to Tomato Spotted Wilt Virus. Plant Dis 81 : 1410 – 1415 .
Culbreath A. K. , Todd J. W. , Gorbet D. W. , Branch W. D. , Sprenkel R. K. , Shokes F. M. , and Demski J. W. 1996 Disease progress of spotted wilt in selected peanut cultivars and advanced breeding lines. Plant Dis 80 : 70 – 73 .
Culbreath A. K. , Todd J. W. , and Demski J. W. 1992a Productivity of Florunner peanut infected with Tomato spotted wilt virus. Peanut Sci 19 : 11 – 14 .
Culbreath A. K. , Todd J. W. , and Demski J. W. 1992b Comparison of hidden and apparent spotted wilt epidemics in peanut. Proc. Am. Peanut Res. Ed. Soc 24 : 39 (abstr.).
Culbreath A. K. , Csinos A. S. , and Brenneman T. B. 1991 Association of Tomato spotted wilt virus with foliar chlorosis of peanut in Georgia. Plant Dis 75 : 863 .
Eckel C. S. , Cho K. , Walgenbach J. F. , Kennedy G. G. , and Moyer J. W. 1996 Variation in thrips species composition in field crops and implications for tomato spotted wilt epidemiology in North Carolina. Entomol. Exp. App 78 : 19 – 29 .
German T. L. , Ullman D. E. , and Moyer J. W. 1992 Tospoviruses: diagnosis, molecular biology, phylogeny, and vector relationships. Annu. Rev. Phytopathol 30 : 315 – 348 .
Groves R. L. , Walgenbach J. F. , Moyer J. W. , and Kennedy G. G. 2003 Seasonal dispersal patterns of Frankliniella fusca (Thysanoptera: Thripidae) and Tomato spotted wilt virus occurrence in central and eastern North Carolina. J. Econ. Entomol 96 : 1 – 11 .
Groves R. L. 2001 The role of weed hosts and tobacco thrips, Frankliniella fusca Hinds (Thysanoptera: Thripidae), in the epidemiology of tomato spotted wilt tospovirus. Ph.D. diss North Carolina State University Raleigh .
Hurt C. A. , Brandenburg R. L. , Jordan D. L. , Kennedy G. G. , and Bailey J. E. 2005 Management of spotted wilt vectored by Frankliniella fusca (Thysanoptera: Thripidae) in virginia market-type peanut. J. Econ. Entomol 98 : 1435 – 1440 .
Hurt C. A. 2003 Evaluation of cultural practices to reduce the incidence of Tomato spotted wilt virus in North Carolina peanut (Arachis hypogaea). M.S. Thesis North Carolina State University Raleigh .
Jordan D. L. 2006 Peanut Production Practices. 15 – 34 In Peanut Information, North Carolina Coop. Ext. Ser. Series AG-331.
Lyerly J. H. , Stalker H. T. , Moyer J. W. , and Hoffman K. 2002 Evaluation of Arachis species for resistance to Tomato spotted wilt virus. Peanut Sci 29 : 79 – 84 .
Mandal B. , Pappu H. R. , Culbreath A. K. , and Todd J. W. 2001 Movement and accumulation of Tomato spotted wilt virus in peanut (groundnut). Phytopathology 91 : S57 (abstr.).
Marois J. J. and Wright D. L. 2003 Effect of tillage system, phorate, and cultivar on tomato spotted wilt of peanut. Agron. J 95 : 386 – 389 .
Mitchell F. L. 1996 Implementation of the IPM planting window for management of Tomato spotted wilt virus and avoidance of peanut yellowing death. Final Compliance Report, Texas Pest Management Association, Biologically Intensive Integrated Pest Management Grant Program http://stephenville.tamu.edu/fmitchel/ento/tswv3.pdf.
Mound L. A. 1996 The Thysanoptera vector species of tospoviruses. Acta Hortic 431 : 298 – 309 .
Murakami M. , Gallo-Meagher M. , Gorbet D. W. , and Meagher R. L. 2006 Utilizing immunoassays to determine systemic tomato spotted wilt virus infection for elucidating field resistance in peanut. Crop Prot 25 : 235 – 243 .
Rowland D. , Dorner J. , Sorensen R. , Beasley J. P. , and Todd J. 2005 Tomato spotted wilt virus in peanut tissue types and physiological effects related to disease incidence and severity. Plant Pathol 54 : 431 – 440 .
SAS Institute 1999 User's manual, version 7. SAS Institute Cary, NC .
Shew B. Peanut Disease Management. Ext. Ser. Series AG-331 79 – 104 In 2006 Peanut Information, North Carolina Coop.
Tillman B. L. , Gorbet D. W. , Culbreath A. K. , and Todd J. W. 2006 Response of Peanut cultivars to seeding density and row patterns. Online. Crop Management doi:10.1094/CM-2006-0711-01-RS.
Todd J. W. , Culbreath A. K. , Chamberlin J. R. , Beshear R. J. , and Mullinix B. G. 1995 Colonization and population dynamics of thrips in peanuts in the southern United States. 453 – 460 In Parker B. L. , et al Thrips Biology and Management Plenum Press NY .
Todd J. W. , Culbreath A. K. , Demski J. W. , and Beshear R. 1990 Thrips as vectors of TSWV. Proc. Am. Peanut Res. Ed. Soc 22 : 81 (abstr.).
Ullman D. E. 1996 Thrips and tospoviruses: Advances and future directions. Acta. Hortic 431 : 310 – 324 .
Ullman D. E. , German T. L. , Sherwood J. L. , Westcot D. M. , and Cantone F. A. 1993 Tospovirus replication in insect vector cells: immunocytochemical evidence that the nonstructural protein encoded by the S RNA of tomato spotted wilt tospovirus is present in thrips vector cells. Phytopathology 83 : 456 – 463 .
Wetering Fvande , Goldbach R. , and Peters D. 1996 Tomato spotted wilt tospovirus ingestion by first instar larvae of Frankliniella occidentalis is a prerequisite for transmission. Phytopathology 86 : 900 – 905 .
Wijkamp I. , Almarza N. , and Peters D. 1995 Median latent period and transmission of tospoviruses vectored by thrips. 153 – 156 In Parker B. L. , et al Thrips Biology and Management Plenum Press NY .
Wijkamp I. and Peters D. 1993 Determination of the median latent period of two tospoviruses in Frankliniella occidentalis, using a novel leaf disk assay. Phytopathology 83 : 986 – 991 .
Notes
- First and second authors: Res. Agron. , USDA-ARS and Agric. Res. Statistician; Coastal Plain Exp. Sta., Tifton, GA 31793-0748. Third author: President and CEO, Hebert Green AgroEcology; Asheville, NC 28801. [^]
- Former Graduate Research Assistant and Professor, respectively, Dept. of Entomology, North Carolina State Univ., Box 7613, Raleigh, NC 27695-7613 [^]
- Professor, Dept of Entomology, North Carolina State Univ., Box 7630, Raleigh NC 27695-7630 [^]
- Professor, Dept. of Crop Science, North Carolina State Univ., Box 7629, Raleigh, NC 27695-7629 [^]
- Associate Professor, Dept. of Crop Science, North Carolina State Univ., Box 7620, Raleigh NC 27695-7620 [^] *Corresponding author (e-mail: steven.riniker@bayercropscience.com).
Author Affiliations