Introduction
The role of starch granules and their development in peanut cotyledonary cells are poorly understood. Pattee and Stalker (1991) observed differences in starch grain concentration and cytoplasmic stranding organization between Arachis hypogaea cultivars and the wild Arachis species. They suggested these differences have a significant impact on energy availability at syngamy and the subsequent early cell division of the embryo. Although starch is not normally considered a reserve source in peanut seeds, peanut seed may accumulate starch more rapidly than lipids in the early stages of maturity (Pattee et al., 1974). Furthermore, starch reserves in peanuts are rapidly depleted during the first day of germination (Vyas et al., 1967). This observation is consistent with the idea that starch reserves may provide a sugar source for peanut seed germination. The objective of this study was to examine the ultrastructure of the developing starch granules in peanut cotyledonary cells and cellular material involved in the synthesis of starch.
Materials and Methods
Materials
Ten randomly selected peanut plants (cv. NC 7) were harvested from the Central Crops Research Station, Clayton, North Carolina. Peanut pods were opened, and twenty seeds were classified into early and late developmental stages according to criteria modified from Pattee et al., (1974). The peanut seeds were ranked developmentally based on interior pericarp surfaces, testa characteristics, and seed size and shape. Peanut seeds were classified as being in an early developmental stage (stages 6 -7) according to the following criteria: (1) pods with light-colored interior pericarp surfaces, (2) testa with longitudinal wrinkles, and (3) seeds approximately 8 mm in length and having a length/width ratio average of 2.2. Peanut seeds were classified as being in a late developmental stage (stages 12 to 13) according to the following criteria: (1) pods with dark-colored interior pericarp surfaces, (2) smooth testa, and (3) seeds approximately 20 mm in length and having a length/width ratio average of 1.6.
Methods
Testa was carefully removed from seeds with a razor blade. The individual seeds were separated into the two cotyledons that comprise each seed. Tissue blocks (1 mm3) of mid-region cotyledon were cut with a razor blade and fixed in Karnovsky's fixative (Karnovsky, 1965) as modified by Young and Schadel (1989). The modified fixative was prepared by mixing 25 mL of 8% formaldehyde, 3.6 mL of 70% glutaraldehyde and 28.6 mL of 0.l M sodium phosphate buffer. The tissue blocks were fixed under vacuum for 30 min at 23 C and then fixed at atmospheric pressure for 48 hr at 4 C. Following six changes of 0.1 M sodium phosphate buffer (4 C, pH 7.0), the material was post-fixed for l hr in l% osmium tetroxide in 0.1 M buffer (4 C, pH 7.0) and dehydrated at room temperature at 15 min intervals in a graded series of aqueous ethanol (10, 25, 50, 75 and 95%) and then finally at two 30 min intervals in absolute ethanol.
Dehydrated tissue for scanning electron microscopy was critical point dried in a Tousimis unit (Ladd Research, Williston, VT) using liquid carbon dioxide. Dried sections were mounted on aluminium specimen stubs with double-sided tape and silver conducting paint. Tissue blocks on stubs were coated with 30 nm of gold-palladium alloy with a Hummer V sputter-coater fitted with a Technics digital thickness monitor. Specimens were viewed with a Phillips 505T SEM at a working distance of 15 mm and an accelerating voltage of 15 kV.
Dehydrated tissue for transmission electron microscopy was also embedded in Spurr's resin for long pot-life (Spurr, 1969). Ultrathin sections cut with a Reichert ultramicrotome were stained with 4.0% uranyl acetate for 45 min, followed by 0.4% lead citrate for 4 min. Sections were examined with a JEOL 100S transmission electron microscope.
Results
Transmission Electron Microscopy
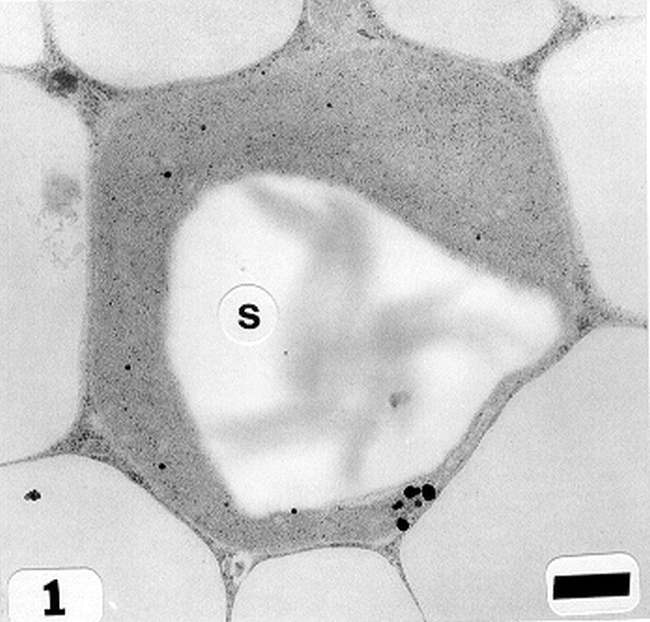
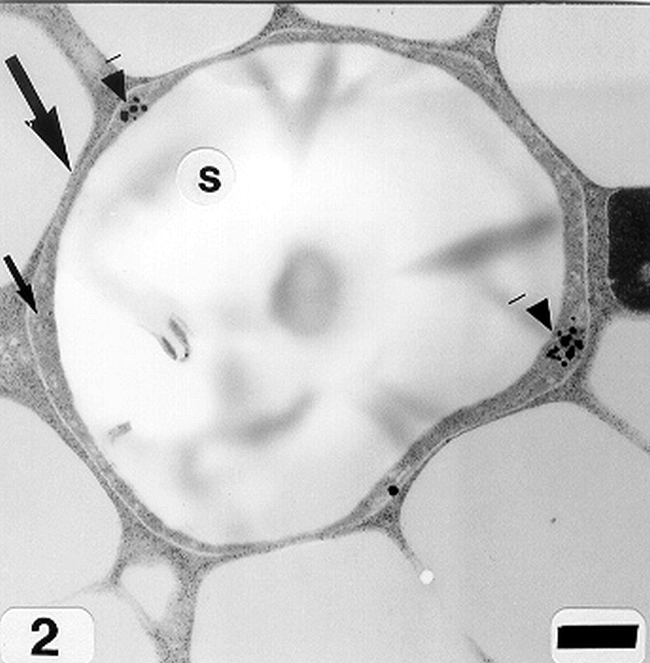
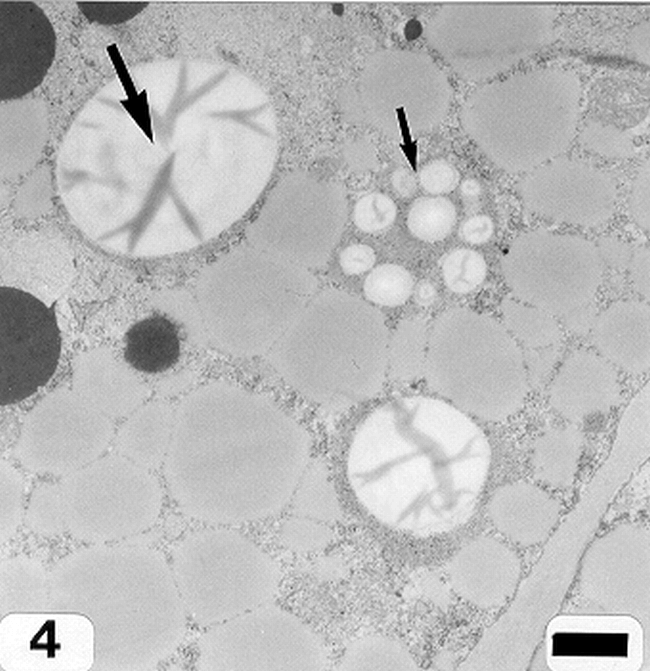
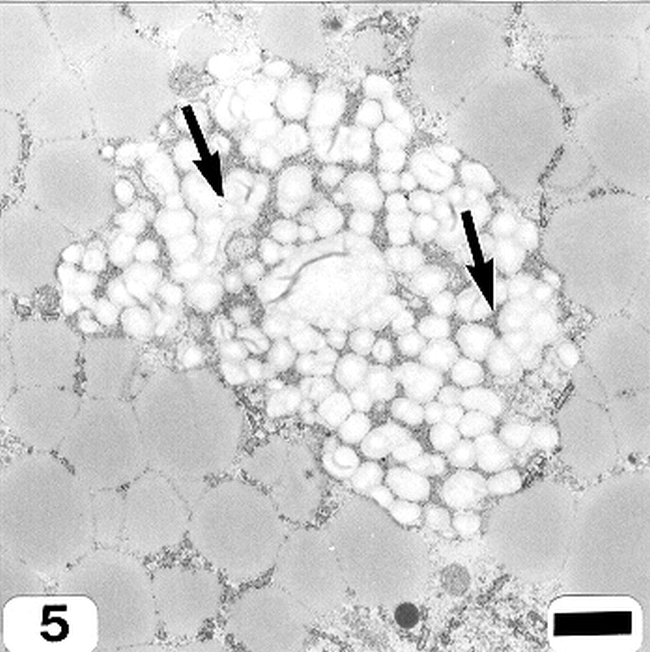
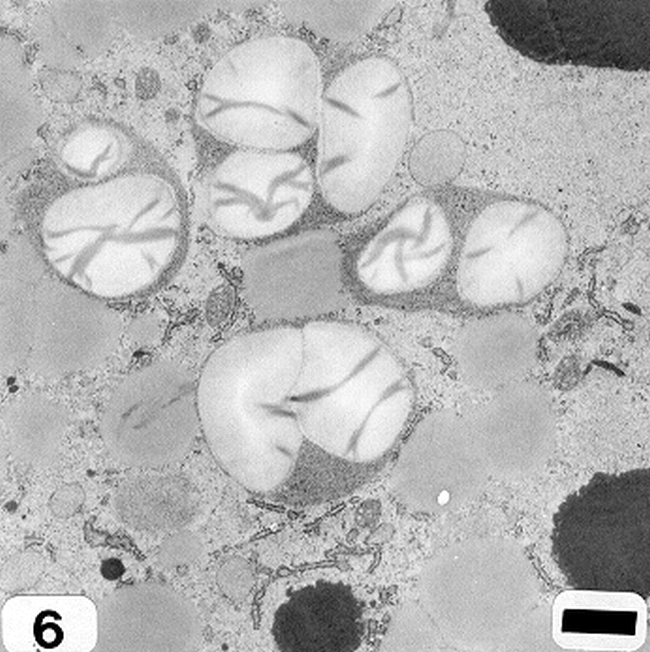
Peanut cotyledonary cell starch granules were observed to originate and grow in amyloplasts (Fig. 1). The amyloplast consists of a double membrane enclosing a ground substance, the stroma, in which the electron translucent starch granule develops. Electron dense material believed to be enzyme complexes were observed within the stroma (Fig. 2). Fully developed as well as developing starch granules were observed during early stages of seed development (Fig. 3). Simple starch granules (single granule per amyloplast) as well as compound starch granules (more than one granule per amyloplast) were noted (Fig. 4). The potential for increase in size of starch granules was observed to result from the deposition of starch material on the surface of pre-existing granules (Fig. 5). The compound granules in which the granuli remained distinct and separate showed no evidence of such aggregation (Fig. 6).
An amyloplast with its double membrane (large arrow) enclosing a ground substance, the stroma (small arrow), in which an electron translucent starch granule(s) has developed. Note the presence of electron dense material believed to be enzyme complexes (pointers) within the stroma of an amyloplast (cotyledonary cell late stage of seed development). Bar = 0.75 μm.
Scanning Electron Microscopy
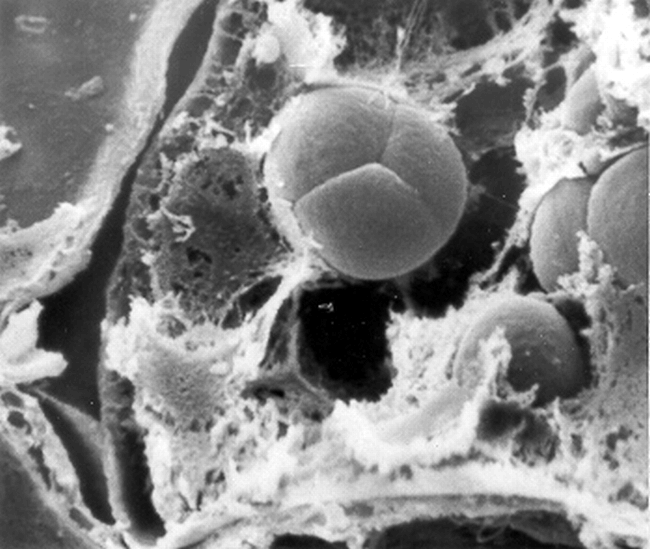
Due to the similar spherical nature of protein bodies and simple starch granules, SEM was capable of distinguishing only the compound starch granules (Fig. 7) in peanut cotyledonary cells.
Discussion
The way in which the structure of starch granules is determined remains one of the least understood aspects of starch synthesis in storage organs such as peanut seed. It is generally accepted that granule growth occurs in a zone at the surface of the granule and that the synthesis in this zone of two different kinds of polymers, namely amylose and amylopectin, is a function of the existence of multiple isoforms of starch synthetase and starch-branching enzyme with different properties and spatial locations (Smith et al., 1995). Furthermore, it is likely that carbon for starch synthesis enters the amyloplast as hexose phosphate and that transporters in the amyloplast envelope probably catalyze a hexose-phosphate/phosphate exchange (Smith et al., 1995). There is strong evidence that ADP glucose pyrophosphorylase is required for starch synthesis and that the activity of this enzyme is plastidial (Okita, 1992).
Electron dense material within the stroma of the developing starch granules in peanut cotyledonary cells was observed and the spatial location of this electron dense material may be ultrastructural evidence of the location of the enzymes involved in the synthesis of starch within the peanut cotyledonary cell amyloplast. Future prospects for progress for understanding the regulation of starch synthesis in peanut cotyledonary cells should include not only the study of enzymes of starch synthesis but also these enzymes' relationship to the chemistry of starch polymers and to the ultrastructure of starch granules.
Acknowledgements
We are grateful to Ms. Valerie M. Knowlton of the North Carolina State University Electron Microscopy Center for assistance with transmission electron microscopy. The research reported in this publication was a cooperative effort of the Agric. Res. Ser. of the U.S. Dept. of Agric. and the NC Agric. Res. Ser., Raleigh, NC 27695-7643. The use of trade names in this publication does not imply endorsement by the United States Department of Agriculture or the North Carolina Agricultural Research Service of the products named, nor criticism of similar ones not mentioned.
Literature Cited
Karnovsky M. J. 1965 A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J. Cell Biol 27: 137A.
Okita T. W. 1992 Is there an alternative pathway for starch synthesis? Plant Physiol 100: 560- 564.
Pattee H. E., Johns E. B., Singleton J. A., and Sanders T. H. 1974 Composition changes in peanut fruit parts during maturation. Peanut Science 1: 57 - 62.
Pattee H. E. and Stalker H. T. 1991 Comparative embryo sac morphology at anthesis of cultivated and wild species of Arachis. Ann. Bot 68: 511 - 517.
Smith A. M., Denyer K., and Martin C. R. 1995 What controls the amount and structure of starch in storage organs? Plant Physiol 107: 673 - 677.
Spurr A. R. 1969 A low-viscosity epoxy resin-embedding medium for electron microscopy. J. Ultrastructure Res 26: 31 - 43.
Vyas D. N., Patel K. C., and Patel R. D. 1967 Carbohydrate contents during germination of growth regulators treated groundnut. Die Starche 19: 410 - 415.
Young C. T. and Schadel W. E. 1989 A method for light and scanning electron microscopy of drought-induced damage of resting peanut seed tissue. Food Microstructure 8: 253 - 256.
Notes
- Professor (deceased), Dept. of Food Science, Box 7624; Res. Chemist, U.S. Dept. of Agric. Res. Serv., Dept. of Botany, Box 7625, Microscopist, Dept. of Food Science, Box 7624, Supv. Plant Physiologist, U.S. Dept. of Agric. Res. Serv., Dept of Food Science, Box 7624, North Carolina State Univ., Raleigh, NC 27695 [^] *Corresponding author Email: harold_pattee@ncsu.edu.
Author Affiliations