Introduction
Cultivated peanut (Arachis hypogaea L.) is a geotropic annual, self-pollinating, herbaceous legume native to South America (Hammons, 1982). Debrah and Waliyar (1996) reported that 25.7 million tons, from 21 million hectares of land, are cropped with peanut worldwide with Asia alone accounting for about 70% and Africa 20% of total production. In West Africa, important producing countries include the Burkina Faso, Gambia, Ghana, Mali, Nigeria, Niger, and Senegal.
Minton and Baujard (1993) reported that nematodes damage peanut in all production regions of the world. Nematode infestation of peanut lead to various symptom expression and damage. M. arenaria infected plants become yellow and stunted as early as 40 days after planting (Zhang, 1985). Meloidogyne spp. cause galling on peanut roots, pods, and pegs (Taylor and Sasser, 1978). Lesions caused on roots, pods, and pegs by Pratylenchus brachyurus permit easy entry of fungi and bacteria to cause peg and pod rot (Jackson and Sturgen, 1973). Sasser and Freckman (1987) reported that annual losses caused by nematodes to peanut were estimated at 12%, translating into losses of over one billion US dollars.
Peanut is an important food and oil crop in Ghana. Principally, peanut is cultivated in the forest and savanna transitional zones of southern Ghana and the savanna regions of northern Ghana. In a survey of peanut production problems in five communities in northern Ghana, about 50% of respondents implicated insect pests as major constraint to production (Salifu, 1996). However, the importance of nematodes in peanut production has not been thoroughly defined.
Rodriguez-Kàbana et al. (1994) reported that M. arenaria damage to peanut could be so severe that continuous production of the crop is impossible in fields with high populations of this nematode. Nematicides and crop rotation are frequently employed to manage plant parasitic nematodes. Oudejans (1991) reported that the number of nematicides available for use by farmers is limited, especially in West Africa, and the cost of nematicides can be cost prohibitive and not sustainable (ACES, 1993). Additionally, human exposure and environmental hazards associated with nematicide application are a concern in developing countries (Anon, 2000; Thomas, 1996).
Nematodes have extensive host ranges (Saka and Carter, 1987), and benefits of rotation are not always realized because of sustained survival on crops and weeds within the same field. Incorporating host-plant resistance is often the least expensive and most effective approach to reducing disease and nematode severity in crop fields. Incorporating host-plant resistance into peanut production systems used by low-resource farmers in Africa is the most sustainable approach to managing disease and nematodes (FAO, 1982).
Presence of nematodes in four principal peanut-growing regions in southern Ghana was surveyed to identify the nematode pests associated with peanut. These data are currently being used to develop appropriate pest management strategies for peanut production for the region. Research was also conducted to determine colonization of cultivars grown in southern Ghana by native parasitic nematodes. The ultimate goal is to incorporate germplasm with substantial resistance into integrated pest management systems to sustain and increase peanut production in Ghana.
Materials and Methods
Survey of Peanut Fields.
Field sampling of nematode in Ghana during the 1999 and 2001 cropping seasons included twelve farms in the Ashanti and Brong, fourteen farms in Eastern region, and sixteen farms in the Volta region. Soil and peanut root samples were collected from the 54 farms at harvest by randomly selecting five samples at three locations within the rhizosphere of peanut using a 5-cm diameter auger to a depth of 20 cm. Samples were bulked and thoroughly mixed before a composite sample of 200 cm3 was stored in a sealed polyethylene bag until nematode extraction was performed. Samples from all 54 farms were assayed for nematodes to compare frequency of occurrence and relative abundance of parasitic nematodes.
Nematodes were extracted from 200 cm3 of soil using Cobb's decanting and sieving method combined with a blender-cotton wool filter procedure (Southey, 1986; Schouten and Arp; 1991). Nematode suspension was concentrated to 25 cm3 by siphoning off the supernatant. Nematodes were relaxed in water at 60 C for 3 min and fixed with formalin:acetic acid:distilled water solution (10:1:89). Nematode suspension from 1-cm3 aliquots were placed in a counting dish and the nematodes identified with a binocular stereoscopic and compound microscopes using standard reference (CIH, 1975). Nematode genera were counted and expressed as number per 200 cm3 of soil. In the case of peanut root, 5 cm3 samples replicated three times were assayed for nematodes using only the Blender-cotton wool filter method (Schouten and Arp, 1991). Sample size was variable in the case of millipedes, which were sampled from the rhizosphere of peanut due to differences in age and size of millipedes. The gut system of millipedes was extracted using the method described previously.
Host-Plant Resistance to Nematodes.
Twenty-one peanut cultivars and experimental lines from the Savanna Agricultural Research Institute (SARI) and Crops Research Institute (CRI) in Ghana and from North Carolina State University in the US were collected and screened for nematode resistance during 2000 and 2001 in field trials located at Kwadaso near Kumasi, Ghana. Cultivars included: AADRO 93, AT 120, Georgia Green, GK 7 High Oleic, ICGX-SM 89029, ICGV-SM 86047, ICGV 87160, ICGV 86556, NC 7, NC 10 C, NC-V 11, NC 12 C, RRR-MDR-8-19, RRR-UGA-9, RRR-MDR-8-16, RRR-M 576-79, RRR-M249-74, Shitoachi, Sinkarzei, Southern Runner, and VA 93 B. Cultivars and experimental lines were seeded in rows spaced 76 cm apart in conventionally prepared seedbeds. Plot size was five rows by 5 m in length. The experimental design was randomized complete block with 4 replications.
During land preparation, soil and roots of weed species on the experimental site were randomly sampled and processed for nematodes using Cobb's decanting and sieving method combined with a blender-cotton wool filter procedure (Southey, 1986; Schouten and Arp, 1991). At harvest, soil samples were randomly taken from the rhizosphere of peanut in addition to samples of peanut roots for extraction. Throughout the investigation, nematodes were extracted from 200 cm3 of soil and 5 cm3 of root samples. Nematodes were relaxed in warm water (60 C) for 3 min and fixed with 40:1:89 (formalin:glacial acetic acid:distilled water) solution. One-cm3 aliquots were removed and nematodes were identified using a binocular stereoscopic and compound microscopes using standard reference (Anon, 1975). Nematodes were counted and expressed as number per 200 cm3 of soil. Data were log transformed {log (X+1)} before analysis using SAS. Means were separated by the Student-Newman-Keuls Test at p ≤ 0.05.
Results and Discussion
Survey of Peanut Fields.
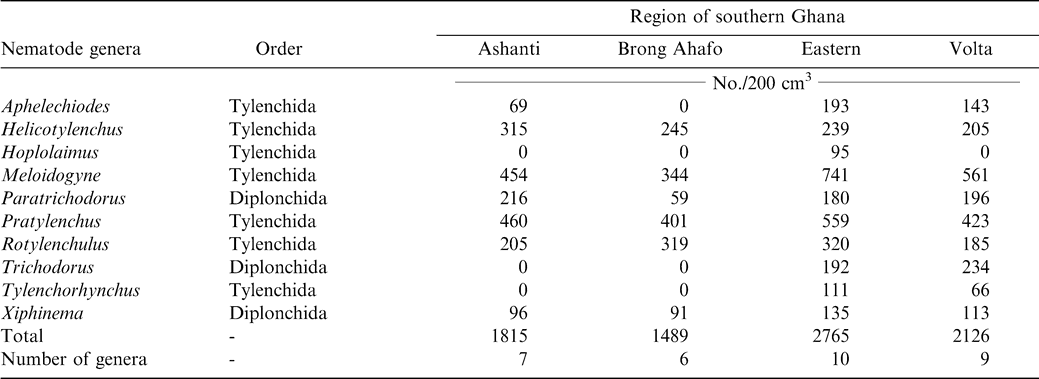
Ten genera of plant parasitic nematodes were found belonging to the orders Tylenchida and Dorylaimida (Table 1). Nematodes were found associated with ten different cultivars of peanut grown in the four production regions. Peanut cultivars grown in the Ashanti region were Konkoma, Broni, Kowoka, and China. In Brong Ahafo region, cultivars included Konkoma, Bremawuo, Afromo, and China. In Eastern region cultivars included Konkoma and Cameroon with Klukluklui, Kpedevi, and Goroga grown in the Volta region. Ten, nine, seven, and six genera of nematodes were found in the Eastern, Volta, Ashanti, and Brong Ahafo regions, respectively (Table 1). Six genera; Helicotylenchus, Meloidogyne, Paratrichodorus, Pratylenchus, Rotylenchulus, and Xiphinema, were found in all the four regions (Table 1). Hoplolaimus was found only in the Eastern region. The genera Trichodorus and Tylenchorhynchus were not found in Ashanti or Brong Ahafo regions but were present in Eastern and Volta regions.
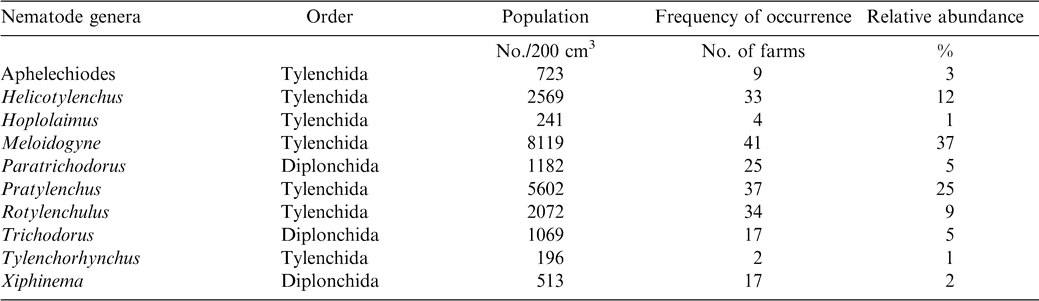
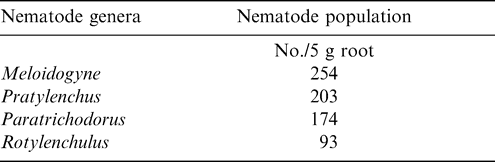
Meloidogyne (juveniles) alone constituted approximately 37% of the total population and occurred in 41 of the 54 farms sampled (Table 2). Pratylenchus, Helicotylenchus, Pratylenchus, and Helicotylenchus consisted of 25, 12, 37, and 33% of the nematode genera. Relative abundance of Tylenchorhynchus, Hoplolaimus, Aphelenchoides, and Xiphinema was below 5% while Tylenchorhynchus was found in two fields with a relative abundance of less than 1%. The number of nematodes associated with peanut roots ranged from 93 to 254/5 g root (Table 3). The highest mean population of 104 Rhigonema was extracted from millipedes sampled from the rhizosphere of peanut in the Brong Ahafo region while the lowest mean of 48 was recovered from millipedes found in the Volta region (Table 4).
Results of these surveys indicate that plant parasitic nematodes occur in all the four regions of southern Ghana. Nematodes encountered during the survey such as the lesion nematode (Pratylenchus brachyurus), peanut rootknot nematode (Meloidogyne arenaria), testa nematode (Aphelenchoides arachidis), and spiral nematode (Helicotylenchus multicinctus) have been reported previously (Sharma, 1985). However, absence of particular nematodes from a region does not imply that the nematodes are non-existent in the region. A possible suggestion might be that peanut is not a favorable host. Also, different biotic and abiotic stresses might explain why some nematodes were found in some regions but absent from others. However, it is evident that the ten different cultivars of peanut grown in the four regions support large populations of Helicotylenchus, Meloidogyne, and Pratylenchus. Meloidogyne arenaria, which is endoparasitic, was the most abundant nematode found in peanut root system. Pratylenchus, Paratrichodorus, and Rotylenchulus followed in that order. Rhigonema species have been reported to parasitize the gut system of millipedes (Hunt, 1998). Results in the current study are in concert with those from other West African regions; Bos (1977) found Aphelenchoides arachidis on peanut in northern Nigeria. Criconemella species have been reported on peanut in Gambia (Merny et al., 1974). In Senegal, Netscher (1975) reported that Meloidogyne species reproduced on peanut.
Additional research is needed to establish interactions among nematodes and other pathogenic organisms in the development of disease complexes in peanut (Patel et al., 1985). For sustainable peanut production in southern Ghana, plant parasitic nematodes must be managed effectively, and results from these surveys will assist practitioners in germplasm development and selection and formulation of management strategies that utilize control measures in addition to host plant resistance.
Host-Plant Resistance to Nematodes.
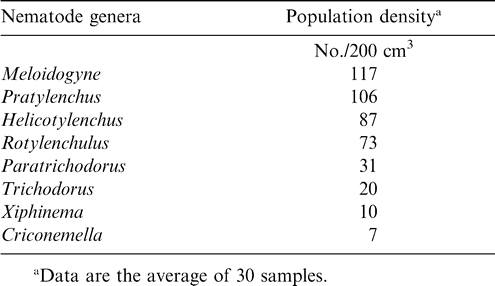
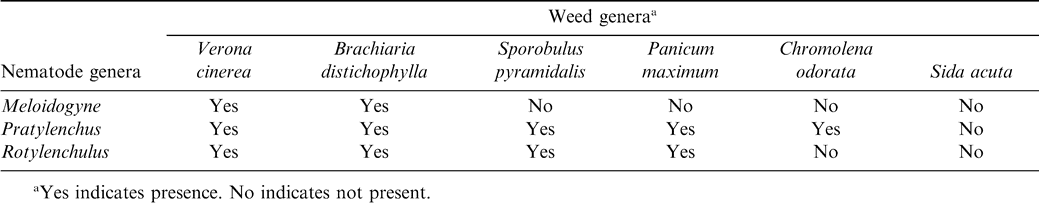
Eight genera of nematodes were identified at the beginning of the trial, with Meloidogyne being the most abundant and Criconemella being the least abundant (Table 5). Six weed species, Verona cinerea, Sida acuta, Panicum maximum, Brachiaria distichophylla, Chromolaena odorata, and Sporobulus pyramidalis, were predominant at this location. Some of these weed species were also found to be infected with the same three genera of nematodes found in peanut roots (Table 6.). Verona cinerea gave the highest nematode population. Nematodes were not extracted from Sida acuta samples. The isolation of nematodes from weed species collaborated previous findings demonstrating that weeds serve as alternate hosts of nematodes (Khan and Khan, 1985). Nematode's ability to survive on native flora makes management of these pests extremely difficult to manage using crop rotation only. Significant crop loss can occur when nematodes found in these experiments are not controlled or suppressed (Minton and Baujard, 1993). The nematode Trichodorus similis was found during the initial soil sampling but was not present at harvest (Sharma, 1985).
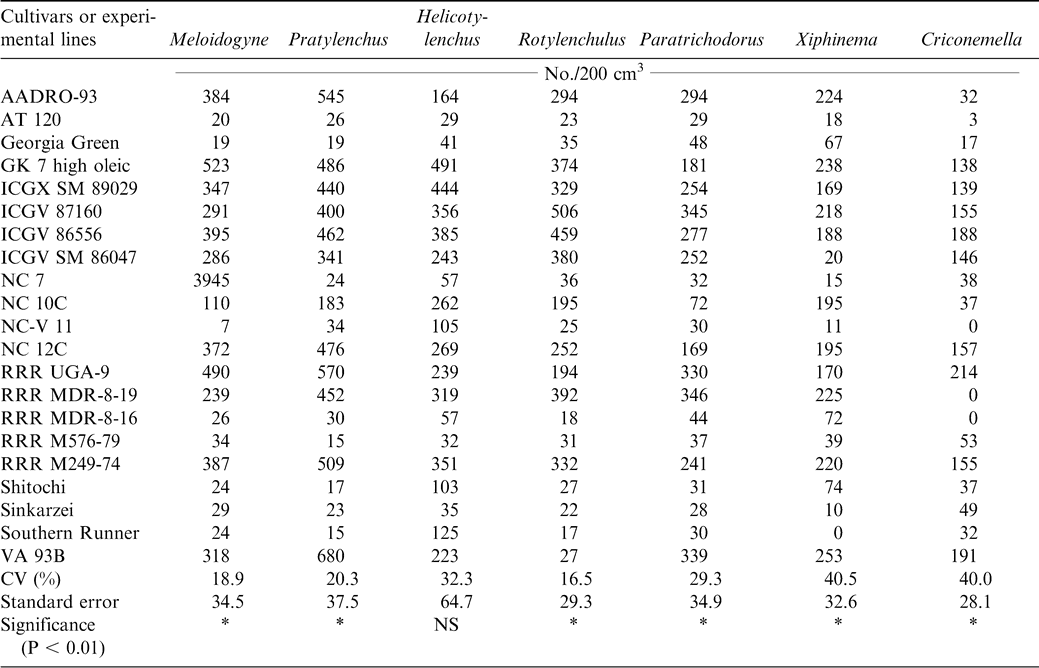
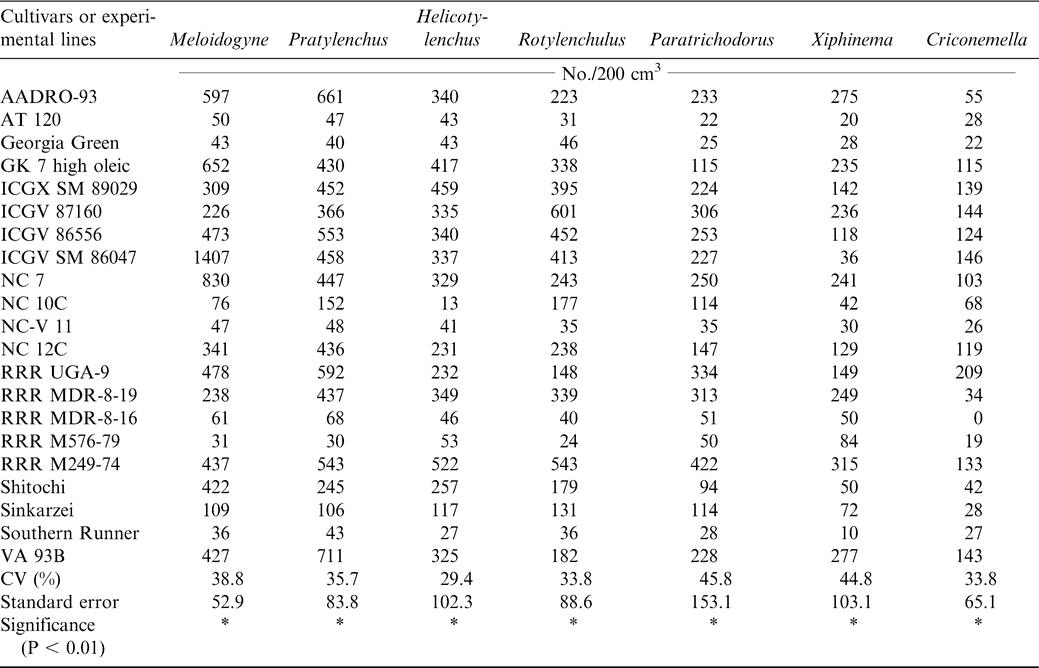
At harvest, lesion nematode (Pratylenchus brachyurus), peanut root-knot nematode (Meloidogyne arenaria), reniform nematode (Rotylenchulus reniformis), dagger nematode (Xiphinema elongatum), ring nematode (Criconemella ornata, Paratrichodorus minor), and spiral nematode (Helicotylenchus multicintus) were extracted from the peanut rhizosphere. Three endoparasitic nematodes, Pratylenchus brachyurus, Meloidogyne arenaria, and Rotylenchulus reniformis, were isolated from peanut roots (Table 7). The cultivars or experimental lines ICGX-SM 87057, AT 120, NC-V 11, Southern Runner, RRR M 576-79, RRR - MDR-8-16, Georgia Green, and Shitaochi had lower nematode populations at harvest during 2000 compared with the other plant entries (Table 7). The experiment was repeated the following year (2001) and all the eleven cultivars identified the previous year except ICGX-SM 87057 and Shitaochi had lower nematode populations (Table 8).
Host plant resistance represents the inherent ability of crop plants to restrict, retard or overcome pest infestations (Kumar, 1984) and thereby, improve the yield and /or quality of the harvestable crop product. Resistant cultivars are economical and environmentally safe method for control of root knot nematodes (Dent, 1990; Netscher and Mauboussin, 1973; Roberts and Thomason, 1986), although races may exist which are able to break resistance (Fargette, 1987). Additional research under a wider range of edaphic and environmental conditions is needed to determine nematode resistance under production systems in Ghana.
Acknowledgements
This research was supported by funds administered through the Peanut CRSP, USAID grant no. LAG-G-00-96-90013-00.
Literature Cited
Alabama Cooperative Extension Services (ACES) 1993 Alabama pesticide handbook. Circular ANR 500 Auburn University Auburn, Alabama .
Anon 1975 Descriptions of Plant Parasitic Nematodes Commonwealth Institute of Helminthology St. Albans, Herts, England .
Anon 2000 Protection of stratospheric ozone: incorporation of Clean Air Act Amendments for reductions in Class I, Group VI controlled substances. Fed. Reg 65 : 70795 – 804 .
Bos W.S. 1977 A preliminary report on the distribution and host range of the nematode Aphelenchoides arachidis in the North of Nigeria. Samaru Agricultural Newsletter 19 : 21 – 23 .
CIH 1975 Descriptions of Plant Parasitic Nematodes Commonwealth Institute of Helminthology St. Albans, Herts, England .
Debrah S.K. and Waliyar F. 1996 Groundnut production and Utilization in western Africa: past trends; projections and opportunities for increased production. Paper delivered at the fifth Regional Groundnut Workshop for West and Central Africa, 18-21 Nov, 1996. Accra, Ghana.
Dent D. 1990 Host Plant Resistance. Insect Pest Management. 213 – 294 C. A. B. International Wallingford, U.K 604 .
FAO 1982 Breeding for durable disease and pest resistance. FAO Plant Production and Protection Paper No. 55: 167.
Fargette M. 1987 Use of the esterase phenotype in taxonomy of the genus Meloidogyne. 2. Esterase phenotypes in West African populations and their characterization. Revue de Nematologie 10 : 45 – 55 .
Hammons R.O. 1982 Origin and early history of the peanut. 1 – 20 IN Patte H.E. and Young C.T. eds. Peanut Science and Technology American Peanut Research and Education Society Yoakun, TX.
Hunt D.J. 1998 Rhigonematidae from the Indo-Malayan region: Two new species of Rhigonema Cobb, 1898 (Nematoda: Rhigonematida). International Journal of Nematology 8 / 1 : 33 – 39 .
Jackson E.E. and Sturgen R.V. 1973 Effects of nematicides upon rootknot lesion nematode populations. Journal of the American Peanut Research and Education Association 5 : 178 – 181 .
Khan A.A. and Khan M.W. 1985 Root-knot nematodes infecting some common weeds in vegetable fields of western Uttah Pradesh, India. International Nematological Network Newsletter 2 / 4 : 15 – 16 .
Kumar R. 1984 Insect Pest Control with Special Reference to African Agriculture Edward Arnold London .
Merny G. , Fortuner R. , and Luc M. 1974 Plant parasitic nematodes in Gambia. Agronomic Tropicale, Nogent 29 : 702 – 707 .
Minton N.A. and Baujard P. 1993 Nematode Parasites of Peanut. 285 IN Plant Parasitic Nematodes in Subtropical and Tropical Agriculture Cambrian Printers, Ltd Aberystwyth .
Netscher C. and Mauboussin J.C. 1973 Résultats d'un essai concernant l'efficacite comparée d'une variéte résistance et de certains nématicides contre Meloidogyne javanica. Série Biologie 21 : 97 – 102 .
Netscher C. 1975 Studies on the resistance of groundnut to Meloidogyne spp. Série Biologie 10 : 227 – 232 .
Oudejans J.N. 1991 Pesticide Toxicity and Residues. In Agro-pesticides, properties and functions in integrated crop protection 68 United Nations Bangkok .
Patel H.R. , Vaishnao M.U. , and Dhruj I.U. 1985 Interaction of Meloidogyne arenaria and Fusarium solani on groundnut. Indian Journal of Nematology 15 : 98 – 99 .
Roberts P.A. and Thomason I.J. 1986 Variability in reproduction of isolates of Meloidogyne incognita and M. javanica on resistant tomato genotypes. Plant Disease 70 : 547 – 551 .
Rodríguez-Kàbana R. , Kokalis-Burelle N. , Robertson D.G. , King P.S. , and Wells L.W. 1994 Rotations with Coastal Burmudagrass, Cotton and Bahiagrass for Management of Meloidogyne arenaria and Southern Blight in Peanut. Journal of Nematology 26 / 4 : 665 – 667 .
Saka V.W. and Carter C.C. 1987 Hosts and non-hosts of root-knot nematode Meloidogyne incognita. Crop Nematode Research and Control Project 6 – 53 .
Salifu A.B. 1996 Status of groundnut entomofauna in Northern Ghana. Paper delivered at the fifth Regional Groundnut Workshop for West and Central Africa, 18-21 Nov, 1996. Accra, Ghana.
Sasser J.N. and Freckman D. 1987 A world perspective on Nematology. The role of the society. 7 – 14 IN Veech J.A. and Dickson D.W. eds. Vistas on Nematology Hyattsville, M.D., USA Society of Nematologists .
Schouten A.J. and M K.K. 1991 A comparative study on the efficiency of extraction methods for nematodes from different forest litters. Pedobiologia 35 : 393 – 400 .
Sharma S.B. 1985 A world list of nematode pathogens associated with chickpea, groundnut, pearl millet, pigeon pea and sorghum. Pulse Pathology Progress Report 42. International Crops Research Institute for the Semi-Arid Tropics 5 – 8 .
Southey J.F. 1986 Laboratory methods for work with plant and soil nematodes Ministry of Agriculture, Fisheries and Food. Reference Book 402 London, UK .
Taylor A.L. and Sasser J.N. 1978 Biology, identification and control of root-knot nematodes (Meloidogyne species). A Cooperative publication of the Department of Plant Pathology, North Carolina State University and USAID. North Carolina State University Graphics. 111 .
Thomas W.B. 1996 Methyl bromide: effective pest management tool and environmental threat. Supplement to the Journal of Nematology 28 : 586 – 590 .
Zhang Y. 1985 Occurrence and control of peanut root-knot diseases in non-irrigated Slopin.g fields of Zhangjiang District. Agricultural Science of Guamgdong Province 6 : 48 – 49 .