Introduction
Foliar-applied of peanut, Arachis hypogaea L, in the southeastern United States. Typical fungicide programs consist of four to eight applications per season depending on local disease risk (Nutter and Shokes, 1995). These fungicide programs are highly effective in protecting peanut from fungal diseases as typified by yield responses in excess of 1,000 kg/ha (Bowen et al., 1997; Grichar et al. 2005). Early leaf spot, Cercospora arachidicola Hori, and late leaf spot, Cercosporidium personatum (Berk. and Curt.), are the primary foliar diseases which can cause severe defoliation and pod loss from deterioration of the pegs (gynophores) which attach pods to the peanut plant (Nutter and Shokes, 1995). Soilborne diseases such as southern stem rot, Sclerotium rolfsii Sacc., and Rhizoctonia limbrot, Rhizoctonia solani Kühn AG-4, are known to attack peanut pegs (gynophores) and pods directly, thereby contributing to further yield loss (Melouk and Bachman, 1995). In addition to protecting pegs from deterioration caused by fungal infection, there are also anecdotal claims that some foliar-applied fungicides produce physiological increases in peg strength. Such increases in peg strength at harvest maturity would be advantageous in reducing potential losses during crop inversion even in the absence of disease.
Previous studies of factors influencing peg strength have included evaluations of cultivar differences (Bauman and Norden, 1971; Troeger et al., 1976; Thomas et al., 1983; Johnson George et al., 1988a, 1988b) and the anatomical basis for such differences (Thomas et al., 1983; Tiwari et al., 1988). Ito et al. (1970), Steele et al. (1972), and Troeger et al. (1976) also studied the effect of pod age on peg strength. Bauman and Norden (1971) evaluated the effect of plant growth regulators on peg strength. The primary objective of our study was to measure the effect of fungicide treatment programs on peanut peg strength. We also quantified the effect of pod maturity and disease incidence on peg strength.
Materials and Methods
Experimental Design & Crop Production
Tests were conducted on NC-V11 cultivar during the 2003 and 2004 growing seasons at the Edisto Research and Education Center (Barnwell County, SC). The soil type was a Varina sandy loam (clayey, kaolinitic, thermic, Plinthic Paleudults). The experimental design was a randomized complete block with five replicates of each fungicide treatment. The experimental unit was a plot eight rows wide (0.96-m row spacing) by 12 m long. The middle four rows of each plot were not subjected to traffic after planting in order to reduce experimental error on rows evaluated for disease incidence and harvested for yield and grade. Peanuts were produced using standard practices for virginia type cultivars in conventional tillage (Chapin and Thomas, 2005). Planting was on 16 May in 2003 and 17 May in 2004. In-furrow aldicarb (5.6 kg ai/ha Temik 15G, Bayer Corp., Kansas City, MO) was the only insecticide used. Herbicides consisted of S-metolachor (1.39 kg ai/ha Dual Magnum, Syngenta Crop Protection, Greensboro) and flumioxazin (0.1 kg ai/ha Valor, Valent USA Corp., Walnut Creek, CA) at planting. Imazapic (0.07 kg ai/ha Cadre DG, BASF Corp., Research Triangle Park, NC) and clethodim (0.21 kg ai/ha Select 2 EC, Valent USA Corp) were applied postemergence.
Fungicide Treatments
In 2003 six fungicide applications were made at about 15-day intervals for each of five tested programs except the nontreated check. In 2004 five total fungicide applications were made at about 15-day intervals for each of eight programs except the nontreated check. Complete descriptions of the tested fungicide programs with active ingredient rates and dates of application are listed in Table 1. For brevity, programs will be referred to in the following text and figures by the abbreviated program names listed in Table 1. With the exception of the Bravo program, which only controls foliar diseases, the timing and combinations of fungicides tested were typical of programs required to control the complex of soilborne and foliar fungal diseases under South Carolina production conditions. Fungicide treatments were applied to all eight plot rows with a 4-row tractor-mounted boom. In 2003 TX 8 hollow cone nozzles (Spraying Systems, Wheaton, IL) were used (93.5 L/ha at 344 Kpa). In 2004 the same boom was used with 8003 flat fan nozzles (187 L/ha at 241 Kpa).
Peg Strength
Peg tensile strength was measured with a Shimpo DFS-50 digital force gauge (Shimpo Instruments, Nidec-Shimpo America Inc., Itasco IL). The gauge was mounted on a board and each tested pod was placed in an alligator clip attached to the gauge. The clip allowed pods to rotate slightly as initial tension was placed on the pegs. Pods were placed in the clip such that when pegs were pulled along a reference line on the board, the peg would be normal to the surface of the pod at the point of peg attachment. The peg was then slowly pulled by hand until it either broke along the length of the peg, or more typically detached near the pod. The gauge recorded the peak force required for the peg to fail. After the pod was removed from the clip, the upper surface or “saddle” area of the pod was scraped with a knife to categorize pod mesocarp color as dark yellow, orange, brown, or black. This sequence of mesocarp colors is indicative of advancing pod maturity from the yellow through black stages (Williams and Drexler, 1981). All of the tested pods would be considered harvestable pods in that all of these four mesocarp color categories produce sound mature kernels, although pods in the dark yellow category would produce a relatively lower percentage of sound mature kernels than more mature pod categories. The point of peg failure also was recorded in one of two categories: the break point occurred either along the length of the peg or at the point of pod attachment. All peg strength measurements were taken in the field within 10 min of uprooting plants and collecting pods. The accuracy of the gauge over the range of data collected was tested by mounting the gauge vertically and suspending 5 replicates of standard weights. The gauge read within 1.49 ± 0.43% of the weight standards.
Pods were collected for testing by using a pitchfork to uproot plants as gently as possible. About 0.5 m of row was lifted from each of rows two and seven in each 8-row plot after the middle four rows had been harvested for yield. Pods were cut from plants with scissors, leaving as much peg as possible attached to the pod. In 2003, 20 healthy (asymptomatic for disease), full-sized pods were collected from each plot (100 per fungicide treatment). In 2004, 25 such healthy pods were collected from each plot (125 per treatment). Five over-mature pods were also collected from each plot (25 per treatment) in both years. Over-mature pods were identified by the presence of a slight anthocyanin pigmentation on the pod exterior. These pods were subsequently found to have a coal black mesocarp and tan-brown coloration on the seed coat. Over-mature pods also typically had some visible deterioration of the peg. In 2003, pods were collected with southern stem rot symptoms, as defined by the presence of characteristic mycelia or sclerotia. Invariably these diseased pods also had some visible deterioration of the peg. An attempt was made to collect five southern stem rot symptomatic pods from each plot (125 total), but only 85 were collected because fewer could be found in some of the more efficacious soilborne disease treatments. In 2003 we also collected 120 pods symptomatic for tomato spotted wilt, approximately five from each plot. Pods symptomatic for tomato spotted wilt were identified based on a typical orange color and corky texture of the pod exterior, as well as finding the characteristic foliar ring spot symptoms on the plant (Demski and Reddy, 1997).
In 2003 peg strength measurements were taken from 10–15 Oct (147–152 DAP) and in 2004 from 12–18 Oct (148–154 DAP). However, all treatments within a replicate were collected and tested on the same day.
Disease Sampling
Southern stem rot incidence was rated within two hr of digging by scanning two rows per plot and counting the total row length symptomatic for this disease. Plots were examined for the presence of early leaf spot and late leaf spot within one wk prior to harvest. Two observers scanned the middle four rows of each plot and estimated percent defoliation. Although both leaf spot diseases were present, late leaf spot was predominant and caused the observed defoliation. Stunting from tomato spotted wilt virus affected less than an estimated 5% of plants, and therefore no individual plot ratings were taken for this disease.
Yield, Grade, and Crop Value
The middle four rows of each plot were inverted with a KMC peanut digger (Kelly Manufacturing Company, Tifton, GA) on 8 October (145 DAP in 2003, 144 DAP in 2004). These rows were subsequently harvested with a two-row Hobbs 525 combine (Hobbs Manufacturing Company, Albany, GA) modified with a bagging attachment. Samples were weighed in the field and a subsample (~1,500 gm) was removed for grading. Grade samples were dried at approximately 32 C and then stored at room temperature until graded in accordance with USDA standards (USDA 1998). Yields were adjusted to 7.0% moisture before statistical analysis.
Data Analysis
The data were subjected to analysis of variance (PROC GLM, SAS Institute Inc., 1985). Where a significant treatment effect was measured by ANOVA, means were separated with a protected LSD test. The significance level for all statistical tests was P = 0.05. Percentage data for disease severity variables and grade were transformed using arcsin (x) prior to analysis.
Results
Fungicide Peg Strength Effects
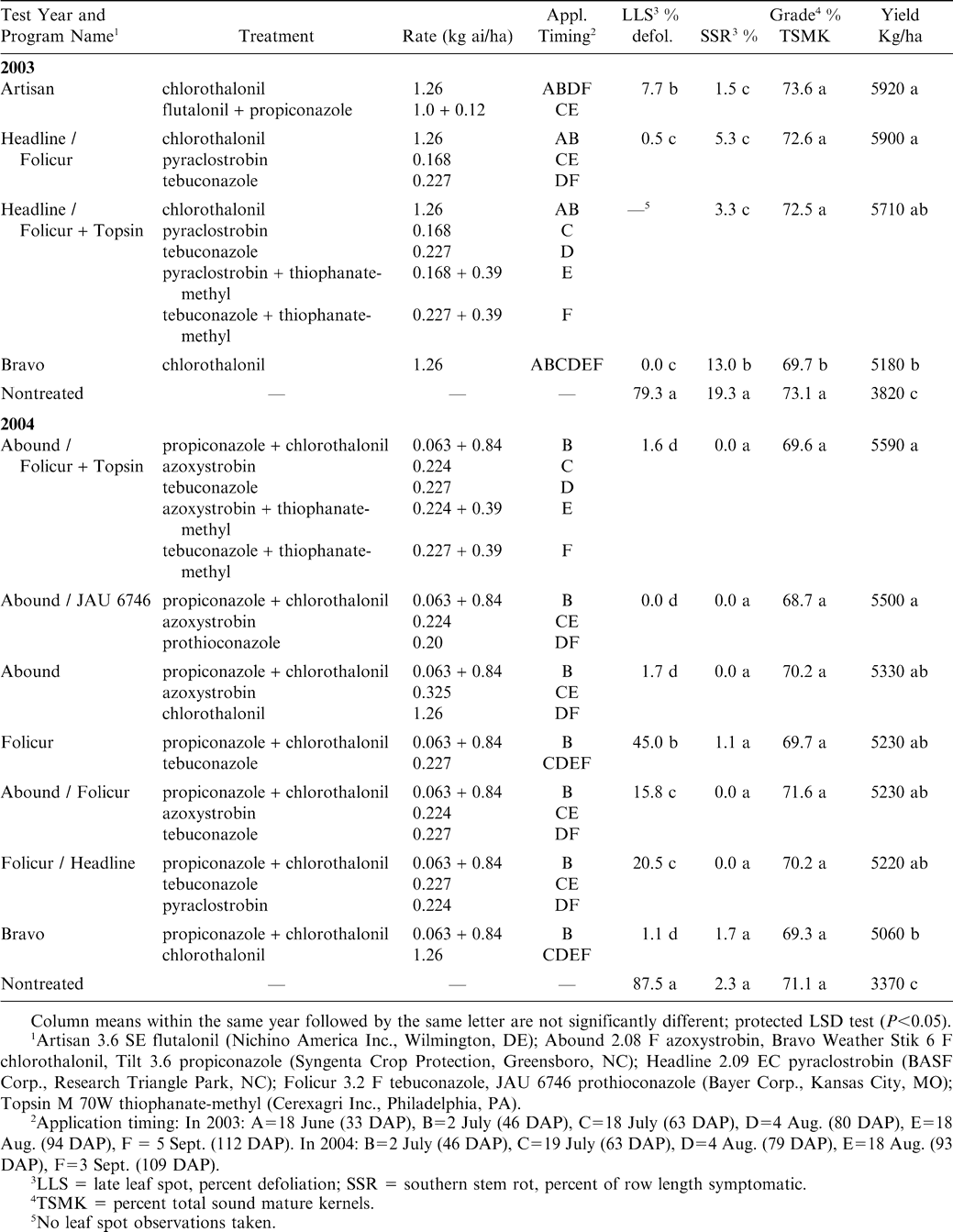
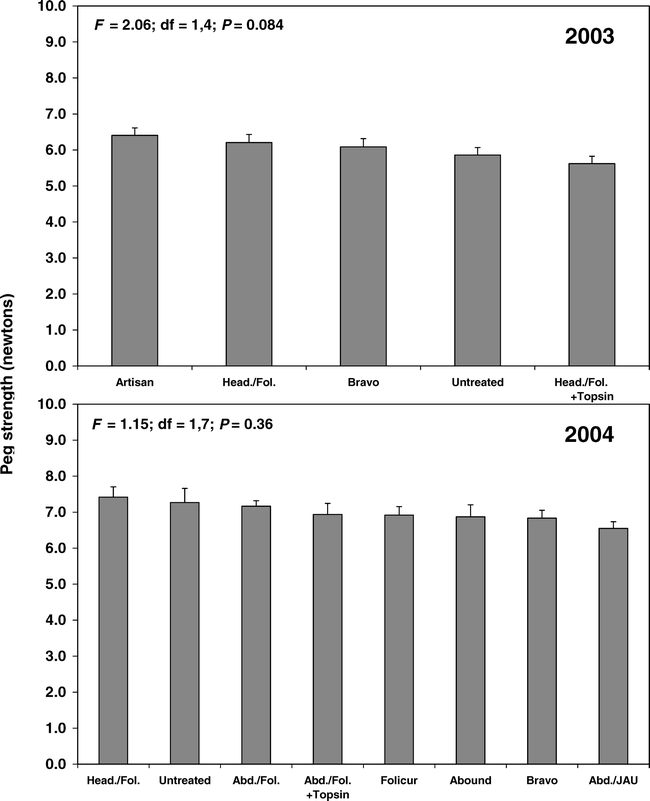
None of the fungicide treatments in either year had a significant effect on peg strength when healthy pods were compared (Fig. 1). When pods symptomatic for southern stem rot were compared, the Artisan program and Headline / Folicur + Topsin program had greater peg strength than the nontreated check (Fig. 2). When over-mature pods were compared, fungicide treatment had no effect on peg strength in 2003 (F = 0.24; df = 1,4; P = 0.913) or 2004 (F = 1.10; df = 1,7; P = 0.362).
Effect of fungicide treatment programs on the peg strength of healthy (disease asymptomatic) harvest-mature peanut pods in 2003 and 2004. Maturity category of tested pods ranged from dark yellow to black mesocarp color. Differences are non-significant as indicated by the F test. Vertical lines indicate standard errors of the means. N = 100 and 125 for each treatment in 2003 and 2004, respectively. See Table 1 for detailed descriptions of the fungicide programs.
Effect of fungicide treatment programs on the peg strength of peanut pods symptomatic for southern stem rot in 2003. Bars sharing the same letter are not significantly different (protected LSD test, P = 0.05). Vertical lines indicate standard errors of the means. N = 5, 20, 25, 15, and 20 for Artisan, Head./Fol. + Topsin, Bravo, Head./Fol., and nontreated, respectively. See Table 1 for a description of the fungicide programs.
Pod Maturity Effects
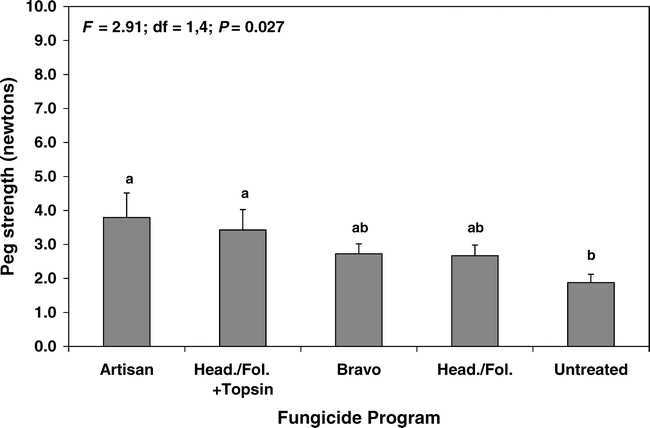
Results for pod maturity were pooled over years because there was no significant interaction between year and pod maturity (F = 2.44; df = 4, 31; P = 0.067). There were no measurable differences in peg strength due to pod maturity in the yellow through orange, brown, and black mesocarp color categories (Fig. 3). However, over-mature pods had significantly lower peg strength than all other maturity categories, and the strength of over-mature pods was only about 32% that of fully mature, black mesocarp pods (Fig. 3).
Effect of pod maturity, as indicated by mesocarp color, on the peg strength of healthy (disease asymptomatic) peanut pods in 2003 and 2004 (pooled data). Over-mature pods were defined by a coal-black mesocarp, tan-brown seed coat, and slight anthocyanin pigmentation. Bars sharing the same letter are not significantly different (protected LSD test, P = 0.05). N = 33, 316, 520, 656, and 330 for dark yellow, orange, brown, black, and over-mature categories, respectively. Vertical lines indicate standard errors of the means.
Disease Effects
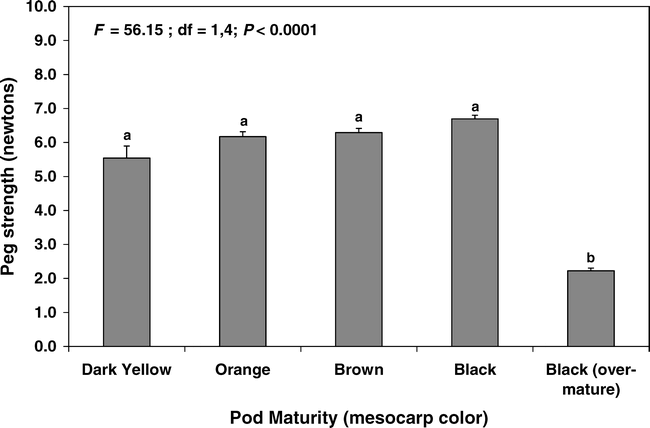
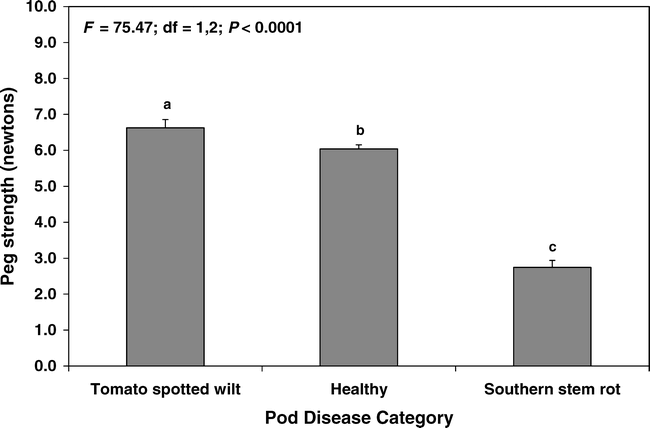
Pods symptomatic for southern stem rot had significantly weaker pegs, being only about 45% the strength of healthy pods (Fig. 4). In contrast, pods with tomato spotted wilt symptoms had significantly stronger pegs than those of healthy pods (Fig. 4).
Effect of disease incidence on the peg strength of peanut pods in 2003. Bars sharing the same letter are not significantly different (protected LSD test, P = 0.05). N = 120, 500, and 85 for tomato spotted wilt, healthy, and southern stem rot categories, respectively. Vertical lines indicate standard errors of the means.
Peg Breaking Point
On 98% of healthy mature pods, the peg broke at the point of attachment to the pod; whereas on over-mature pods, 60% of the pegs broke proximal to the point of attachment, that is, along the length of the peg rather than at the point of pod attachment. On pods symptomatic for southern stem rot, 45% of the pegs broke proximal to the point of pod attachment. On pods symptomatic for tomato spotted wilt, only 3% of pegs broke proximal to the point of pod attachment.
Disease Severity, Fungicide Efficacy, Yield and Grade Effects
Late leaf spot and southern stem rot infection levels were both significant in 2003 when late leaf spot caused 79% defoliation of the nontreated check and southern stem rot was symptomatic on 19% of the untreated check (Table 1). All fungicide treatments effectively suppressed late leaf spot in 2003; however, the Bravo program was not effective in suppressing southern stem rot. Not surprisingly, the highest yields (1880–2,100 kg/ha greater than the nontreated check) were recorded for programs that suppressed both late leaf spot and southern stem rot.
In 2004 late leaf spot caused 87% defoliation of the nontreated check (Table 1). However, southern stem rot symptoms were present on only 2% of the nontreated check. In 2004 the Folicur program was least effective in preventing defoliation from late leaf spot, and both the alternated Folicur / Abound and Folicur / Headline programs were less effective than Abound / Folicur + Topsin, Abound / JAU 6746, Abound, or Bravo programs. All fungicide programs increased yield over the nontreated check in 2004, with yield improvement ranging from 1,690–2,220 kg/ha.
Discussion
Although the tested fungicide programs were highly effective in preventing pod loss from foliar disease or a combination of foliar and soilborne diseases under severe infection conditions, there was no evidence that any of the fungicide treatments increased peg strength in the absence of disease. Even on over-mature pods with inherently weaker pegs, there was no indication that fungicide treatment enhanced peg strength. Some fungicide programs did increase the peg strength of pods which were symptomatic for southern stem rot, presumably because fungicide treatments efficacious against southern stem rot would slow disease progress on infected pods. In addition, pods symptomatic for southern stem rot were much more difficult to find in plots treated with fungicides efficacious against this disease. Therefore the diseased pods selected for testing from these plots may have had less obvious or advanced symptoms. The relatively poor efficacy of tebuconazole (Folicur) against late leaf spot was representative of tebuconazole performance in replicated experiments and S. C. grower fields in 2003 and 2004. Tebuconazole has previously been consistently effective against late leaf spot since becoming available for grower use in 1994. Subsequent field tests in 2005 confirmed that tebuconazole is no longer effective against late leaf spot in South Carolina.
Our data indicate that for NC-V11 cultivar there was no decline in peg tensile strength as pods matured through the dark yellow to black mesocarp stages. In fact peg strength values for fully mature black pods were numerically greater than those of less mature pods. Thus on healthy plants and assuming equivalent digging conditions (soil moisture), there would seem to be little risk of increased harvest loss until the first over-mature pods with visibly deteriorated pegs are present. This is counter to the commonly held assumption that pegs of mature pods (black mesocarp) are inherently weaker than pegs of less mature pods. Our results are in contrast to Troeger et al. (1976) who found that peg strength decreased with increasing maturity when maturity categories were determined by the ratio of kernel dry weight to total pod dry weight. Ito et al. (1970) reported cultivar differences in the relationship between peg strength and maturity. All cultivars demonstrated an increase in peg strength up to a peak at 60–80 d after planting depending on cultivar. Subsequently peg strength declined to harvest maturity in some cultivars but remained almost constant in others.
Tomato spotted wilt virus causes significant yield reduction in peanut due to severe plant stunting and pod deformity (Brown et al., 2005). However, based on our results, this disease does not cause additional loss by weakening the peg. This is consistent with our observation that when severe pod loss occurs due to delayed digging, a disproportionate number of the remaining attached pods have tomato spotted wilt symptoms. Southern stem rot is well-known to cause peg and pod deterioration and therefore the 55% reduction in peg strength we quantified is not surprising. The defoliation associated with late leaf spot is also known to cause significant pod loss, presumably due to premature weakening of pegs (Nutter and Shokes, 1995). Yet the peg strengths we measured from disease asymptomatic pods on plants with severe late leaf spot defoliation were not measurably different from treatments with no leaf spot defoliation. Deteriorated pegs and pod loss were much more prevalent in treatments with late leaf spot symptoms, but there was no indication that fungicide treatment had any effect on peg strength other than disease prevention.
Our measures of peg strength were relatively low compared to previous studies. Bauman and Norden (1971) recorded peg strengths of about 10 N for both Florunner and Florigiant cultivars. In a study of 48 cultivars, Johnson George et al. (1988a) categorized peg strengths in the 5.7–8.4 N range as low. Virginia market types in general exhibit relatively weak peg strength (Johnson George et al., 1988a, and 1988b). Troeger et al. (1976) reported that virginia market type cultivars had lower peg strength than Spanish types, and that runner types had slightly lower peg strengths than virginia types. Thomas et al. (1983) found that ten commercial cultivars had peg strengths in the 8.3–22.1 N range, and summarizing results from a series of previous studies, they reported a 4.9–14.7 N range of peg strengths in other cultivars. Despite the relatively low mean peg strength values (~ 6.0–7.4 N) measured for healthy NC-V11 pods, this variety is preferred by growers for its consistent yield performance in South Carolina. Characteristics such as a relatively small pod size and stable peg strength in mature pod stages may be factors in limiting harvest loss in this cultivar.
Our observation that pegs of healthy pods typically broke at the point of pod attachment is consistent with previous results (Ito et al., 1970; Steele et al., 1972; and Troeger et al. 1976). The tendency we measured for pegs of over-mature and diseased pods to break proximal to the pod attachment point can be useful in helping growers diagnose loss problems at digging. When pegs break at the point of pod attachment, pieces of the pod exocarp remain attached to the end of the peg and are distinctly visible as bright star-shaped remnants on inverted peanuts. The presence of these pod remnants or “stars” indicates that pod loss was due to physical factors such as soil moisture or digger operation. In contrast, when no pod remnants are visible at the end of pegs, and the shed pods have a short length of frayed peg attached, pod loss was probably due to over-maturity or disease.
In conclusion, our results demonstrate that growers should make fungicide treatment decisions based on disease prevention efficacy rather than on the assumption of any additional physiological peg strengthening benefits. These results may also prove useful in refinement of harvest-timing guidelines based on the distribution of pod maturity as defined by mesocarp color categories. Specifically, the data indicate that given equivalent digging conditions, pod loss risk will not increase until some over-mature pods with deteriorated pegs are present. However, further research is needed to measure the relationship between peg strength and pod maturity in other commercial varieties.
Acknowledgements
We appreciate the support of South Carolina peanut growers and the National Peanut Board. This is Technical Contribution No. 5109 of the Clemson University Experiment Station, and is based on work supported by the CSREES/USDA, under project number SC1700191.
Literature Cited
Bauman R. W. and Norden A. J. 1971 Effect of growth regulators on vegetative and reproductive characteristics of six peanut genotypes 1. J. Amer. Peanut Res. Educ. Assoc 3 : 75 – 86 .
Bowen K. L. , Hagan A. K. , and Weeks J. R. 1997 Number of tebuconazole applications for maximizing disease control and yield of peanut in growers' fields in Alabama. Plant Dis 81 : 927 – 931 .
Brown S. L. , Culbreath A. K. , Todd J. W. , Gorbet D. W. , Baldwin J. A. , and Beasley J. P. 2005 Development of a method of risk assessment to facilitate integrated management of spotted wilt of peanut. Plant Dis 89 : 348 – 356 .
Chapin J. W. and Thomas J. S. 2005 Peanut money-maker production guide – 2005. http://virtual.clemson.edu/groups/peanuts/mmaker05.PDF.
Demski J. W. and Reddy D. V. R. 1997 Diseases caused by viruses. 53 – 59 In Kokalis-Burelle N. , Porter D. M. , Rodriguez-Kabana R. , Smith D. H. , and Subrahmanyam P. eds. Compendium of peanut diseases, Second Edition APS Press St. Paul, MN .
Grichar W. J. , Jaks A. J. , and Besler B. A. 2005 Response of peanuts (Arachis hypogaea) to weather-based fungicide advisory sprays. Crop Protection 24 : 349 – 354 .
Ito K. , Inouye J. , and Yoshida K. 1970 Studies on grain shedding in some crops. On the strength requisite for detaching pod from gynophore in peanut (in Japanese, English summary). Crop Sci. Soc. Japan, Proc 39 : 47 – 53 .
Johnson George K. , Murthy T. G. K. , Sen P. , and Reddy P. S. 1988a High peg strength donors in groundnut (Arachis Hypogaea L.). Current Science 57 : 1006 .
Johnson George K. , Tiwari S. P. , and Reddy P. S. 1988b Genetic variability for peg strength and related characters in groundnut (Arachis Hypogaea). Indian J. Agric. Sci 57 : 141 – 143 .
Melouk H. A. and Backman P. A. 1995 Management of soilborne fungal pathogens. 75 – 82 In Melouk H. A. and Shokes F. M. eds Peanut health management APS Press St. Paul, MN .
Nutter F. W. and Shokes F. M. 1995 Management of foliar diseases caused by fungi. 65 – 73 In Melouk H. A. and Shokes F. M. eds Peanut health management APS Press St. Paul, MN .
SAS Institute 1985 SAS/STAT guide for personal computers, version 6 edition SAS Institute Cary, NC .
Steele J. L. , Wright F. S. , and van Schaik P. H. 1972 Certain physical and mechanical properties of Va. 61R peanuts. J. Amer. Peanut Res. Educ. Assoc 4 : 108 – 119 .
Thomas R. J. , Pettit R. E. , Taber R. A. , and Jones B. L. 1983 Peanut peg strength: force required for pod detachment in relation to peg structure. Peanut Sci 10 : 97 – 101 .
Tiwari S. P. , Murthy T. G. K. , Johnson George K. , and Varmoda D. L. 1988 Path coefficient analysis of anatomical characters affecting peg strength in groundnut. Euphytica 39 : 119 – 121 .
Troeger J. M. , Williams E. J. , and Butler J. L. 1976 Factors affecting peanut peg attachment force. Peanut Sci 3 : 37 – 40 .
[USDA] U.S. Department of Agriculture 1998 Farmers' stock peanuts inspection instructions USDA Washington, DC .
Williams E. J. and Drexler J. S. 1981 A non-destructive method for determining peanut pod maturity. Peanut Sci 8 : 134 – 141 .
Notes
- 1Department of Entomology, Soils, and Plant Sciences, Clemson University, Edisto REC, 64 Research Road, Blackville, SC 29817, USA. jchapin@clemson.edu. [^]