Introduction
Cultivated peanut (Arachis hypogaea L.) is susceptible to many pathogens including nematodes, viruses, insects, and fungi (Melouk and Backman, 1995). Soilborne fungi cause diseases that adversely affect peanut health and productivity throughout the growing areas of the United States (U.S.). Diseases such as pod rot, caused by Rhizoctonia solani Kühn and Pythium myriotylum Drechsler, crown rot, caused by Aspergillus niger Tiegh., and southern blight, caused by Sclerotium rolfsii Sacc., occur in all areas of the U.S. where peanuts are produced. Other diseases such as Sclerotinia blight (caused by Sclerotinia minor Jagger) are limited to certain geographic regions.
In 1971, Sclerotina blight of peanut was first observed in Virginia (Porter and Beute, 1974) and has since become a major concern to peanut producers in the Southwest U.S. Depending upon severity of field infection, yield losses due to Sclerotinia blight may be as high as 50% (Melouk and Backman, 1995). Onset and progression of the disease depends mainly on presence of the fungus and environmental conditions. Optimal conditions for the germination of S. minor sclerotia include a temperature of 20 to 25 C and relative humidity of at least 95% (Dow et al., 1988a). Onset of the disease usually begins as full plant canopy development approaches and infection usually begins at the point of contact of stems and the soil. Early symptoms of Sclerotinia blight include wilting and stem lesions with white mycelial growth and may occur on the lower main stem or “crown”, but may also be found on side branches or “limbs” of the peanut plant. Progression of the disease can be rapid under optimal environmental conditions, including a cool damp dense plant canopy, ultimately resulting in light tan lesions on stems, stem shredding, and plant death. The sclerotia of S. minor can persist in the soil for 4–5 yr in the absence of peanut and are easily spread to other fields by animals, farm machinery and water (Melouk and Backman, 1995). Due to this fact, the disease is not readily managed via crop rotation and most often requires fungicide application for effective control. The results of this study have implications regarding the most effective placement of fungicide application.
Plant growth habit can also be an important contributor to field resistance or susceptibility to Sclerotinia blight (Coffelt and Porter, 1982, Chappell et al., 1995). Spanish-type peanuts with upright growth habits are generally less susceptible than are runner and virginia-types which usually have a spreading growth habit. Cultivar Southwest Runner (Kirby et al., 1998) has an upright growth habit and is extremely tolerant to S. minor infection, presumably due to its open canopy, which does not create optimal environmental conditions for disease progression.
Much research has been done to develop effective management of Sclerotinia blight on peanut (Goldman et al., 1995; Jackson and Sholar, 1997; Butzler et al., 1998; Langston et al., 2001). The effect of crop pruning or thinning on Sclerotinia blight incidence has been investigated (Dow et al., 1988b; Bailey and Brune, 1997) and this practice has been integrated into a management strategy along with selective canopy morphology (genotypic) and fungicide sprays. Complete control this disease has not been achieved, but current management strategies include cultivar selection and fungicide application (Melouk and Backman, 1995). Information regarding the impact of initial disease location on plant yield would be helpful in improving Sclerotinia blight management programs. It is possible that prevention of initial infection on a specific area of the peanut plant by focusing fungicide applications to that area may decrease production loss. To determine whether or not influencing fungicide placement on the plant is rational, the effects of location of the S. minor lesions on peanut plant integrity or productivity must first be examined. Therefore, the objectives of this study were to compare the effects of the timing of onset of Sclerotinia blight and lesion location on peanut production parameters.
Materials and Methods
Field tests were conducted for two growing seasons (2001, 2002) at the Oklahoma State University Caddo Research Station in Ft. Cobb, OK. The cultivar Okrun (Banks et al., 1989) is highly susceptible to Sclerotinia blight and was therefore chosen for this study. The soil in the test plot area was a menofine sandy loam, pH 6.0., and has been used repeatedly to test peanut lines for S. minor resistance due to the high level of soil infestation which allows for objective assessment of disease resistance without artificial inoculation. Disease inoculum levels endogenous to test plots were assessed each test year by determining the number of S. minor sclerotia present in soil samples (96 total per yr) taken from the test plots within 2 wk after planting. Viable sclerotial density was determined from the top 5 cm of soil by a modified elutriation technique (Porter and Steele, 1983).
In this study, the experimental unit is the individual test plant and all factors were measured separately for each plant. The rows of Okrun plants used in this study were randomly distributed within a larger study (Chenault et al., 2005) which was designed as a randomized complete block with three replications. Plots within that study consisted of eight 6.1-m rows with 0.91-m row spacing. All seeds were treated with fungicide (90% thiophanate-methyl) at 2.5 g per kg seed before planting to reduce seed transmission (Bowen et al., 2000). Planting dates were 15 May 2001 and 15 May 2002. Seeds were hand planted 23 cm apart. No fungicide for control of Sclerotinia blight was applied to the test area. After planting, no herbicide was applied for weed control but all test plants were hand-weeded on a weekly basis and assessed for disease.
Test plants were assessed individually for Sclerotinia blight by the presence of visible above-ground symptoms on three dates, hereafter termed early, mid, and late. The early rating refers to the time of initial onset of disease in the test area. Mid and late season ratings were taken after weather events were favorable to initiation of fungal infection. Only newly infected plants (small lesions < 24 hr old) were flagged for use in the experiment. In 2001, readings were taken at 106, 120 and 134 days after planting (DAP), and in 2002, readings were taken at 123, 137, and 144 DAP. On an individual plant basis, date of initial disease onset was recorded and location of infection was noted as either “crown” or “limb”. Since only one initial infection per plant was possible, plants were tagged and labeled with date and location of infection to ensure that only initial infections were recorded and that these infections were recorded only once.
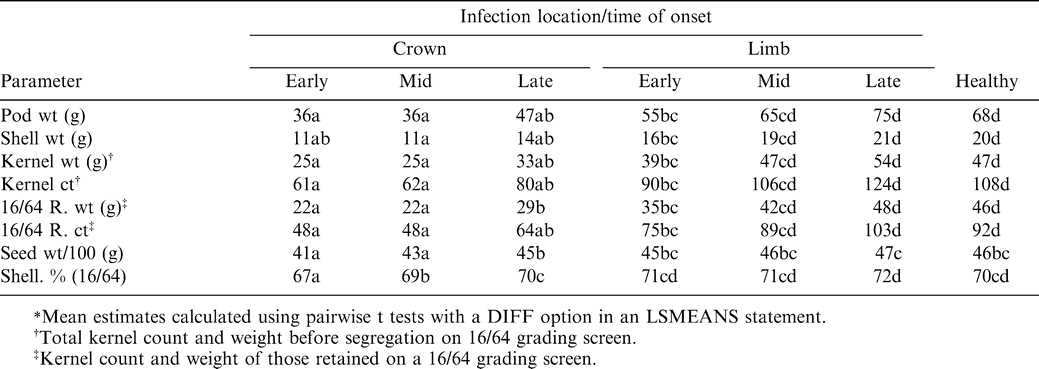
Plants were harvested (lifted carefully by hand after loosening soil with a pitch fork) individually on 15 October 2001 and 15 October 2002, and returned to the laboratory for analysis. Production parameters were measured and reported on an individual plant basis. Parameters included pod mass, shell weight, total kernel weight, total kernel count, 16/64 grading screen (19 mm × 6 mm) retained weight and count, seed weight per 100 seed, and 16/64 screen shelling percentage. This study was designed as a randomized complete block with a factorial arrangement of early- mid- and late-season infection onset across the two infection loci.
Statistical treatment of experimental data for Table 2 included using the MIXED procedure of PC SAS Version 8.2 (SAS Institute, Cary, NC) to conduct the analysis of variance. Year of study was included in the model statement as a random factor. The least-squares means for the combinations of infection location and date were computed using LSMEANS, and pairwise t-tests performed with the DIFF option.
Results
For both years of this study, the mean number of viable sclerotia of S. minor was 3 sclerotia/100 g of soil and the sclerotia were uniformly distributed throughout the test plot area (data not shown). A total number of 1643 and 1844 plants were analyzed in 2001 and 2002, respectively. Initial onset of disease was earlier in 2001 than in 2002. Initial infections on test plants are hereafter described as early- mid- or late-season (Tables 1 and 2). The final percentage of disease recorded for 2001 and 2002 was 42% and 59%, respectively (Table 1). In 2001, disease progressed steadily from the date of initial onset until the last reading was taken at 134 DAP. In 2001, an early freeze at 140 DAP prevented accurate disease readings after 134 DAP. In 2002, disease progression followed a bell-shaped curve with the peak of new infections occurring at 137 DAP and then dropping off at 144 DAP. In each year of this study, the number of crown infections equaled the number of limb infections (Table 1).
Data on plant production parameters for the 2-yr study were combined over years (Table 2), due to the fact that there was no significant interaction of year by infection location or by date of infection onset. In general, plants experiencing early-, mid- or late-season crown infections were significantly less productive than those with limb infections occurring at any sampling date. Mean estimates for all production parameters were significantly lower when plants were diagnosed with early- or mid-season crown infections, compared to late-season crown infections which did not significantly reduce production parameters more than early-season limb infections. Early and mid-season crown infections decreased most parameters by 42–48% when compared to limb infections occurring at the same time. Seed weight per 100 seed and 16/64 shelling percentage were least affected by infection location, with crown infections decreasing their values by an average of 6% and 4%, respectively.
Parameters directly related to yield (total kernel weight, pod weight, and seed count) were most severely affected by early- and mid-season crown infections. Plant production parameters were similar from plants with early- and mid-season limb infections. With the exception of seed weight/100 seed and 16/64 screen shelling percentage, all production parameters were significantly lower for those plants encountering early- rather than late-season limb infections. With the exception of seed weight/100 seed and 16/64 shelling percentage, significant decreases were seen in all productivity parameters measured for plants with crown infections and early onset limb infections when compared to those measured for non-infected plants. However, productivity parameters measured for plants with mid- and late-season limb infections were not significantly different for those of healthy plants.
Discussion
Despite efforts to develop efficient management strategies, control of Sclerotinia blight in the Southwestern U.S. has been only moderately successful. Most methods of control in infested fields involve a combination of cultivar selection and fungicide application. Cultivars with morphology-based resistance such as Tamspan 90 (Smith et al., 1991) have been planted in infested fields in the past, but a large number of growers in this region have turned to runner-type peanuts which have a greater yield potential and unit value (Damicone and Jackson, 2001). Runner-type peanuts, however, are more susceptible to Sclerotinia blight due to their spreading growth habit, which creates a more dense plant canopy with many lateral branches along the soil surface. Southwest Runner has a high level of tolerance to Sclerotinia blight. Other runner varieties released with measurable tolerance to Sclerotinia blight include Tamrun 98 (Simpson et al., 2000). Unfortunately, Tamrun 98 and Southwest Runner are not currently acceptable for commercial use due to both varieties having a small seed size and poor yield.
Many studies have examined the effect of fungicide application method on soilborne fungal disease incidence in peanuts (Sturgeon, 1990; Brenneman et al., 1991; Smith et al., 1992; Brenneman and Culbreath, 1994; Damicone and Jackson, 2001; Lemay et al., 2002). Sturgeon (1990) reported that southern blight incidence in peanuts was reduced when fungicide was applied in conjunction with a canopy opener. Damicone and Jackson (2001) found that reduced incidence of Sclerotinia blight and increased total yield were possible, depending on the fungicide applied, if fungicide was concentrated into a band over the center of the plant row and used with a canopy opener. Such reports are further supported by the results of this study which suggest that productivity greatly decreases when Sclerotinia blight infection occurs on the crown of the plant verses the limb. Canopy spreaders and single nozzle concentrated fungicide sprayers focus the chemical application to the center of the plant in bands, decreasing the incidence of crown infections.
Date of initial disease onset and disease progression varied among years. In both years of this study, uniform distribution of disease inoculum (viable sclerotia) was observed throughout the test plot area. It is possible that if not halted by an early freeze, additional disease readings in 2001 would have paralleled those recorded in 2002 by showing a decline in new infections recorded towards the end of the growing season. Regardless of the difference in disease onset profile from year to year, newly discovered infection sites occurred equally on the crown and limb of the test plants, but crown infections reduced plant productivity severely, regardless of date of onset. This result may be explained by the fact that crown infections often lead to a more rapid decline in plant vigor, thus reducing overall yield quality and quantity. Earlier attempts were made to develop algorithms to predict the timing and severity of soilborne fungal pathogen infection in peanut fields, including Sclerotinia blight (Brenneman and Culbreath, 1994; Butzler et al., 1998; Langston et al., 2001). Factors that have been used to predict such outbreaks include vine growth, canopy density, available moisture, relative humidity, and temperature. The results of this study suggest that limiting infection of the plant crown area, without necessarily trying to prevent limb infections, may increase plant productivity up to 48%. Therefore, focusing or banding fungicide application to the crown area early in the growing season could potentially increase yield without necessarily increasing production costs. This practice has been discussed by others (Damicone and Jackson, 2001), but the possible benefits of reducing or eliminating crown infections has now been shown in this study. Considering these findings, including the parameter of initial location of infection in prevention programs may lead to more effective means of Sclerotinia blight control.
Acknowledgements
The authors would like to express their gratitude to the Oklahoma State University Agricultural Experiment Station for their aid in providing land and personnel to conduct these studies. Research assistance provided by Lisa Myers and Doug Glasgow are also deeply appreciated. Mention of company or trade names is for the purpose of description only and does not imply endorsement by the U.S. Department of Agriculture.
Literature Cited
Bailey J. E. and Brune P. D. 1997 Effect of crop pruning on Sclerotinia blight of peanut. Plant Dis 81 : 990 – 995 .
Banks D. J. , Kirby J. S. , and Sholar J. R. 1989 Registration of ‘Okrun’ peanut. Crop Sci 29 : 1574 .
Bowen C. , Melouk H. A. , Jackson K. E. , and Payton M. E. 2000 Effect of a select group of seed protectant fungicides on growth of Sclerotinia minor in vitro and its recovery from infested peanut seed. Plant Dis 84 : 1217 – 1220 .
Brenneman T. B. , Murphy A. P. , and Scinos A. S. 1991 Activity of tebuconazole on Sclerotium rolfsii and Rhizoctonia solani, two soilborne pathogens of peanut. Plant Dis 75 : 744 – 747 .
Brenneman T. B. and Culbreath A. K. 1994 Utilizing a sterol demethylation inhibiting fungicide in an advisory program to manage foliar and soilborne pathogens of peanut. Plant Dis 78 : 866 – 872 .
Butzler T. M. , Bailey J. , and Beute M. K. 1998 Integrated management of Sclerotinia blight in peanut: utilizing canopy morphology, mechanical pruning, and fungicide timing. Plant Dis 82 : 1312 – 1318 .
Chappell G. F. , Shew B. B. , Ferguson J. M. , and Beute M. K. 1995 Mechanisms of resistance to Sclerotinia minor in selected peanut genotypes. Crop Sci 35 : 692 – 696 .
Chenault K. D. , Melouk H. A. , and Payton M. E. 2005 Field reaction to Sclerotinia blight among transgenic peanut lines containing antifungal genes. Crop Sci 45 : 511 – 515 .
Coffelt T. A. and Porter D. M. 1982 Screening peanuts for resistance to Sclerotinia blight. Plant Dis 71 : 811 – 815 .
Damicone J. P. and Jackson K. E. 2001 Effects of application method and rate on control of Sclerotinia blight of peanut with iprodione and fluazinam. Peanut Sci 28 : 28 – 33 .
Dow R. L. , Porter D. M. , and Powell N. L. 1988a Effect of environmental factors on Sclerotinia minor and Sclerotinia blight of peanut. Phytopathology 78 : 672 – 676 .
Dow R. L. , Powell N. L. , and Porter D. M. 1988b Effects of modifications of the plant canopy environment on Sclerotinia blight of peanut. Peanut Sci 15 : 1 – 5 .
Goldman J. J. , Smith G. D. , Simpson C. E. , and Melouk H. A. 1995 Progress in breeding Sclerotinia blight resistant runner-type peanut. Peanut Sci 22 : 109 – 113 .
Jackson K. E. and Sholar J. R. 1997 Effect of planting time and fungicides applied at planting on peanut pod yields. 75 – 83 Res. Rep. P-956 Oklahoma Agricultural Experiment Station Stillwater, OK .
Kirby J. S. , Melouk H. A. , Stevens T. E. , Banks D. J. , Sholar J. R. , Damicone J. P. , and Jackson K. E. 1998 Registration of ‘Southwest Runner’ peanut. Crop Sci 38 : 545 – 546 .
Langston D. B. , Phipps P. M. , and Stipes R. J. 2001 An algorithm for predicting outbreaks of Sclerotinia blight of peanut and improving the timing of fungicide sprays. Plant Dis 86 : 118 – 126 .
Lemay A. V. , Bailey J. E. , and Shew B. B. 2002 Resistance of peanut to Sclerotinia blight and the effect of acibenzolar-S-methyl and fluazinam on disease incidence. Plant Dis 86 : 1315 – 1317 .
Melouk H. A. and Backman P. A. 1995 Management of Soilborne Fungal Pathogens. 75- 85 In Melouk H. A. and Shokes F. M. eds. Peanut Health Management APS Press St. Paul, MN.
Porter D. M. and Beute M. K. 1974 Sclerotinia blight of peanuts. Phytopathology 64 : 263 – 264 .
Porter D. M. and Steele J. L. 1983 Quantitative assay by elutriation of peanut field soil for sclerotia of Sclerotinia minor. Phytopathology 73 : 636 – 640 .
Simpson C. E. , Smith O. D. , and Melouk H. A. 2000 Registration of ‘Tamrun 98’ peanut. Crop Sci 40 : 859 .
Smith O. D. , Simpson C. E. , Grichar W. J. , and Melouk H. A. 1991 Registration of ‘Tamspan 90’ peanut. Crop Sci 31 : 1711 .
Smith F. D. , Phipps P. M. , and Stipes R. J. 1992 Fluazinam: a new fungicide for control of Sclerotinia blight and other soilborne pathogens of peanut. Peanut Sci 19 : 115 – 120 .
Sturgeon R. V. 1990 Improved southern blight control with peanut canopy opener and banded reduced fungicide rates. Proc. Amer. Peanut Res. Educ. Soc 22 : 36 (abstr.) .