Introduction
Peanut (Arachis hypogaea) is an indeterminate plant on which new fruit continues to be set throughout the growing season. Therefore, the determination as to when to dig plants is critical to ensure maximum yield because many of the heaviest pods can be lost during digging if allowed to become “over-mature”. Even when harvest occurs at the optimum time, plants contain pods of varying physiological maturity, including some that are very immature. In certain years the percentage of immature pods can be relatively high, and this presents a variety of problems to different segments of the peanut industry. To the grower, a high percentage of immature pods means reduced yield and grade. To the buying point manager, it means farmers' stock loads are more difficult to dry without decreasing overall quality because of the higher moisture content associated with immature pods. Also, it is difficult to determine when a load has reached a kernel moisture content (KMC) that is safe for storage because individual KMCs can vary widely around the mean. To the warehouseman and/or sheller there is greater potential for aflatoxin contamination by Aspergillus flavus and A. parasiticus because higher preharvest aflatoxin concentrations are associated with immature kernels (Dorner et al., 1989) and their higher KMC increases the risk of further contamination during storage. Finally, the manufacturer experiences problems associated with the different roasting characteristics of immature as compared to mature kernels as well as less desirable flavor.
In addition to the association of immature pods with higher levels of preharvest aflatoxin (Dorner et al., 1989), it also has been shown that kernel water activity (aw), or equilibrium relative humidity, is a major factor in aflatoxin contamination (Diener and Davis, 1968; Dorner et al., 1989). The aw of a food is defined as the ratio of the vapor pressure of the food to the vapor pressure of pure water at the same temperature and pressure (Scott, 1957), and it is equal to the equilibrium relative humidity divided by 100. The ability of A. flavus to grow in a substrate and produce aflatoxin is governed more accurately by aw, not total KMC (Northolt and Bullerman, 1982). This is because aw is a measure only of water that is actually available for fungal growth, while KMC includes water that is bound and unavailable. However, most determinations of the water content of peanuts are made by measuring total KMC only. Data generally are not available to convert total KMC to aw because those relationships vary considerably among substrates. This presents difficulties in understanding the process of aflatoxin contamination in peanuts, particularly preharvest contamination resulting from extended late-season drought. The relationship between kernel aw and total KMC is understood in general terms, and some KMC-aw curves for peanuts have been published (Pixton, 1967; Pixton and Warburton, 1971); however, those curves were likely based on mature peanuts (no maturity designations were given) and they were confined to peanuts well below a KMC of 20% (aw < 0.90). Dorner et al. (1989) pointed out that when the aw of peanuts was high, there were large differences in total KMC among different maturity stages. However, there was no indication of how those differences changed as peanuts dehydrated under drought conditions. The purpose of this paper is to present relationships between KMC and aw for freshly harvested peanuts in various maturity stages covering a wide KMC range.
Materials and Methods
Cultivar Florunner peanuts were grown in plots at the National Peanut Research Laboratory equipped with a mechanized roof system to withhold rainfall during the last 40 days of the growing season to induce drought stress (Blankenship et al., 1983). Prior to that time peanuts received full irrigation either from rainfall or an irrigation system mounted under each roof. Irrigation was optimized using the Irrigator Pro expert system (Davidson, et al., 1998; Lamb et al., 2004). During the last 4 weeks of the growing season, pod samples were collected periodically by digging several plants exhibiting varying degrees of stress based on visual assessment. Pods were hand-picked and placed in a wet impact blaster (Williams and Monroe, 1986) to remove the exocarp and expose the color in the mesocarp. Color and structural differences in the mesocarp were used to separate pods into five maturity stages (Henning, 1983; Williams and Drexler, 1981). This is commonly referred to as the hull scrape method and is the most commonly used method to determine the optimum time for digging peanuts. In order of increasing maturity, stages were yellow 1, yellow 2, orange, brown, and black, which correspond to stages 3–7, respectively, as described by Williams and Drexler (1981). The designations used here (yellow 1 to black) are those that appear on peanut profile boards that are widely used in the southeastern US and are available at most county extension offices in peanut-growing areas.
Pods were hand-shelled and individual kernels were placed in small air-tight containers and allowed to equilibrate to 25 C. Kernels were then weighed to the nearest 0.1 mg, and aw was determined with an AquaLab model CX-2 water activity meter (Decagon Devices, Inc., Pullman, WA). KMC (wet weight) was then determined according to ASAE standard 410.1 (ASAE, 1993) by drying individual kernels for 6 h at 130 C. After drying, kernels were placed in a desiccator for temperature equilibration and then re-weighed. KMC was calculated as: (initial weight – final weight)/initial weight × 100.
Results and Discussion
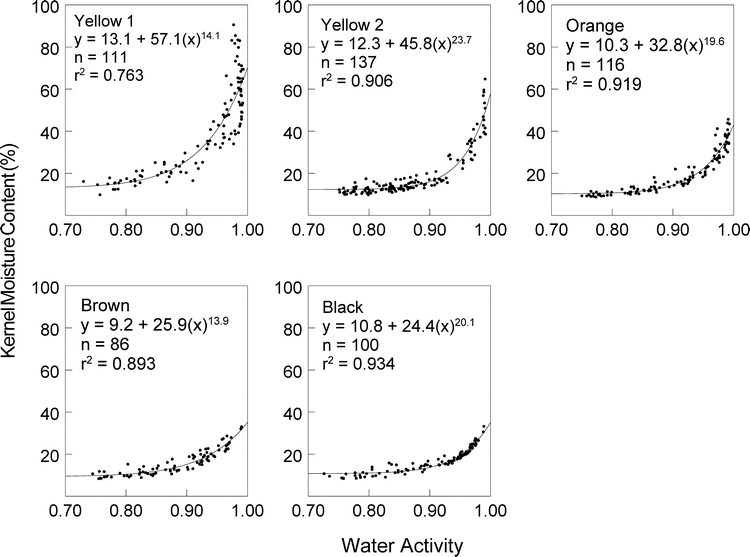
The KMC and aw of 550 kernels were measured, ranging from n = 86 for the brown maturity stage to n = 137 for the yellow 2 stage. The power function was used to describe the relationships between KMC and aw for the five maturity stages, and curves and equations for those relationships are shown in Figure 1. The general relationship between KMC and aw was similar among maturity stages, demonstrating a strong correlation between KMC and aw. The strength of the correlation generally increased with increasing maturity.
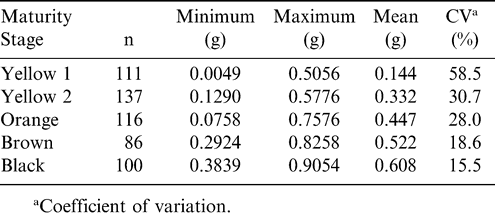
When the aw was relatively high, there were large differences in KMC among maturity stages with the more immature stages having much higher total KMC than mature stages. In addition, the immature stages had much more variation in KMC at high aw levels. When the aw was > 0.97, KMC in the yellow 1 stage ranged from 34.0 to 90.6%, but the degree of variation decreased as maturity increased. The larger variation in KMC associated with the more immature stages corresponds to rapid compositional changes that are occurring, such as with starch, sugar, and lipid contents (Pattee et al., 1974). Statistics based on the dry weight of kernels are shown in Table 1 and illustrate decreasing variation in dry weight as pods mature. The pattern of decreasing variation in KMC at high aw with increasing maturity (Figure 1) coupled with a similar decrease in variation associated with dry weight (Table 1) indicates that as pods mature within a maturity stage, rapid changes in total KMC occur in the immature stages with much smaller changes occurring in the more mature stages (brown and black).
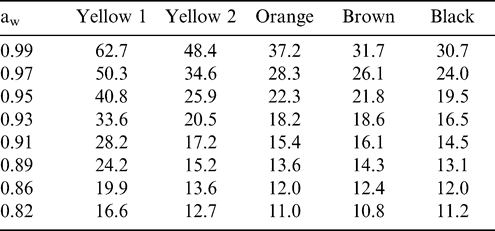
Equations in Figure 1 were used to predict % KMC for a variety of aw values (Table 2). There was extremely wide variation in % KMC among maturity stages at high aw, ranging from 62.7% for the yellow 1 class to 30.7% for the black class at an aw of 0.99. As the aw decreased so did the variation in KMC among maturity stages, but even at the relatively low aw of 0.82, the total moisture of kernels from more immature pods was still higher than that from the more mature orange, brown, and black stages.
These KMC-aw relationships have particular implications in studies of preharvest aflatoxin contamination of peanuts resulting from infection and growth by A. flavus and A. parasiticus. It has been reported that the minimum aw for aflatoxin production in peanuts is about 0.85 (Diener and Davis, 1968). Furthermore, kernels from immature pods are more likely to be contaminated with aflatoxin than kernels from mature pods (Dorner et al., 1989). When kernels are at high aw (> 0.95), they are unlikely to accumulate aflatoxin, presumably because of the ability of kernels to produce antifungal phytoalexins (Sobolev et al., 1995; Sobolev et al., 2007). As the aw decreases during a period of extended drought, kernels eventually lose the capacity to produce phytoalexins and become contaminated (Dorner et al., 1989). The loss of phytoalexin-producing capacity occurs between an aw of 0.98 and 0.95, making kernels particularly susceptible to aflatoxin contamination when the aw is close to 0.95. As kernel aw continues to decrease, the growth rate of the aflatoxigenic fungi and amounts of aflatoxins produced become limited. Therefore, the critical aw for preharvest aflatoxin production in peanuts appears to be in the range of about 0.95–0.90. Although aflatoxin can be produced at aw lower than 0.90, most of the preharvest aflatoxin that is produced in individual kernels probably accumulates as the kernel dehydrates through the optimum aw range for A. flavus growth and aflatoxin production after phytoalexin production shuts down.
Although one cultivar was used in this study, it is expected that other cultivars and genotypes would react similarly as long as the composition (primarily oil content) is similar. In a comparison of equilibrium relative humidity (equivalent to aw ) and moisture relationships for various products, Pixton (1967) showed that differences were slight for different non-oily cereals, but for high-oil products differences were much greater. The major differences seen in this study among maturity groups is likely a function of their different oil contents. As oil content increased and became more stable with increasing maturity, differences in the KMC-aw relationships became less.
The results presented here can be useful in aflatoxin modeling and breeding programs that are focused on development of peanut cultivars with greater resistance to aflatoxin contamination. One possible approach in development of such cultivars is to identify genotypes and germplasm that can maintain high kernel aw for longer periods of time during a period of late-season drought (Dorner et al., 1991). Monitoring aw during late-season drought, particularly in the more susceptible immature kernels, could help identify genotypes with the potential for reduced aflatoxin accumulation without the costly aflatoxin analyses that often yield extremely variable results. If measuring KMC is more convenient or cost-effective than measuring aw, the equations in Figure 1 can be used to calculate aw for a better assessment of susceptibility.
Acknowledgements
The technical contributions of Valerie Orner, Sam Hilton, III, and Robert A. Tennille are gratefully acknowledged.
Literature Cited
ASAE 1993 S410.1: Moisture measurement – Peanuts. ASAE Standards, 40th ed ASAE St. Joseph, MI .
Blankenship P. D. , Cole R. J. , Sanders T. H. , and Hill R. A. 1983 Environmental control plot facility with manipulable soil temperature. Oléagineux 38 : 615 – 620 .
Davidson J. I. , Bennett C. T. , Tyson T. W. , Baldwin J. A. , Beasley J. P. , Bader M. J. , and Tyson A. W. 1998 Peanut irrigation management using EXNUT and MOISNUT computer programs. Peanut Sci 25 : 103 – 110 .
Diener U. L. and Davis N. D. 1968 Effect of environment on aflatoxin production in freshly dug peanuts. Trop. Sci 10 : 22 – 28 .
Dorner J. W. , Cole R. J. , Sanders T. H. , and Blankenship P. D. 1989 Interrelationship of kernel water activity, soil temperature, maturity, and phytoalexin production in preharvest aflatoxin contamination of drought-stressed peanuts. Mycopathologia 105 : 117 – 128 .
Dorner J. W. , Cole R. J. , Yagen B. , and Christiansen B. 1991 Bioregulation of preharvest aflatoxin contamination of peanuts. 352 – 360 In Hedin P. A. Naturally Occurring Pest Bioregulators American Chemical Society Washington .
Henning R. J. 1983 The hull-scrape method to determine when to dig peanuts. The Peanut Farmer 19 : 11 – 14 .
Lamb M. C. , Masters M. H. , Rowland D. , Sorensen R. B. , Zhu H. , Blankenship P. D. , and Butts C. L. 2004 Impact of sprinkler irrigation amount and rotation on peanut yield. Peanut Sci 31 : 108 – 113 .
Northolt M. D. and Bullerman L. B. 1982 Prevention of mold growth and toxin production through control of environmental conditions. J. Food Prot 45 : 519 – 526 .
Pattee H. E. , Johns E. B. , Singleton J. A. , and Sanders T. H. 1974 Composition changes of peanut fruit parts during maturation. Peanut Sci 1 : 57 – 62 .
Pixton S. W. 1967 Moisture content — its significance and measurement in stored products. J. Stored Prod. Res 3 : 35 – 47 .
Pixton S. W. and Warburton S. 1971 Moisture content relative humidity equilibrium, at different temperatures, of some oilseeds of economic importance. J. Stored Prod. Res 7 : 261 – 269 .
Scott W. J. 1957 Water relations of food spoilage microorganisms. Adv. Food Res 7 : 83 – 127.
Sobolev V. S. , Cole R. J. , Dorner J. W. , and Yagen B. 1995 Isolation, purification, and liquid chromatographic determination of stilbene phytoalexins in peanuts. J. AOAC Int 78 : 1177 – 1182 .
Sobolev V. S. , Guo B. Z. , Holbrook C. C. , and Lynch R. E. 2007 Interrelationship of phytoalexin production and disease resistance in selected peanut genotypes. J. Agric. Food Chem 55 : 2195 – 2200 .
Williams E. J. and Drexler J. S. 1981 A non-destructive method for determining peanut pod maturity. Peanut Sci 8 : 134 – 141 .
Williams E. J. and Monroe G. E. 1986 Impact blasters for peanut pod maturity determination. Trans. ASAE 29 : 263 – 266 .
Notes
- First and second authors: Res. Agron. , USDA-ARS and Agric. Res. Statistician; Coastal Plain Exp. Sta., Tifton, GA 31793-0748. Third author: President and CEO, Hebert Green AgroEcology; Asheville, NC 28801. [^]
- Research Microbiologist, USDA, ARS, National Peanut Research Laboratory, 1011 Forrester Dr., SE, Dawson, GA 39842 [^] *Corresponding author (email: Joe.Dorner@ars.usda.gov)
Author Affiliations