Introduction
Moisture can be introduced to peanut products intentionally or unintentionally during processing, transport, or storage. It has been suggested that moisture alters the quality of roasted peanut products (Felland, 1993). High moisture content roasted peanut products contain objectionable soggy nut flavor (Woodroof, 1983). Felland and Koehler (1997) observed that moisture added to peanut butter that was subsequently stored at different temperatures affected the sensory attributes. Young and Heinis (1989) noted that the addition of honey or corn syrup altered peanut butter flavors and viscosity.
Reed et al. (2002) reported high oleic and normal oleic peanuts with a water activity (aw) of 0.19 demonstrated the lowest peanutty flavor loss as compared to those samples stored at 0.60 aw. For both peanut types, the 0.19 aw samples had the highest painty, cardboardy, and bitter attributes during storage.
St. Angelo et al. (1972, 1973) observed that water could act as a proxidant or antioxidant in peanut butter samples when different concentrations of water were added to them after a storage period of 2 mo. Brannan et al. (1999) observed that defatted roasted peanuts, which usually have higher moisture contents, have lower roasted peanutty scores, which decreased during storage as compared to full-fat roasted peanuts. They also observed that defatted peanuts had intense rancid-related attributes, which increased during storage as compared to full-fat roasted peanuts.
Previous studies have shown that factors such as roasting time (Buckholtz et al., 1980), seed size (Pattee et al., 1982), maturity (McNeill and Sanders, 1998), relative humidity (Reed et al., 2002), and storage temperature (Pattee et al., 1999) affect peanut flavor during storage. However, there are no full sensory studies on the combinations of common ingredients such as water, sugar, and antioxidants and their influences on peanut flavor during storage. The objective of this study was to determine the effect of added moisture, sugar, and antioxidant on sensory flavor attributes of a peanut paste during storage, which acted as a model peanut confection system.
Materials and Methods
Sample Preparation and Storage
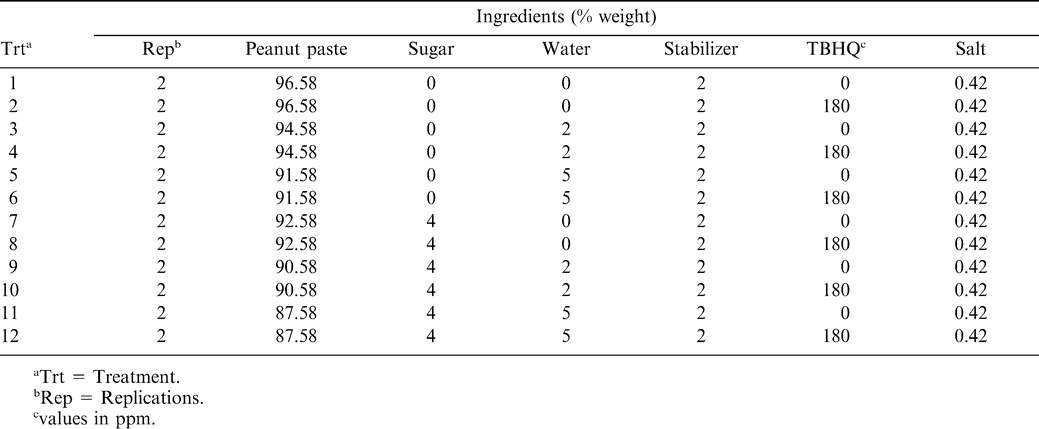
Fresh, untreated, certified, aflatoxin-negative peanut paste was supplied by Seabrook Ingredients (Edenton, NC). Initial analysis of the peanut paste showed it to contain 53.94% oil, 0.86% water, and 0.13% free fatty acid, with a fineness of grind of 35 mils first scrape and 3 mils second scrape, and a coliform MPN <3. Upon receipt, it was placed in storage at −10 C until used. The peanut paste was first mixed using a model SPY-1824 paddle mixer (Marion Mixers, Inc., Marion, IA) to ensure that there was no oil separation before sample preparations. A factorial statistical design was used to specify the levels of peanut paste and ingredients. Twenty-four batches were prepared with three levels of added moisture (0, 2, and 5%), two levels of sugar (0 and 4%), two levels of antioxidant, tertiary butylhydroquinone (TBHQ) (0 and 180 ppm), and two replications (Table 1). Approximately 5.44 kg of peanut paste was heated inside a Sharp microwave oven [model R-510CK (Sharp Electronics Corp., Mahwah, NJ)] to a temperature of 83 C, and then placed in a model KSMC50S KitchenAid mixer equipped with a flat beat paddle (KitchenAid, St. Joseph, MI). A heating mantle maintained the temperature at 83 C. Sucrose (Domino Sugar Co., New Orleans, LA), water, TBHQ (Eastman Chemical, Kingsport, TN) in refined peanut oil (CNT Refinery, Charlotte, NC), Dritex RC hydrogenated vegetable oil stabilizer (AC Humko, Memphis, TN) melted at 83 C, and salt (Cargill, Minneapolis, MN) were added slowly while mixing, for a total mixing time of 2 min. The resulting peanut pastes were poured into a series of small plastic petri dishes (30 mm diam.×10 mm depth), where the samples were allowed to cool and harden. The samples were covered with plastic lids pre-drilled with 1 mm holes to allow air circulation.
Storage and Sampling
Samples were placed in controlled temperature (21 C) and humidity chambers at a relative humidity close to 100 times the aw of the sample. By this approach, we were able to limit the amount of moisture lost or gained by the sample due to moisture transfer with the surroundings. Two replicate samples were randomly pulled for sensory evaluation at 0, 4, 8, 16, 32, and 52 wk of storage.
Water Activity and Moisture Measurement
Water activity of the sample was determined on each sampling day. The water activity meter (Aqua Lab Series 3, Pullman, WA) was standardized at 21 C with the saturated salts 8.5 molal LiCl and 13.3 molal LiCl, with aw values of 0.25 and 0.50, respectively. Moisture was calculated as percent weight of moisture to the original weight of the sample (Ab 2-49 AOCS, 1993). The procedure was modified in that approximately 5.0 g of peanut paste sample was thinly spread on an aluminum pan and dried in a convection oven (Precision Mechanical Oven, Chicago, IL) for 24 hr at 100 C.
Sensory Evaluation
Sensory Panel
Nine panelists, four males and five females from the Dept. of Food Sci. & Technol. at the Univ. of Georgia, were trained in descriptive analysis as described by Meilgaard et al. (1991). The recruitment criteria for the panelists were that they a) be between 18 and 50 yr of age, b) not be allergic to peanut butter, c) consume peanut butter at least once a month, d) were able to pass screening test that consisted of two sections, a taste test and aroma test, and e) be available and willing to participate during training and testing sessions.
Training Sessions
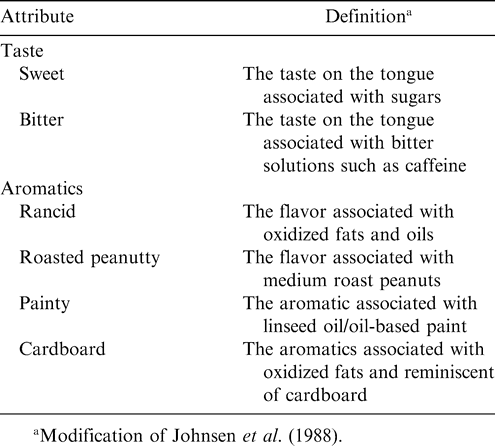
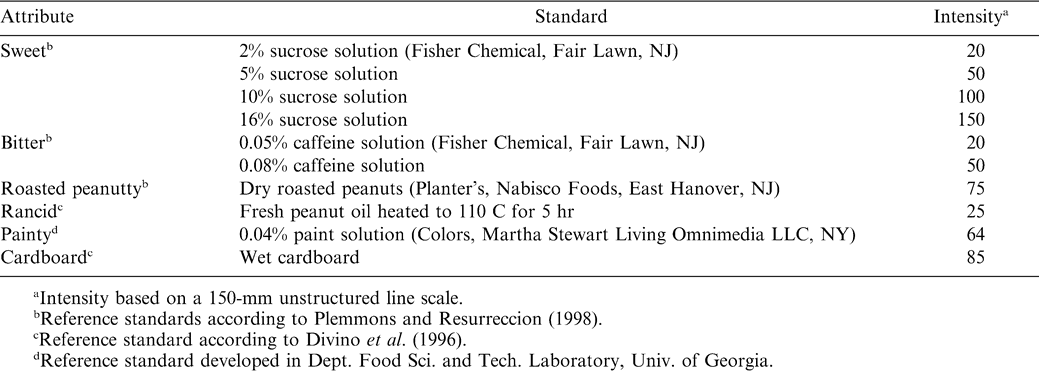
The panelists were trained for seven 90 min sessions. Panelists were presented with fresh and older commercial peanut butter samples in order to acquaint them with different peanut flavor attributes. A lexicon of established peanut flavor descriptors (Johnsen et al., 1988) was used as a guide during terminology development. A final list of six peanut paste sensory attribute definitions was retained after a consensus by panel members (Table 2). Panelists were trained using reference standards corresponding to points on a 150-mm unstructured line scale (Table 3). Calibration of the panel was conducted by first obtaining an average panel rating. Those not rating within 10 points of the average were then asked to re-evaluate the sample and adjust their rating until a consensus was reached. The group, as a whole, was considered to be calibrated if the group's standard deviation was within 10 points of the mean attribute rating (Malundo et al., 1996).
Testing Sessions
Peanut paste samples were removed from storage 1 hr before sensory evaluation and were equilibrated to room temperature. Evaluations were carried out in partition booths illuminated by fluorescent lighting. The panelists rated attributes using 150-mm unstructured line scales. In each booth, the panelists were presented with approximately 5.0 g peanut paste samples in petri dishes that were labeled with three digit random numbers, along with reference standards, water, unsalted crackers, and expectorant cups. The panelists were instructed to evaluate six peanut paste sensory attributes, sweet, bitter, roasted peanutty, rancid, painty, and cardboard. The panelists were told to first familiarize themselves with the reference standard intensities (Table 3), and then place a half-teaspoon of peanut paste in their mouth to evaluate the flavor attributes. They were also instructed to evaluate the samples in the order shown on the score sheets, and to eat unsalted crackers and drink distilled water between samples to clear the palate. Each panelist evaluated twelve peanut paste samples the same day in two separate sessions, with six randomly assigned in the morning and six randomly assigned in the afternoon for each panelist. Each panel session was replicated after 24 hr using the same procedure, but with different random orders of presentation for each panelist.
Statistical Analysis
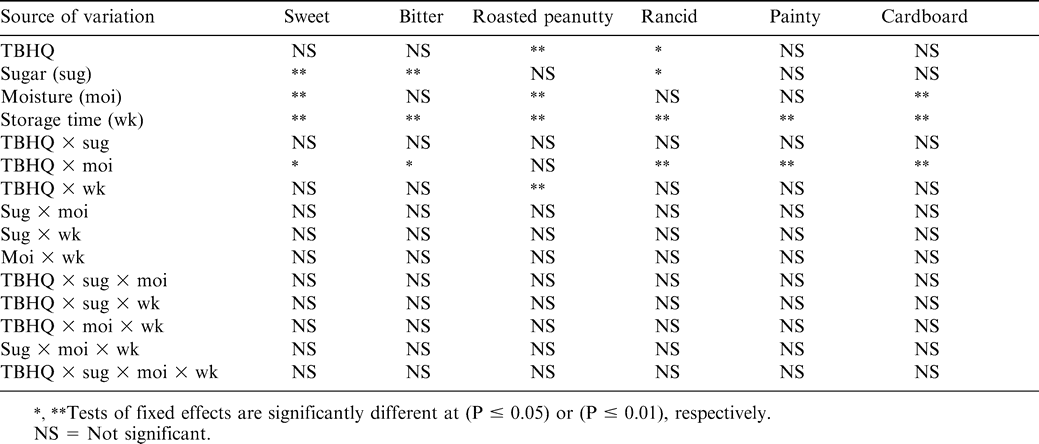
Data were analyzed using PROC MIXED in the SAS statistical software system (SAS Institute, Cary, NC). The sources of variation and corresponding tests of significance for each of the fixed effects in the analysis are shown in Table 4. The LSMEANS statement with the PDIFF option was used for mean separation. The random effects consisted of Reps, session (rep), panelists, panelist × rep, and panelist × session (rep).
Results and Discussion
Sweetness
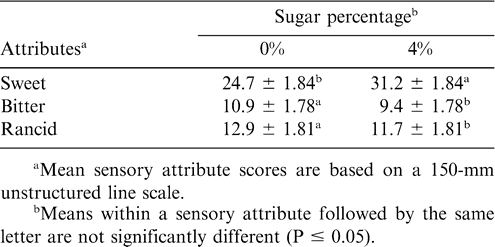
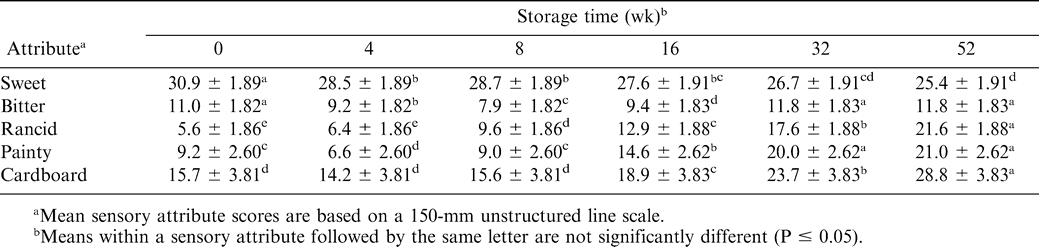
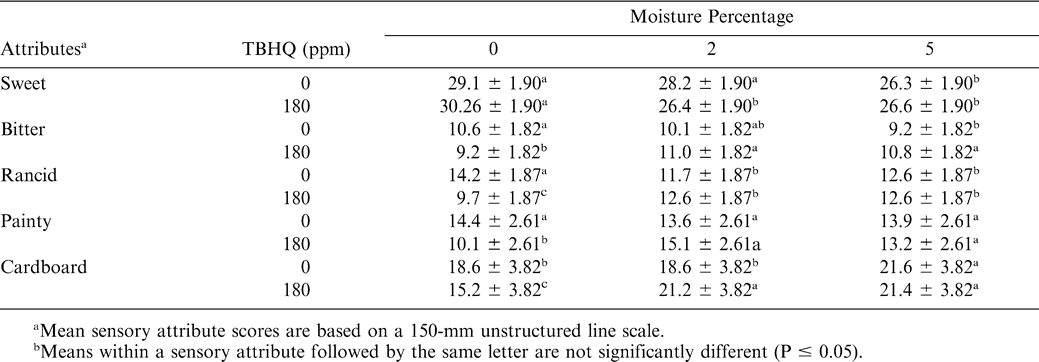
The perception of sweetness was not affected by the presence of antioxidant (Table 4). Obviously, samples with higher sugar levels had higher sweet ratings (Table 5). There was significant TBHQ and moisture interaction (Table 4). Treatments with higher moisture levels had lower perceived sweetness (Table 7). As sucrose is readily soluble in water, the decrease in sweetness with added moisture may be related to the dilution of sugar by the moisture. A rough calculation based on composition suggested that the aqueous phase of the 4% sugar, 0% added moisture samples (0.86 residual moisture) would be saturated with sucrose, with a significant portion of that sucrose remaining undissolved. For the 2 and 5% added moisture treatments, the concentration of sugar in the aqueous phase would be 66 and 44%, respectively, and little if any sugar would remain undissolved. For the sweet attribute, storage time was a significant factor (Table 4). In general, sweet scores decreased with time, although the changes were not large (Table 6). Muego-Gnanasekharan and Resurreccion (1992) found no change in sweet taste during storage of peanut paste samples that were stored at 30, 40, and 50 C for up to 1 yr. Since the treatments were maintained so as to limit moisture changes, the reason for the decrease in sweetness over time is uncertain. It may be that changes in other flavor components mask or decrease the perception of sweetness.
Bitterness
The presence of additional sugar in peanut butter had a significant effect on the perception of bitterness. Those treatments with 4% sugar added were perceived as less bitter (Table 5). The interactions between sweet and bitter flavors are well known (Walters and Roy, 1994). Sweet materials can be used to mask bitter ones to some extent, and sweet and bitter tastes rely on similar stereochemical mechanisms. Previous studies (Pattee et al., 1998) suggested that sweetness could be effective in simultaneously enhancing the roasted peanut attribute and decreasing the bitterness attribute in peanuts. There was significant TBHQ and moisture interaction for bitterness perception (Table 4). Generally, treatments with added moisture had slightly higher bitter scores as compared to treatments with no moisture addition. Bitter score was significantly lower for treatments with an addition of 0% moisture and 180 ppm TBHQ as compared to samples with 0% moisture and no TBHQ addition (Table 7). Generally, bitterness increased with storage time (Table 6). This is probably due to increased protein hydrolysis during storage, which sometimes liberates bitter peptides (Damodaran, 1996). The increase in bitterness may be responsible for the decreased sensation of sweetness.
Roasted Peanutty Flavor
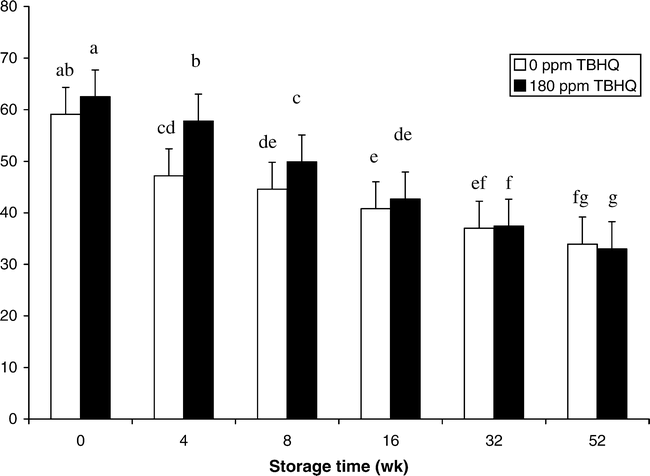
The roasted, browned, and nutty flavor notes of roasted peanuts have been attributed to the alkylpyrazines. These compounds are formed through Maillard and other thermal degradation reactions between amino acids and sugars (Mason et al., 1966; Johnson et al., 1971; Bett and Boylston, 1992). Moisture affected the perception of roasted peanutty flavor. Samples with intermediate and high moisture had the greatest change in roasted peanutty flavor (Fig. 1). Samples with 5% moisture added had a 13.3% decrease in roasted peanutty scores compared to 0% moisture samples. Felland and Koehler (1997) also found that roasted odor and roasted flavor scores decreased with the addition of moisture in peanut butters. There was significant interaction between TBHQ and storage week (Table 4). Treatments with TBHQ had significantly higher roasted peanutty scores on weeks 4 and 8 than those without TBHQ (Fig. 2). For samples both with and without TBHQ, roasted peanutty scores decreased during storage. For treatments with TBHQ, after 52 wk, roasted peanutty scores dropped by 47.2% (62.5 to 33.0%) (Fig. 2). Felland and Koehler (1997) found decreases in roasted odor and roasted flavor of peanut butters during 30 d of storage, while Pattee et al. (1999) observed decreased roasted peanut attribute of peanut paste after it was stored at −10 and −23 C for up to 13 mo. Brannan et al. (1999) observed that for full-fat and reduced-fat peanuts stored at 25 C, roasted peanutty flavor continued to decrease during 12 wk of storage, and Warner et al. (1996) also found that roasted peanut flavor decreased slightly during 68 d of storage at 65 C. Bett and Boylston (1992) suggested that the decrease in roasted peanut flavor during storage may be attributed to the degradation of peanut flavor volatile compounds by either degradation by lipid radicals and peroxides or flavor entrainment by complexes between proteins and lipid hydroperoxides or their secondary products.
Lipid Oxidation-related Flavors
Lipids comprise 52% of the dry weight of peanuts. Over 75% of the fatty acids present in peanuts are unsaturated, with 48% oleic and 31% linoleic acids. Lipid oxidation continues during storage, resulting in increases in the content of aliphatic aldehydes, ketones, alcohols, and other products of lipid oxidation (Bett and Boylston, 1992). In our study, lipid oxidation-related attributes were grouped into rancid, painty, and cardboard. For the rancid attribute, there was a significant sugar effect (Table 4). Treatments with 4 % added sugar had significantly lower rancid scores compared to no sugar addition (Table 5). This could possibly be due to the sensation of sweetness masking or softening the sensation of rancid flavor.
Storage time was significant for rancid, painty, and cardboard attributes (Table 4). Differences in rancid scores developed after 8 wk of storage, and after 16 wk for painty and cardboard attributes (Table 6). Samples with TBHQ still developed rancid, painty, and cardboard flavors over time.
There was significant TBHQ and moisture interaction for rancid, painty, and cardboard attributes (Table 4). Generally, intermediate and high moisture treatments had higher rancid, painty, and cardboard scores (Table 7). It was also observed that rancid, painty, and cardboard scores were significantly higher for treatments with 0% moisture addition and 0 ppm TBHQ compared to the 0% moisture and 180 ppm TBHQ treatments. In a study of peroxidation of peanut butter samples stored for 2 mo, St. Angelo and Ory (1973) observed that when water was added at 4.8% (w/w) it had an apparent antioxidant effect, while at 1.2% (w/w) the rate of peroxidation did not differ from the control sample. However, when water was added at 0.6% (w/w), it behaved as a proxidant. TBHQ might have slowed lipid oxidation in 0% moisture added treatments. Although not strictly dealing with oxidation, Felland and Koehler (1997) found off-odors and flavors were higher in peanut butter samples containing 5% moisture. However, they also measured very low TBA values, and concluded that off-flavor was probably not due to oxidation alone. As roasted peanut flavor was found to decrease with moisture, this may have left heightened sense of off-flavor and aromas.
Summary and Conclusions
Roasted peanutty attribute decreased over storage time. Oxidation-related attributes, such as rancid, painty, and cardboard, intensified over storage time. Sweet and roasted peanutty attributes in intermediate and high moisture samples were scored low. Bitter, rancid, painty, and cardboard attributes were rated higher in intermediate and high moisture samples, possibly due to roasted peanutty flavor loss resulting in off-flavor. Treatments with added TBHQ resulted in higher roasted peanutty scores. In low moisture treatments, addition of TBHQ resulted in lower rancid, painty, and cardboard scores. Addition of 4% sugar resulted in higher sweet scores, as well as lower bitter and rancid scores. Significant two-way interactions were observed for all attributes. The level of moisture, sugar, and antioxidant that was considered in this study is relevant to the confection industry, which uses peanut products. The moisture levels considered could be picked up intentionally or unintentionally during processing and storage, and could interact with the sugar and antioxidant present, thus resulting in flavor changes in peanut confections during storage.
Acknowledgements
This study was supported in part by the Pennsylvania Manufacturing Confectioners' Assoc. The authors express their appreciation to Doug Manning and Rick Boyce for their advice and support. Sensory panel members were instrumental in making the sensory data possible.
Literature Cited
Amer. Oil Chem. Soc 1993 Official Method of American Oil Chemists' Society. 4th Ed Champaign, IL .
Bett K. L. and Boylston T. D. 1992 Effect of storage of roasted peanut quality – Descriptive sensory analysis and gas-chromatographic techniques. Amer. Chem. Soc. Symposium Series 500 : 322 – 343 .
Brannan G. L. , Koehler P. E. , and Ware G. O. 1999 Physico-chemical and sensory characteristics of defatted roasted peanuts during storage. Peanut Sci 26 : 44 – 53 .
Buckholtz L. L. , Daun H. , Stier E. , and Trout R. 1980 Influence of roasting time on sensory attributes of fresh roasted peanuts. J. Food Sci 45 : 547 – 554 .
Damodaran S. 1996 Amino acids, peptides, and proteins. In Fennema O. R. ed. Food Chemistry Marcel Dekker, Inc New York 321- 430.
Divino G. L. , Koehler P. E. , and Akoh C. C. 1996 Enzymatic and autoxidation of defatted peanuts. J. Food Sci 61 : 112 – 115 .
Felland S. L. 1993 Sensory, chemical, and physical changes in a higher water activity peanut butter product M.S. thesis. Univ. of GeorgiaAthens, GA.
Felland S. L. and Koehler P. E. 1997 Sensory, chemical, and physical changes in increased water activity peanut butter products. J. Food Quality 20 : 145 – 156 .
Johnsen P. B. , Civille G. V. , Vercellotti J. R. , Sanders T. H. , and Dus C. A. 1988 Development of a lexicon for the description of peanut flavor. J. Sensory Stud 3 : 9 – 17 .
Johnson B. R. , Waller G. R. , and Burlingane A. L. 1971 Volatile components of roasted peanuts: Basic fraction. J. Agric. Food Chem 19 : 1020 – 1024 .
Malundo T. M. M. and Resurreccion A. V. A. 1993 Optimization of liquid whitener from peanut extract. Lebensm.-Wiss. U-Technol 26 : 552 – 557 .
Mason M. E. , Johnson B. R. , and Hamming M. C. 1966 Flavor components of roasted peanuts. J. Agric. Food Chem 14 : 454 – 460 .
McNeill K. L. and Sanders T. H. 1998 Maturity effects on sensory and storage quality of roasted virginia-type peanuts. J. Food Sci 63 : 366 – 369 .
Meilgaard M. , Civille G. V. , and Carr T. 1991 Sensory Evaluation Techniques. 2nd Ed CRS Press, Inc Boca Raton, FL .
Muego-Gnanasekharan K. F. and Resurreccion A. V. A. 1992 Physicochemical and sensory characteristics of peanut paste stored at different temperatures. J. Food Sci 57 : 1385 – 1389.
Pattee H. E. , Giesbrecht F. G. , and Isleib T. G. 1999 Sensory attribute variation in low-temperature stored roasted peanut paste. J. Agric. Food Chem 47 : 2415 – 2420 .
Pattee H. E. , Isleib T. G. , and Giesbrecht F. G. 1998 Variation in intensity of sweet and bitter sensory attributes across peanut genotypes. Peanut Sci 25 : 63 – 69 .
Pattee H. E. , Pearson J. L. , Young C. T. , and Giesbrecht F. G. 1982 Changes in roasted peanut flavor and other quality factors with seed size and storage time. J. Food Sci 47 : 455 – 456 460 .
Plemmons L. E. and Resurreccion A. V. A. 1998 A warm-up sample improves the reliability of responses in descriptive analysis. J. Sensory Stud 13 : 359 – 376 .
Reed K. A. , Sims C. A. , Gorbet D. W. , and O'Keefe S. F. 2002 Storage water activity affects flavor fade in high and normal oleic peanuts. Food Res. Intl 35 : 769 – 774 .
SAS 1997 SAS/STAT®: Changes and enhancements through Release 6.12. SAS Inst. Inc Cary, NC.
Angelo St A. J. and Ory R. L. 1973 Investigation of the causes and prevention of fatty acid peroxidation in peanut butter. J. Amer. Peanut Res. Educ. Assoc 5 : 128 – 133 .
Angelo St A. J. , Ory R. L. , and Brown L. E. 1972 A comparison of minor constituents in peanut butter as possible source of fatty acid oxidation. J. Amer. Peanut Res. Educ. Assoc 4 : 186 – 194 .
Walters D. E. and Roy G. 1996 Taste interactions of sweet and bitter compounds. Flavor-Food Interactions, Amer. Chem. Soc. Symp. Ser 633 : 130 – 142 .
Warner K. J. H. , Dimick P. S. , Ziegler G. R. , Mumma R. O. , and Hollender R. 1996 ‘Flavor-fade’ and off-flavors in ground roasted peanuts as related to selected pyrazines and aldehydes. J. Food Sci 61 : 469 – 472 .
Woodroof J. G. 1983 Peanuts: Production, Processing, Products The AVI Publishing Co Westport, CT .
Young C. T. and Heinis J. J. 1989 Manufactured peanut products and confections. In Lusas E. W. Erickson D. R. , and Nip W. K. eds. Food Uses of Whole Oil and Protein Seeds Amer. Oil Chem. Soc Champaign, IL 177- 190.
Notes
- 1 Assistant Manager, Scientific Regulatory Affairs, Ajinomoto Corporate Services LLC, 1120 Connecticut Ave., N.W., Washington, D.C. 20036. [^]
- 2 Associate Professor and 3Professor, Department of Food Science Technology, University of Georgia, Athens, GA 30602-7610. [^] *Corresponding author (email: wlkerr@uga.edu).
Author Affiliations


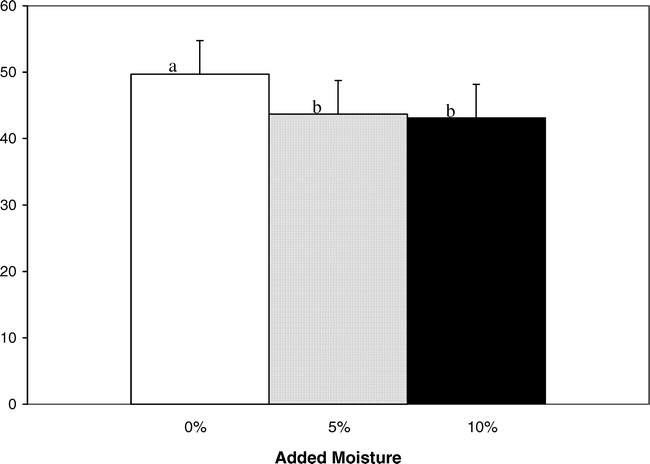
 2%, and ▪5% added moisture.Means with the same letter are not significantly different (P ≤ 0.05). Mean sensory attribute scores are based on a 150-mm unstructured line scale.
2%, and ▪5% added moisture.Means with the same letter are not significantly different (P ≤ 0.05). Mean sensory attribute scores are based on a 150-mm unstructured line scale.