Introduction
Boron (B) is an essential element needed for normal growth and development of peanut (Arachis hypogaea L.) (Gascho and Davis, 1995; Harris and Brolman, 1966a, 1966b, 1966c). Deficiencies of B in crops are often associated with production on sandy soils that are droughty and prone to leaching of nutrients (Gascho and Davis, 1995). Boron influenced flowering of peanut (Harris and Brolmam 1966a) and affected kernel formation (Harris and Brolman, 1966b). Boron is associated with sugar transport, polysaccharide formation, and pectin metabolism (Gardner et al., 1985). Deficiencies of B can be corrected with B-containing fertilizers applied topically during flowering (Gascho and Davis, 1995).
A variety of commercial formulations of B are available, with the range of elemental B applied per unit area varying considerably when these products are applied at the manufacturer’s suggested use rate. Some manufacturers claim that absorption of B is enhanced by their formulation. Determining the relative difference in the amount of elemental B accumulation in plant tissue following foliar application of B fertilizer is important in determining which formulation is the most beneficial for plant growth.
Determining compatibility of agrichemicals is important when developing pest management strategies for peanut. Interactions of graminicides with herbicides that control broadleaf weeds and purple nutesedge (Cyperus rotundus L) and yellow nutsedge (Cyperus esculentus L.) in peanut have been evaluated (Burke et al., 2004). Additionally, compatibility of agrichemicals that are routinely applied to peanut has been defined partially (Lancaster et al., 2005a; Lanier et al., 2003; Jordan et al., 2003). Beam et al. (2002) reported possible interactions of prohexadione calcium, a plant growth regulator applied to peanut to manage vine growth, with herbicides, insecticides, fungicides, foliar fertilizers, and other plant growth regulators registered for use in peanut. However, B did not affect efficacy of prohexadione calcium (Beam et al., 2002).
Reduced herbicide efficacy may be a result of chemical interactions in the spray solution. Cations in the spray solution can adversely affect efficacy of glyphosate and sethoxydim (Nalewaja et al., 1991; Wanamarta et al., 1993). In these situations, efficacy of some herbicides can be improved by the addition of ammonium sulfate prior to herbicide (Wanamarta et al., 1993). Additionally, Prostko et al. (2003) reported that soybean [Glycine max (L.) Merr.] injury was not increased when disodium octaborate was applied with glyphosate or diflubenzuron.
Interactions of fertilizers containing B with herbicides used in peanut have not been clearly defined. Lancaster et al. (2005b) reported that interactions among graminicides and disodium octaborate were inconsistent with respect to large crabgrass control. In other experiments, efficacy of 2,4-DB was not affected by disodium octaborate (Lancaster et al., 2005c). Interactions of B with fungicides and certain other agrichemicals have not been clearly defined.
Determining if B affects efficacy of postemergence herbicides and fungicides will help growers make better decision relative to applying mixtures of agrichemicals. Determining which formulations of B provide the most elemental B in plants will help when attempting to correct deficiencies when a wide range of products are commercially available. Research was conducted to determine: if differences in B accumulation occur in peanut leaf tissue when various commercially available B products are applied, if these B products affect weed and disease control when applied in mixtures with postemergence herbicides and fungicides, and if herbicides, fungicides, and adjuvants affect B accumulation in peanut leaves when applied in mixture with disodium octaborate.
Materials and Methods
Influence of Formulation on B Accumulation in Peanut Tissue
The experiment was conducted in two separate fields located near Edenton, NC during both 2001 and 2002 on a Roanoke silt loam soil (clayey, mixed, thermic, Typic Ochraquepts). The cultivar NC-V 11 was seeded at 130 kg/ha in conventionally prepared seedbeds on rows spaced 91 cm apart. Plot size was two rows by 5 m. Two non-treated rows separated each plot.
Treatments consisted of no B, a dry formulation of disodium octaborate (Solubor® 17.5DF, U.S. Borax, Inc., Valencia, CA) at 2.8 kg/ha (17.5% B), a liquid complex of B containing 3.3% B and 4.5% nitrogen (N) formulated at 1.14 kg B/L (N-BoronTM, Clawel Division, Brant Consolidated, Pleasant Plains, IL) at 0.38 L/ha, and a liquid formulation of B containing 5.0% B formulated at 1.10 kg B/L (Boron Xtra©, Custom Agricultural Formulations, Fresno, CA) at 0.12 L/ha applied during late flowering in late July. These rates are consistent with those recommended by the manufacturer and those applied by peanut producers in North Carolina. The concentration of B in a spray volume of 140 L/ha from these respective B formulations was 589, 316, and 3469 g B/L aqueous solution (data not shown). Boron was applied using a CO2-pressurized backpack sprayer equipped with 80015 regular flat fan nozzles (Spraying Systems Co., Wheaton, IL).
In 2001, ten leaves consisting of four leaflets per leaf were removed at random from the top one-third of the peanut canopy 6 h after application and were subjected to a 30 second leaf wash in 1 L tap water. Leaves were also removed 2 and 14 d after application but were not subjected to a water wash. A rainfall event of 32 mm occurred 1 d after application in 2001. In 2002, ten leaves were removed 1 wk after application and were washed in 1 L chloroform for 1 minute. Rainfall did not occur between the time treatments were applied and leaflets were harvested in 2002. Chloroform removes the epicuticular wax, and the amount of B present in the leaf tissue following this leaf wash would reflect the amount that was absorbed. In 2001, stems and pods were removed from three randomly selected plants within a plot for B determination.
In 2005, a B formulation containing 9% B (NutriSol 9% BoronTM, Coastal Agrobusiness, Inc., Greenville, NC) at 1.16 L/ha, boric acid (Boric AcidTM, SQM North America Corp., Atlanta, GA) at 3.4 kg/ha (17.4% B), and disodium octaborate at 2.8 kg/ha were applied in early July. The experiment was conducted in two fields near Edenton on the soil described previously for this location and at the Upper Coastal Plain Research Station near Rocky Mount. Ten leaves were removed from each plot 7 days after application as described previously and subjected to a 30 second wash in 1 L tap water. Leaf, stem, and pod tissue were dried and ground to pass through a 1 mm screen prior to B concentration determination using a Perkin Elmer 3300 Argon Plasma Emission Spectrophotometer (Perkin Elmer, Sulton, CN).
Influence of Postemergence Pesticides on B Accumulation in Leaf Tissue
The experiment was conducted in two fields at the Peanut Belt Research Station located near Lewiston-Woodville, NC in 2004 on a Norfolk sandy loam soil (fine-loamy, siliceous, thermic, Aquic Paleudults). The cultivar Gregory was seeded at 170 kg/ha in conventionally prepared seedbeds. Plot size was 2 rows (91-cm spacing) by 5 m. Treatments consisted of disodium octaborate applied alone, with crop oil concentrate (Agri-Dex®, Helena Chemical Co., Memphis, TN) at 1.0% (v/v), or with nonionic surfactant (Induce®, Helena Chemical Co., Memphis, TN) at 0.25% (v/v). Additional treatments included disodium octaborate applied with clethodim (Select 2EC herbicide, Valent USA Corp., Walnut Creek, CA) plus crop oil concentrate, imazapic (Cadre herbicide BASF Corp.Research Triangle Park, NC) plus nonionic surfactant, imazethapyr (Pursuit herbicide, BASF Corp., Research Triangle Park, NC) plus nonionic surfactant, sethoxydim (BASF Corp., Research Triangle Park, NC) plus crop oil concentrate, and 2,4-DB (Butyrac 200, Agri Star Ankeny, IA) without adjuvant. Clethodim, imazapic, imazethapyr, sethoxydim, and 2,4-DB were applied at 140, 70, 70, 220, and 220 g ai/ha, respectively.
Experiments were conducted during 2005 in separate but adjacent fields at the Peanut Belt Research Station located near Lewiston-Woodville to determine the influence of fungicide and insecticide on B accumulation in peanut leaves when disodium octaborate was applied to peanut. Treatments consisted of B (Solubor) applied alone or with the fungicides azoxystrobin (Abound, Syngenta Crop Protection, Greensboro, NC) at 0.43 kg ai/ha, chlorothalonil (Bravo Weather Stik, Syngenta Crop Protection, Greensboro, NC) at 1.25 kg ai/ha, pyraclostrobin (Headline, BASF Corp., Research Triangle Park, NC) at 0.16 kg ai/ha, and tebuconazole (Folicur, Bayer CropScience, Research Triangle Park, NC) at 0.22 kg ai/ha and the insecticide lambda cyhalothrin (Karate Z, Syngenta Crop Protection, Greensboro, NC) at 0.036 kg ai/ha. A non-treated control was included. Additional treatments included B applied with mixtures of lambda cyhalothrin and each of the fungicides listed previously.
Treatments were applied using the equipment and procedures described previously. Ten leaves were removed at random from the top one-third of the peanut canopy 1 wk after application and were washed in 1 L tap water for 1 minute. Concentration of B was determined as described previously.
Influence of B Formulation on Efficacy of Postemergence Herbicides and Fungicides
The experiment was conducted at the Cherry Research Farm located near Goldsboro, NC in 2001 on a Wickam sandy loam (fine-loamy, mixed, semi-active, thermic, Typic Hapludults). The experiment was also conducted at the Central Crops Research Station located near Clayton, NC in 2002 on a Geliad sandy loam soil (Clayey, kaolinitic, thermic, Aquic Hapludults). Experiments were established in tilled fallow areas without a peanut crop. Plot size was 2 by 5 m.
Treatments consisted of clethodim, imazapic, imazethapyr, sethoxydim, and 2,4-DB applied alone or with disodium octaborate, the liquid formulation containing 3.3% B and 4.5% N, and the liquid B formulation containing 5.0% B. Herbicides were applied in separate experiments and were repeated in different fields at the same location. Natural infestations of large crabgrass (Clayton), Palmer amaranth (Goldsboro), and sicklepod (Goldsboro) were used to evaluate efficacy of clethodim and sethoxydim, imazethapyr, imazapic, and 2,4-DB, respectively. Clethodim and sethoxydim were applied when large crabgrass was 12 cm tall with 3 to 4 leaves. Imazethapyr was applied when Palmer amaranth was 60 cm tall. Imazapic and 2,4-DB were applied when sicklepod had 4 to 5 leaves (15 cm). Treatments were applied as described previously.
Visual estimates of percent weed control were determined 2 wk after application using a scale of 0 to 100% where 0 = no control and 100 = complete control. Foliar chlorosis, necrosis, and plant stunting were used when making the visual estimates.
During 2005 in two separate fields at the Peanut Belt Research Station located near Lewiston-Woodville, a liquid commercial formulation of B containing 9% B (NitroSol 9% Boron) at 2.24 L/ha was applied alone or with the fungicides azoxystrobin, chlorothalonil, pyraclostrobin, and tebuconazole in early August. A non-treated control was included, and no other fungicides were applied in the test either before or after applications. Fungicide rates were the same as those applied in the B accumulation experiment. Soil was a Norfolk sandy loam, and plot size was 2 rows (96-cm spacing) by 5 m with one non-treated row separating plots. Visual estimates of percent canopy defoliation caused by late leaf spot were recorded on a scale of 0 to 100% in late September using a scale of 0 = no defoliation to 100 = complete defoliation.
Statistical Analyses
Data for B accumulation (mg B/kg tissue), percent weed control, and percent canopy defoliation were subjected to analysis of variance and were pooled over experiments when possible. Means were separated using Fisher’s Protected LSD Test at p ≤ 0.05.
Results and Discussion
Influence of Formulation on B Accumulation in Peanut Tissue
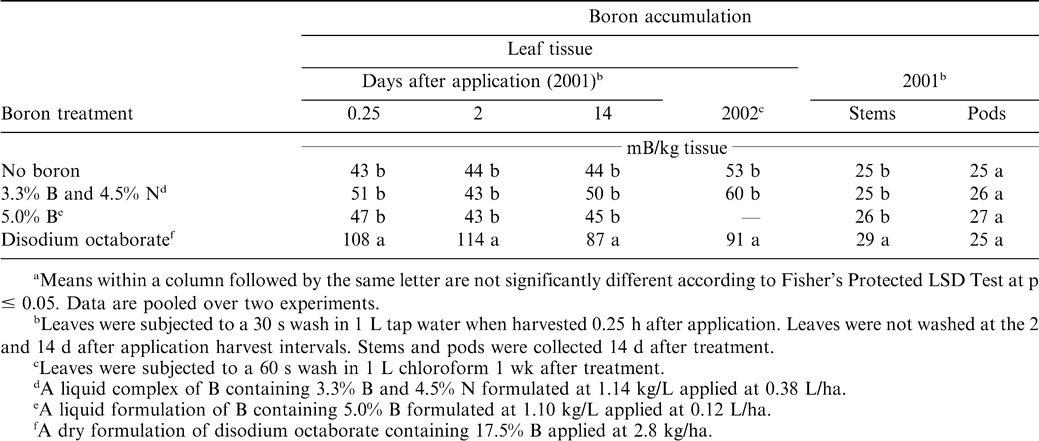
The interaction of B formulation by experiment for B accumulation in leaf tissue was not significant regardless of the timing of leaf harvest or the solvent used to wash leaves in the 2001 and 2002 experiments. Boron accumulation in leaf tissue was higher when disodium octaborate was applied compared with the other formulations of B and the non-treated control (Table 1). This response was noted at all intervals of tissue harvest, regardless of whether the solvent used to wash leaves was tap water or chloroform. This response was not unexpected because a higher rate of B was applied per ha when the dry formulation of disodium octaborate was applied compared with the liquid formulations of B.
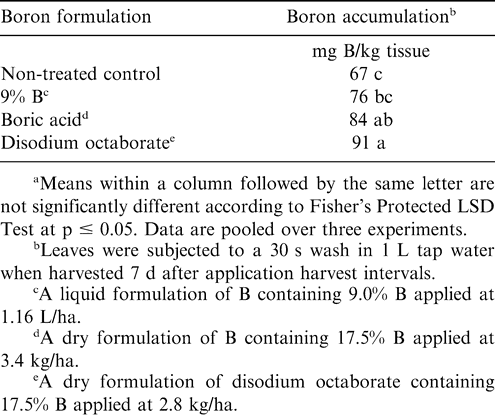
During 2005, the interaction of B formulation by experiment was not significant for B accumulation in leaves one week after application. Accumulation of B was similar when B was applied as disodium octaborate and boric acid, and accumulation exceeded B concentration in non-treated peanut (Table 2). Accumulation of B was similar for the 9% liquid B formulation and boric acid although concentration for the 9% liquid B formulation did not differ from non-treated peanut. Although the concentration of B in the 9% liquid solution was higher than the concentration of liquid formulations used in 2001 and 2002, B accumulation was not increased over that of non-treated peanut.
These data demonstrate that the rates of formulated product recommended by the manufacturer for these liquid formulations are not high enough to increase the B concentration in leaves. Root absorption of B is also a significant entry point, and B products providing a higher amount of B most likely would increase B availability in soil for root uptake.
The concentration of B in stems was higher following application of disodium octaborate compared with application of the other two B formulations and the non-treated control in the 2001 experiments (Table 1). In contrast, the concentration of B in pods did not differ among B formulations and the non-treated control (Table 1). These data indicate that while B accumulation in leaves and stems may be higher when disodium octaborate is applied compared to other B formulations and non-treated peanut, this increase may not result in a corresponding increase in B in pods. Results of B deficiencies are observed in peanut pods (Harris and Brolman, 1966b). However, the mechanism of B function in plants is not completely understood. It is suspected that B influences carbohydrate transport and subsequent polysaccharide deposition and cell wall formation (Gardner at al., 1985). A greater pool of B in leaf and stem tissue may influence carbohydrate transport to the developing fruit. However, postulating further on the physiological mechanism of B function in relation to responses noted in these experiments is beyond the scope of our study.
Influence of Postemergence Pesticides on B Accumulation in Leaf Tissue
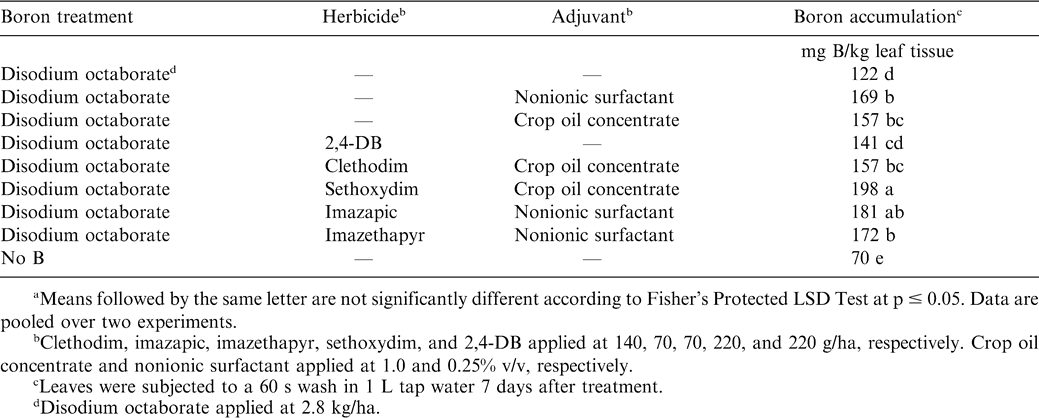
The treatment by experiment interaction was not significant for accumulation of B in leaf tissue 1 wk after application. Applying disodium octaborate increased the concentration of B in leaf tissue regardless of adjuvant or herbicide treatment (Table 3). Nonionic surfactant and crop oil concentrate increased the concentration of B in leaf tissue from 122 g B/kg leaf tissue when adjuvant was not applied to 169 and 157 g B/kg leaf tissue for these respective adjuvants. When disodium octaborate was applied with 2,4-DB, the amount of B in leaf tissue was similar to that by disodium octaborate without adjuvant or with crop oil concentrate but less than that of disodium octaborate plus nonionic surfactant. Boron concentration in leaf tissue following applications of combinations of sethoxydim with crop oil concentrate and disodium octaborate exceeded the concentration noted for clethodim plus crop oil concentrate plus disodium octaborate. However, the concentration of B in leaf tissue following both treatments containing graminicides exceeded that by disodium octaborate without adjuvant or herbicide. When applied with the imidazolinone herbicides imazapic and imazethapyr, the concentration of B in leaf tissue was similar.
These data show that applying disodium octaborate with nonionic surfactant or crop oil concentrate increases absorption of B into epidermis and inner cells of leaves. These data also suggest that applying disodium octaborate with clethodim, imazapic, imazethapyr, sethoxydim, and 2,4-DB will not affect B accumulation in tissue when compared with applications without herbicide or adjuvant.
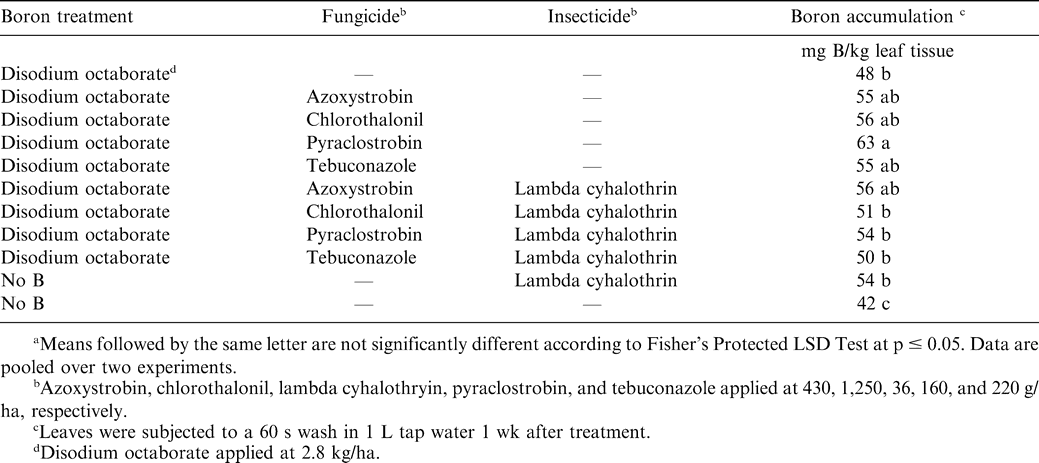
Applying B increased B accumulation in tissue compared with non-treated peanut (Table 4). Boron accumulation was similar when disodium octaborate was applied with azoxystrobin, chlorothalonil, pyraclostrobin, and tebuconazole, and with the exception of pyraclostrobin, B accumulation was similar when comparing B alone or with these fungicides mixed with lambda cyhalothrin. Boron accumulation was higher when B was applied with pyraclostrobin compared with B plus lambda cyhalothrin either alone or with the fungicides chlorothalonil, pyraclostrobin, or tebuconazole.
Influence of B Formulation on Efficacy of Postemergence Herbicides and Fungicides
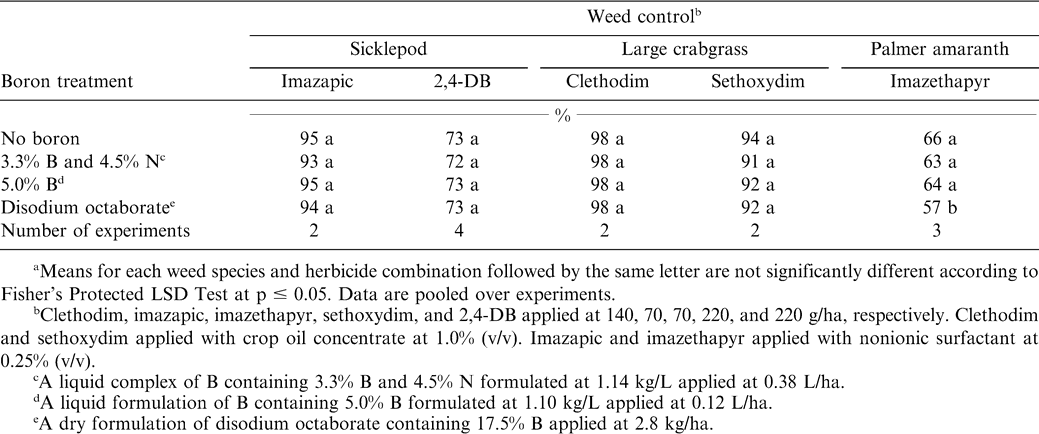
The interaction of experiment by B treatment was not significant for sicklepod, large crabgrass, and Palmer amaranth control by the herbicides evaluated in these experiments. Sicklepod control by imazapic and 2,4-DB was not affected by B treatment (Table 5). Sicklepod control by imazapic ranged from 93 to 95%. These results are consistent with other research suggesting that imazapic controls sicklepod (Jordan, 2004). Although sicklepod control by 2,4-DB was not affected by B, control ranged from 72 to 73%. Single applications of 2,4-DB are generally insufficient to control sicklepod completely (Jordan, 2004). Lancaster et al. (2005c) reported that tall morningglory control by 2,4-DB was not affected by disodium octaborate.
Large crabgrass control by clethodim and sethoxydim ranged from 91 to 98% and was not affected by any of the B formulations (Table 5). Similarly, Lancaster et al. (2005b) reported that disodium octaborate did not affect broadleaf signalgrass (Brachiaria platyphylla Griseb. Nash) or large crabgrass control by clethodim or sethoxydim. However, in some experiments where fungicides were applied in mixture with clethodim, disodium octaborate increased control when it was included in the mixture.
The liquid formulations of B did not reduce Palmer amaranth control by imazethapyr compared with imazethapyr alone (Table 5). In contrast, applying disodium octaborate with imazethapyr resulted in a decrease in control from 63 to 66% when imazethapyr was applied alone or with the liquid B formulations to 57% when imazethapyr was applied with disodium octaborate.
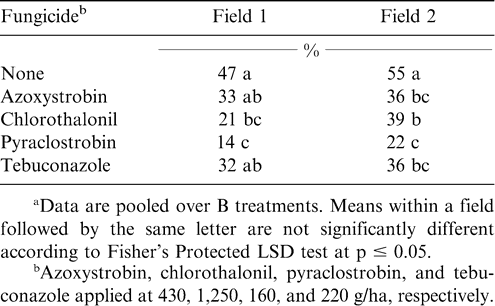
In the experiments evaluating combinations of fungicides and B, the main effect of B and the interaction of fungicide by B were not significant for either field for peanut defoliation. Chlorothalonil and pyraclostrobin reduced defoliation in Field 1 compared with the non-treated control (Table 6). However, azoxystrobin and tebuconazole did not reduce defoliation compared to the non-treated control in this field. In contrast, all fungicides reduced defoliation in Field 2, and pyraclostrobin was more effective than chlorothalonil or tebuconazole (Table 6). Significant defoliation was expected in these fields because after the application of fungicides and B no other fungicides were applied. The plot area in these fields received only one fungicide application, and four or more fungicide sprays were needed to prevent defoliation under weather conditions and field history during 2005 (data not shown). These data do indicate that the liquid B formulation used in this experiment did not affect fungicide efficacy, therefore suggesting that fungicides and B most likely will be compatible.
Collectively, these results suggest that growers should carefully consider the B formulations purchased to ensure adequate B is applied to peanut when B deficiencies are anticipated. While disodium octaborate and boric acid provided the highest concentration of B in leaves and stems, there was no difference in B accumulation in pods 2 wks after application. A better understanding of the mechanism of B function as related to foliar applications of B is needed. These data also demonstrate that B can be applied with herbicides belonging to cyclohexanedione, imidazolinone, and phenoxy families and with nonionic surfactant and crop oil concentrate without adversely affecting B absorption. In fact, results from these studies showed that B absorption can be enhanced when B is applied with certain herbicides and adjuvants. With the exception of imazethapyr applied with disodium octaborate, the B products evaluated in these studies are compatible with most postemergence herbicides and fungicides applied to peanut during middle and latter parts of the peanut growing season.
Acknowledgements
Assistance was provided by staff at the Central Crops Research Station, Cherry Research Farm, the Peanut Belt Research Station, the Upper Coastal Plain Research Station, and by the North Carolina Department of Agriculture and Consumer Services. Boone Farm Supply provided the liquid B formulation Boron Xtra©. The other B formulations were purchased locally. Pesticides were generously provided by manufacturers. This research was supported financially by the North Carolina Peanut Growers Association and USAID Peanut CRSP (LAG –G-00-96-90013-00).
Literature Cited
Beam J. B. , Jordan D. L. , York A. C. , Bailey J. E. , Isleib T. G. , and McKemie T. E. 2002 Interactions of prohexadione calcium with agrichemicals applied to peanut (Arachis hypogaea). Peanut Sci 29 : 29 – 35 .
Burke I. C. , Price A. J. , Wilcut J. W. , Jordan D. L. , and Culpepper A. S. 2004 Annual grass control in peanut (Arachis hypogaea) with clethodim and imazapic. Weed Technol 18 : 88 – 92 .
Gardner F. P. , Pearce R. B. , and Mitchell R. L. 1985 Mineral nutrition. 98 – 131 in Physiology of Crop Plants Iowa State University Press Ames, IA 327 .
Gascho G. J. and Davis J. G. 1995 Soil fertility and plant nutrition. 383 – 418 in Pattee H. E. and Stalker H. T. eds. Advances in Peanut Science Am. Peanut Res. and Educ. Soc Stillwater .
Harris H. C. and Brolman J. B. 1966a Comparison of calcium and boron deficiencies of peanut I. Physiological and yield differences. Agron. J 58 : 575 – 578 .
Harris H. C. and Brolman J. B. 1966b Comparison of calcium and boron deficiencies of peanut II. Seed quality in relation to histology and viability. Agron. J 58 : 578 – 583 .
Harris H. C. and Brolman J. B. 1966c Effect of imbalance of boron nutrition on the peanut. Agron. J 58 : 97 – 99 .
Jordan D. L. 2004 Peanut weed management. 33 – 54 in 2004 Peanut Information North Carolina Coop. Ext. Ser. Pub. 331 106 .
Jordan D. L. , Culpepper A. S. , Grichar W. J. , Tredaway-Ducar J. , Brecke B. J. , and York A. C. 2003 Weed control with combinations of selected fungicides and herbicides applied postemergence to peanut (Arachis hypogaea). Peanut Sci 30 : 1 – 8 .
Lancaster S. , Jordan D. , Brandenburg R. , Royals B. , Shew B. , Bailey J. , Curtis V. , York A. , Wilcut J. , Beam J. , Prostko E. , Culpepper S. , Grey T. , Johnson C. , Kemerait R. , Grichar J. , Baughman T. , Dotray P. , Brecke B. , MacDonald G. , Tredaway-Ducar J. , and Walls B. 2005a Tank mixing chemicals applied to peanut: are the chemicals compatible? North Carolina Coop. Ext. Ser. Pub. AG-W-653R Available at: www.peanut.ncsu.edu.
Lancaster S. R. , Jordan D. L. , York A. C. , Wilcut J. W. , and Monks D. W. 2005b Interactions of clethodim and sethoxydim with selected agrichemicals applied to peanut (Arachis hypogaea). Weed Technol 19 : 456 – 451 .
Lancaster S. H. , Jordan D. L. , York A. C. , Wilcut J. W. , Brandenburg R. L. , and Monks D. W. 2005c Interactions of late-season morninggory (Ipomoea spp.) management practices in peanut (Arachis hypogaea). Weed Technol 19 : 803 – 808 .
Lanier J. E. , Jordan D. L. , Culpepper A. S. , Grichar W. J. , Tredaway-Ducar J. E. , and Brecke B. J. 2002 Interactions of postemergence herbicides with foliar fertilizers and fungicides. Proc. South. Weed Sci. Soc 55 : 185 .
Nalewaja J. D. , Manthey F. A. , Szelezniak E. F. , and Anyska Z. 1989 Sodium bicarbonate antagonism of sethoxydim. Weed Technol 3 : 654 – 658 .
Prostko E. P. , Norsworthy J. K. , and Rayner P. A. 2003 Soybean (Glycine max) response to glyphosate, diflubenzuron, and boron combinations. Weed Technol 17 : 186 – 189 .
Wanamarta G. , Kells J. J. , and Penner D. 1993 Overcoming antagonistic effects of Na-bentazon on sethoxydim absorption. Weed Technol 7 : 322 – 325 .
Notes
- * Assoc. Prof., former Grad. Asst., former Grad. Asst., Ext. Tech., former Grad. Asst., and William Neal Reynolds Prof., Dept. Crop Sci., North Carolina State Univ., Box 7620, Raleigh, NC 27695; Prof., Dep. Entomology, Box 7613, Raleigh, NC; and former and current Chief-Plant/Waste/Solutions Section and Agronomists, respectively, North Carolina Dept. Agriculture and Consumer Services, 4300 Reedy Creek Rd., Raleigh, NC 27607-6465. Current address of S. H. Lancaster, Dept. Soil and Crop Science, Texas A&M Univ., College Station, TX 77843. Current address of J. Beam, North Carolina Coop. Ext. Ser., 115 W. Main St., Lincolnton, NC 28092. Current address of F. R. Walls, FMC Corp., Goldsboro, NC 27533. Current address of S. Casteel, Dep. of Soil Sci., North Carolina State Univ., Box 7619, Raleigh, NC 27695. [^] *Corresponding author E-mail: david_jordan@ncsu.edu.
Author Affiliations