Introduction
Peanut growers throughout the Southeastern Coastal Plain have traditionally utilized conventional tillage systems but are increasing conservation tillage production systems. Growers practicing conservation tillage (including minimum, strip, and no-till) plant peanut directly into residue left by winter cover crops or winter native vegetation. The resulting plant residue left on the surface of the soil protects the soil structure, and aids in reducing erosion in the sandy soils of the Southeastern Coastal Plain (Campbell et al., 2002; Sholar et al., 1995). Additionally, conservation tillage has been credited with better water conservation, increased stand establishment, decreased insect populations, changes in weed densities, and fewer diseases (Campbell et al., 2002; Durham, 2003). Furthermore, Johnson et al., (2001) documented lower incidences of tomato spotted wilt in reduced and minimum tillage peanut systems, which is significant since there is no other control method for this disease. Reduced tillage systems may increase herbicide inputs into a system due to the need for burndown herbicides, but the overall aim is to reduce the overall inputs (time, fertilizer, etc.) into the system.
Reports differ on overall peanut yields in conventional versus conservation peanut production. Wilcut et al., (1987) reported that strip tillage peanut tended to yield more than conventionally grown peanut due to the reduction in weed pressure; however others found that peanut grown in a conventional tillage system yielded better and had a higher gross economic value (Jordan et al., 2001, 2002). Other research also found that minimum tillage systems did not negatively affect peanut quality and yield (Clewis et al., 2002; Colvin and Brecke, 1988; Johnson et al., 2001), which may be due to the fact that peanut planted in raised beds are much easier to dig than peanut planted flat (no raised beds) into stubble (as in conservation tillage). Research in North Carolina on large-seeded Virginia-Carolina (VC) market type peanut indicates that yields are inconsistent under strip tillage conditions because ease of digging depends upon soil type and texture (Jordan et al., 2002). Strip tillage systems tend to cause a weed population shift to small-seeded grasses and broadleaf weeds such as green foxtail [Setaria viridis (L.) Beauv.], Texas panicum (Panicum texanum Buckl.), common lambsquarters, and redroot pigweed (Amaranthus retroflexus L.), which increases the need for research into comprehensive, season-long herbicide programs (Johnson et al., 2002).
Flumioxazin (formerly S-53482 and V-53482), is a soil-applied herbicide used PRE for broadleaf weed control in peanut and soybean [Glycine max (L.) Merr] (Askew et al., 1999; Eyherabide, 1996; Price et al., 2004; Wilcut et al., 2001). The herbicide provides residual control of several problematic broadleaf weeds including Florida beggarweed [Desmodium tortuosum (Sw) DC], common ragweed (Ambrosia artemisiifolia L.), and eclipta (Eclipta prostrata L.) as well as common lambsquarters, and prickly sida (Sida spinosa L.) (Askew et al., 1999; Burke et al., 2002a; Clewis et al., 2002; Eyherabide, 1996). In North Carolina, Askew et al. (1999), found that flumioxazin applied PRE provided better control of common lambsquarters, morningglory and prickly sida than norflurazon and also provided better weed control, and resulted in higher crop yield and net returns than a more traditional system of metolachlor PPI fb EPOST or POST herbicides (Scott et al., 2001). Flumioxazin has also been found to provide ≥ 80% control of common ragweed, and ivyleaf morningglory (Ipomoea hederacea L. Jacq.) in soybean (Niekamp and Johnson, 2001). Conversely, flumioxazin alone does not effectively control nutsedges (Cyperus sp.) (Clewis et al., 2002), or annual grasses (Grichar and Colburn, 1996).
The objective of this research was to evaluate weed control, crop injury, and peanut yield when flumioxazin was applied in combination with PRE and POST herbicides commonly used in strip tillage peanut production.
Materials and Methods
Field experiments were conducted in three locations at the Peanut Belt Research Station located near Lewiston-Woodville, NC in 1999 and 2000 to evaluate weed management systems in strip-tillage peanut. Peanut cultivars ‘NC 10 C’ in 1999 and ‘NC 12 C’ in 2000 were planted 8 cm deep in rows spaced 91 cm wide. Peanuts were planted at 120 to130 kg/ha in early May of both years. Plots measured 6.1 m long and 3.6 m wide, and were arranged in a randomized complete block design with three replications of treatments. Lewiston-Woodville soils are classified as Norfolk sandy loams (fine-loamy, siliceous, thermic Typic Kandiudults) with 1.0% organic matter and pH 5.9 to 6.1.
Paraquat at 0.7 kg ai/ha was applied to all plots 3 wk before planting to control existing vegetation. The PRE herbicide options included: (1) paraquat at 0.7 kg/ha plus dimethenamid at 1.4 kg ai/ha, (2) paraquat at 0.7 kg/ha plus dimethenamid at 1.4 kg/ha plus flumioxazin at 71 g ai/ha or (3) paraquat at 0.7 kg/ha plus flumioxazin at 71 g/ha. At the time of PRE treatment, broadleaf weeds were in the cotyledon to three-leaf stage, and weed densities ranged from 2 to 50 per m2 depending on species (data not shown). For those plots that received an EPOST herbicide treatment, paraquat at 145 g/ha plus bentazon at 0.28 kg ai/ha was applied approximately 15 days after planting. Weed stage and density at time of EPOST was cotyledon to 5-leaf growth stage at densities from 10 to 20 plants per species (data not shown). POST treatments were applied two weeks after the EPOST treatments. At the time of POST treatments, broadleaf weeds were in the cotyledon to seven-leaf stage, and weed densities ranged from 2 to 30 per m2 depending on species (data not shown). POST herbicide options included: (1) a prepackaged mixture3 of bentazon at 0.56 kg/ha and acifluorfen at 0.28 kg ai/ha or (2) imazapic at 71 g ai/ha. A nontreated check was included for comparative purposes.
A nonionic surfactant4 at 0.25% (v/v) was included in all PRE, EPOST, and POST herbicide treatments. Herbicides were applied with a CO2 powered backpack sprayer calibrated to deliver 140 L/ha at 146 kPa. Weeds evaluated at either two or three sites included common lambsquarters (Chenopodium album L.), eclipta, goosegrass [Eleusine indica (L.) Gaertn], ivyleaf morningglory, large crabgrass [Digitaria sanguinalis (L.) Scop.], prickly sida, pitted morningglory (Ipomoea lacunosa L.) and yellow nutsedge (Cyperus escultenus L.). A late POST treatment of clethodim at 0.28 kg ai/ha plus 1.0% COC (crop oil concentrate) was applied in late July to all tests to control escaped goosegrass and large crabgrass.
Peanut injury ratings, based on visual estimations of discoloration, stunting and stand reduction, were made 4 and 12 weeks after planting (WAP). Visual estimates of weed control were recorded early (mid-June) and late season (late August) approximately one month prior to harvest. Weed control and peanut injury ratings were based upon visual estimations of leaf discoloration and biomass reduction when compared to the nontreated control. Ratings are based on a scale of 0 (no injury symptoms) to 100 (complete death of all plants or no plants present) (Frans et al., 1986). The crop was dug and inverted and left to air dry in the field for approximately 2 weeks before being combined. Peanuts were harvested from the center two rows of each plot using a combine modified for small plot research in early November of each year. Data were subjected to ANOVA using the general linear models procedure in SAS (SAS, 2001). PRE and POST herbicide treatment options were analyzed in terms of crop injury, weed control, and crop yield. Data were tested for homogeneity of variance by plotting residuals. The nontreated control was not included in the analyses of visually estimated data. Error was partitioned to evaluate location and associated error terms. Arcsine transformations did not improve variance homogeneity, so nontransformed data were used in analyses and presentation. Mean separations were performed using Fisher's protected LSD test at p≤0.05.
Results and Discussion
Because there were only minor differences in weed control between the two ratings, and weed pressure late in the season is more influential on peanut yield and harvesting efficiency, only the late season ratings are presented
Weed Control
Data were pooled over location and years for those species for which no treatment by location interaction was found, but weed responses to herbicides are discussed separately by location/year in cases where a treatment by location interaction occurred.
Common Lambsquarters
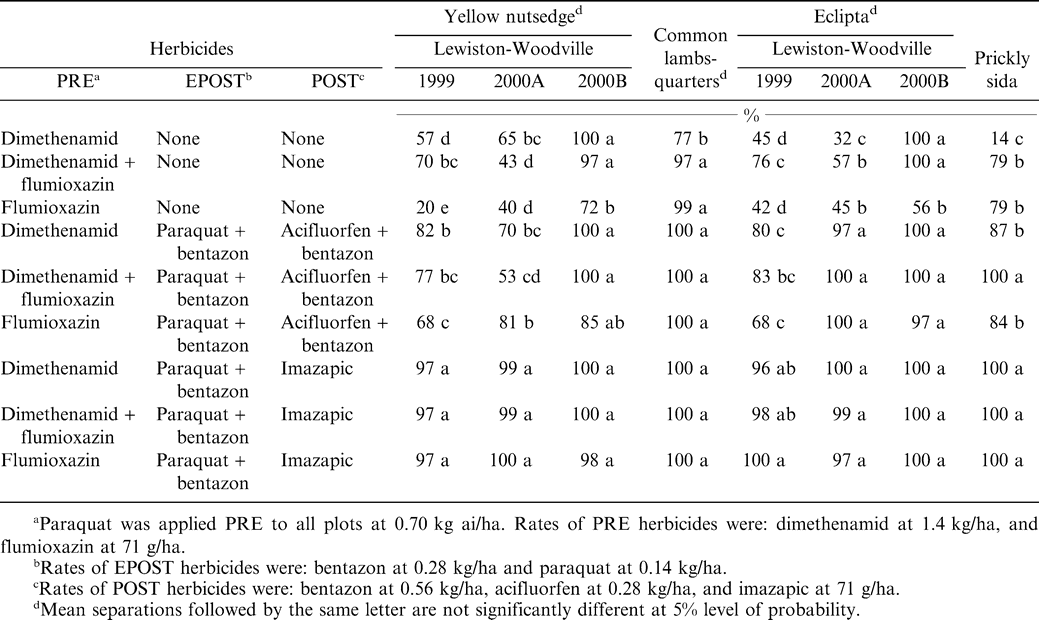
There was no location by treatment interaction for this weed species; therefore the data were combined over locations and years (Table 1). Dimethenamid PRE controlled common lambsquarters 77%, whereas all other PRE, EPOST, and POST treatment combinations controlled common lambsquarters ≥ 97%. Similar results have been seen with these herbicides in conventional tillage peanuts in previous research (Burke et al., 2002a; Clewis et al., 2002; Scott et al., 2001; Wilcut, 1991a, 1991b;Wilcut et al., 1994, 1995).
Ivyleaf, Entireleaf, and Pitted Morningglories
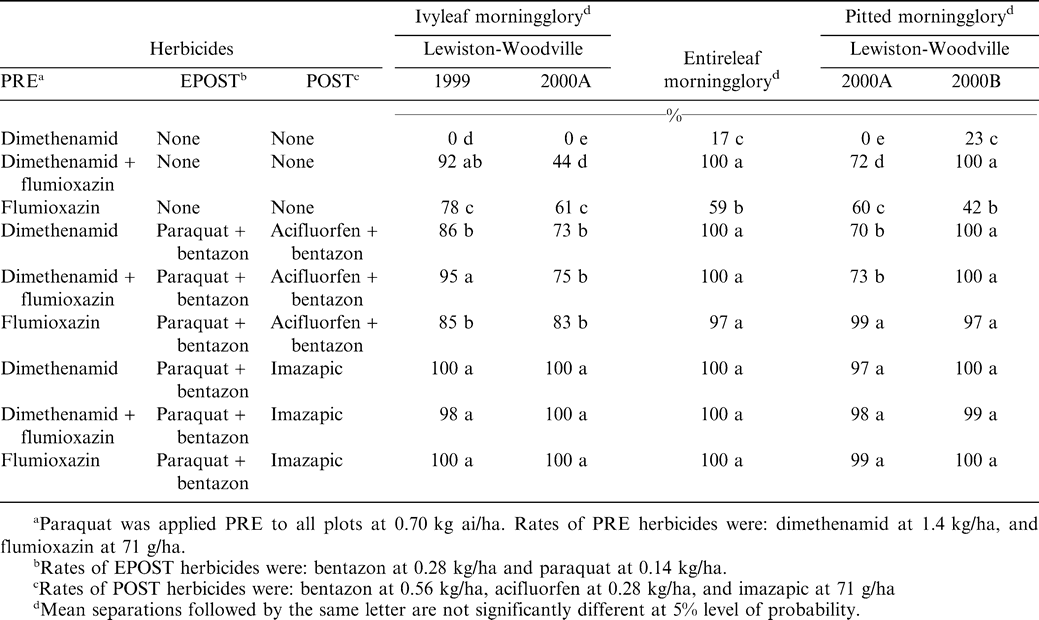
There were treatment by location interactions for control of ivyleaf and pitted morningglories; therefore the data are discussed separately. Entireleaf morningglory (Ipomoea hederacea var. integriuscula Gray) was only found in one location, however control responses were similar to that of pitted morningglory in Lewiston-Woodville 2000B (Table 2). Dimethenamid PRE provided little (< 23%) control of ivyleaf, entireleaf, and pitted morningglory. Flumioxazin PRE and dimethenamid fb flumioxazin PRE provided between 44 and 100% control of all three species. The additional inputs of the EPOST treatments and acifluorfen plus bentazon POST to dimethenamid plus flumioxazin programs controlled greater than 73% of the morningglories, whereas the imazapic POST system controlled greater than 97% of the morningglories at all locations. The residual control properties of imazapic may have contributed to these higher levels of control (Richburg et al., 1995, 1996). Similar results with imazapic (AC 263,222) control of Ipomoea morningglories have been previously reported (Richburg et al., 1995, 1996; Wilcut et al., 1996).
Eclipta
There was a location by herbicide interaction; therefore each location is discussed separately. Dimethenamid or flumioxazin PRE or in PRE tank mixture controlled eclipta variably (Table 1). The addition of EPOST herbicides fb acifluorfen plus bentazon POST controlled eclipta 68 to 100%. Imazapic POST systems, regardless of PRE treatments, controlled eclipta 96 to 100%.
Goosegrass and Large Crabgrass
No treatment by location interactions were present for goosegrass or large crabgrass; therefore the data were combined over locations (data not shown). Dimethenamid PRE controlled goosegrass 95% and large crabgrass 97% initially. However a late application of clethodim was needed for season-long control and to facilitate harvest (≥99% control for both weed species at all locations). Clethodim controls goosegrass and large crabgrass (Burke et al., 2002b, 2004).
Prickly Sida
No treatment by location interactions were seen for prickly sida control; therefore the data were combined over locations (Table 1). Dimethenamid PRE controlled prickly sida 14%. The addition of flumioxazin PRE improved control to 79%. Regardless of PRE herbicide inputs, acifluorfen plus bentazon POST systems controlled prickly sida 84 to 100%. Both imazapic systems controlled prickly sida 100%, regardless of PRE herbicide treatment. Other researchers have also reported excellent control of prickly sida with imazapic (Richburg et al., 1995, 1996; Wilcut et al., 1996).
Yellow Nutsedge
There were treatment by location interactions for yellow nutsedge control, thus each location will be discussed separately. Control by most herbicides was greater in Lewiston-Woodville 2000B than in the other locations. Yellow nutsedge populations at Lewiston 2000B were approximately 10 to 15 plant/m2 while at other locations they ranged from 30 to 50 plants/m2 (data not shown). Dimethenamid PRE alone controlled yellow nutsedge by 57 to 100% depending on location (Table 1). Flumioxazin PRE alone control of yellow nutsedge varied from 20 to 72% depending on location. Flumioxazin plus dimethenamid PRE controlled yellow nutsedge by 43 to 97%, and was not different than the control of dimethenamid PRE. The addition of paraquat plus bentazon EPOST fb aciflourfen plus bentazon POST provided 70 to 100% control depending on location. Paraquat and bentazon EPOST fb imazapic POST controlled yellow nutsedge ≥97% in all locations, regardless of PRE herbicide treatment. These data also show that imazapic POST is an excellent treatment for control of yellow nutsedge in peanut, which agrees with previous research findings (Richburg et al., 1994, 1995, 1996; Wilcut et al., 1994, 1995, 1996).
Peanut Injury
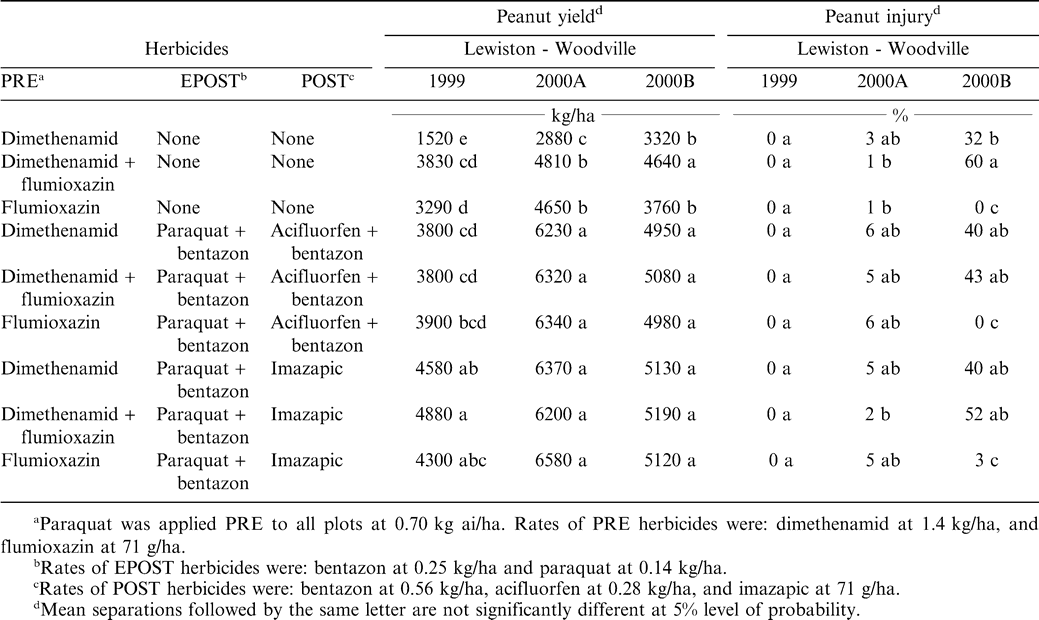
There was a treatment by location interaction for peanut injury (P < 0.05), therefore data are discussed by location. Overall peanut injury was based upon stand reduction due to stunting, leaf discoloration, and spots of necrosis (data not shown). Injury was very erratic, and in 1999 there was no visible injury (Table 3). However, there was significant injury (between 32 and 60% at Lewiston 2000B with treatments that included dimethenamid PRE and in tank mixtures with flumioxazin PRE, regardless of EPOST and POST herbicide treatments. These levels of injury are not typical of dimethenamid PRE and we have no explanation. Other than these exceptions with dimethenamid PRE alone or in tank mixture with flumioxazin, injury levels are consistent with previous research (Wilcut et al., 1994, 1995; Richburg et al., 195, 1996).
Peanut Yield
Location interactions were significant: therefore, each location is discussed separately. Peanut treated with dimethenamid PRE yielded 1,520 to 3,320 kg/ha, depending on location (Table 3). The addition of flumioxazin PRE, or EPOST and POST herbicides always increased yields. The increased yields reflect the increased levels of weed control provided by the additional herbicide inputs (Tables 1 and 2). Dimethenamid or flumioxazin PRE or in PRE tank mixture plus EPOST and POST herbicide treatments yielded between 4,980 and 6,580 kg/ha at both Lewiston locations in 2000. There was no significant correlation between peanut injury and peanut yield. Other research (Main et al., 2003) showed that flumioxazin did not directly result in yield differences between three runner-type cultivars. These data show that weeds in strip-tillage peanut can be controlled with appropriate selection and timely application of PRE, EPOST, and POST herbicide treatments.
Acknowledgements
The authors wish to thank Dr. Cavell Brownie, professor of Statistics, North Carolina State University for statistical assistance. Appreciation is also extended to the station personnel at the Peanut Belt Research Station for their assistance in this research and to the North Carolina Peanut Grower's Association and Valent USA for partial funding of this research.
Literature Cited
Askew S. D. , Wilcut J. W. , and Cranmer J. R. 1999 Weed management in peanut (Arachis hypogaea) with flumioxazin preemergence. Weed Technol 13 : 594 – 598 .
Burke I. C. , Askew S. D. , and Wilcut J. W. 2002a Flumioxazin systems for weed management in North Carolina peanut (Arachis hypogaea). Weed Technol 16 : 743 – 748 .
Burke I. C. , Wilcut J. W. , and Porterfield D. 2002b CGA-362622 anatogonizes annual grass control with clethodim. Weed Technol 16 : 749 – 754 .
Burke I. C. , Price A. J. , Wilcut J. W. , Jordan D. L. , Culpepper A. S. , and Tredaway-Ducar J. 2004 Annual grass control in peanut (Arachis hypogaea) with clethodim and imazapic. Weed Technol 18 : 88 – 92 .
Campbell H. L. , Weeks J. R. , Hagan A. K. , and Gamble B. 2002 Impact of strip-till planting using various cover crops on insect pests and diseases of peanuts. In van Santen E. ed. Making Conservation Tillage Conventional: Building a Future on 25 Years of Research Alabama Agric. Expt. Stn. and Auburn University Proc. of 25th Annual Southern Conservation Tillage Conference for Sustainable Agriculture. Auburn, AL 24–26 June 2002. Special Report No. 1. AL 36849, USA 161 – 164 .
Clewis S. B. , Askew S. D. , and Wilcut J. W. 2002 Economic assessment of diclosulam and flumioxazin in strip- and conventional-tillage peanut. Weed Sci 50 : 378 – 385 .
Colvin D. L. and Brecke B. J. 1988 Peanut cultivar response to tillage systems. Peanut Sci 15 : 21 – 24 .
Durham S. 2003 Drought survival with conservation tillage. Agric. Res 51 : 22 .
Eyherabide J. J. 1996 Evaluation of flumioxazin sprayed alone and in mixtures with other pre-emergent herbicides for weed control in soybeans. Ann. Appl. Biol 128 : 62 – 63 .
Frans R. , Talbert R. , Marx D. , and Crowley H. 1986 Experimental design and techniques for measuring and analyzing plant response to weed control practices. In Camper N. D. ed. Research Methods in Weed Science 3rd Ed Southern Weed Science Society Champaign, IL 37 – 38 .
Grichar W. J. and Colburn A. E. 1996 Flumioxazin for weed control in Texas peanuts (Arachis hypogaea L.). Peanut Sci 23 : 30 – 36 .
Johnson W. C. , Brenneman T. B. , Baker S. H. , Johnson A. W. , Sumner D. R. , and Mullinix B. G. 2001 Tillage and pest management considerations in a peanut-cotton rotation in the southeastern coastal plain. Agron. J 93 : 570 – 576 .
Johnson W. C. , Prostko E. P. , and Grey T. L. 2002 Annoying trends in strip-tillage weed control in peanut: what are our options? In van Santen E. ed. Making Conservation Tillage Conventional: Building a Future on 25 Years of Research Proc. of 25th Annual Southern Conservation Tillage Conference for Sustainable Agriculture. Auburn, AL 24–26 June 2002. Special Report No. 1. Alabama Agric. Expt. Stn. and Auburn University AL 36849, USA 165 – 167 .
Jordan D. L. , Barnes J. S. , Bogle C. R. , Naderman G. C. , Roberson G. T. , and Johnson P. D. 2001 Peanut response to tillage and fertilization. Agron. J 93 : 1125 – 1130 .
Jordan D. L. , Johnson P. D. , Culpepper A. S. , Barnes J. S. , Bogle C. R. , Naderman G. C. , Roberson G. T. , Bailey J. E. , and Brandenburg R. L. 2002 Research in North Carolina with reduced tillage systems for peanut (1997–2001). In van Santen E. ed. Making Conservation Tillage Conventional: Building a Future on 25 Years of Research Proc. of 25th Annual Southern Conservation Tillage Conference for Sustainable Agriculture. Auburn, AL 24–26 June 2002. Special Report No. 1. Alabama Agric. Expt. Stn. and Auburn University AL 36849, USA 336 – 340 .
Main C. L. , Tredway Ducar J. , Whitty E. B. , and MacDonald G. G. 2003 Response of three runner-type peanut cultivars to flumioxazin. Weed. Technol 17 : 89 – 93 .
Niekamp J. W. and Johnson W. G. 2001 Weed management with sulfentrazone and flumioxazin in no-tillage soyabean (Glycine max). Crop Prot 20 : 215 – 220 .
Price A. J. , W Wilcut J. , and Cranmer J. R. 2004 Physiological behavior of root-absorbed flumioxazin in peanut, ivyleaf morningglory, and sicklepod. Weed Sci 52 : 718 – 724 .
Richburg J. S. , Wilcut J. W. , and Wehtje G. R. 1994 Toxicity of AC 263,222 to purple (Cyperus rotundus) and yellow nutsedge (C. esculentus). Weed Sci 42 : 398 – 402 .
Richburg J. S. , Wilcut J. W. , and Wiley G. L. 1995 AC 263,222 and imazethapyr rates and mixtures for weed management in peanut (Arachis hypogae). Weed Technol 9 : 801 – 806 .
Richburg J. S. , Wilcut J. W. , Colvin D. L. , and Wiley G. R. 1996 Weed management in southeastern peanut (Arachis hypogaea) with AC 263,222. Weed Technol 10 : 145 – 152 .
[SAS] Statistical Analysis Systems 2001 SAS/STAT User's Guide. Release 8.2 Statistical Analysis Systems Institute Cary, NC 1028 .
Scott G. H. , Askew S. D. , and Wilcut J. W. 2001 Economic evaluation of diclosulam and flumioxazin systems in peanut (Arachis hypogaea). Weed Technol 15 : 360 – 364 .
Sholar J. R. , Mozingo R. W. , and Beasly J. P. 1995 Peanut cultural practices. In Pattee H. E. and Stalker H. T. eds Advances in Peanut Science Am. Peanut Res. and Educ. Soc Stillwater, OK 354 – 382 .
Wilcut J. W. , Richburg J. S. , Wiley G. L. , and Walls F. R. 1996 Postemergence AC 263,222 systems for weed control in peanut (Arachis hypogaea). Weed Sci 44 : 615 – 621 .
Wilcut J. W. , Askew S. D. , Bailey W. A. , Spears J. F. , and Isleib T. G. 2001 Virginia market-type peanut (Arachis hypogaea) cultivar tolerance and yield response to flumioxazin preemergence. Weed Technol 15 : 137 – 140 .
Wilcut J. W. , Wehtje G. R. , Colvin D. L. , and Patterson M. G. 1987 Economic assessment of herbicide systems for minimum-tillage peanuts. Peanut Sci 14 : 2, 83 – 86 .
Wilcut J. W. 1991a Economic yield response of peanut (Arachis hypogaea) to postemergence herbicides. Weed Technol 5 : 416 – 420 .
Wilcut J. W. 1991b Tropic croton (Croton glandulosus) control in peanut (Arachis hypogaea). Weed Technol 5 : 795 – 798 .
Wilcut J. W. , York A. C. , and Wehtje G. R. 1994 The control and interaction of weeds in peanut (Arachis hypogaea). Rev. Weed Sci 6 : 177 – 205 .
Wilcut J. W. , York A. C. , Grichar W. J. , and Wehjte G. R. 1995 The biology and management of weeds in peanut (Arachis hypogaea). in Pattee H. E. and Stalker H. T. eds. Peanut Science and Technology American Peanut Research and Education Society Stillwater, OK 74078 207 – 244 .
Notes
- Received for publication Date and in revised form Date. [^]
- Storm®, 29% bentazon, and 13.4% Acifluorfen. BASF Corporation. Agricultural Products Group, PO Box 13528, Research Triangle Park, NC 27709. [^]
- Induce nonionic low foam wetter-spreader adjuvant containing 90% non-ionic surfactant (alkylarylopolyoxyalkane ether and isopropanol), free fatty acids, and 10% water. Helena Chemical Company, Suite 500, 6075 Poplar Avenue, Memphis, TN 38137. [^]
- 2Graduate Research Assistant, Graduate Research Assistant, and Professor, respectively, Crop Science Department, North Carolina State University, Raleigh, NC 27695-7620. Corresponding author's E-mail: john_wilcut@ncsu.edu.
Author Affiliations