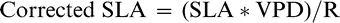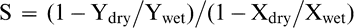Introduction
Water scarcity is a production problem faced by most peanut growers worldwide. Peanut (Arachis hypogaea L.) originates from South America (Hammons, 1982) and is adapted to warm temperatures and long growing seasons. Therefore, peanut is grown almost exclusively in regions prone to extended, or at least partial, periods of drought during the growing season. Irrigation has proven to be a boon to producers, increasing yields by as much as 19% (Lamb et al., 1997), but is often either severely limited or non-existent for many growers. Therefore, research in peanut production needs to be conducted concerning: 1) the sustainable use of limited irrigation; and 2) the development of genotypes that are best adapted to these conditions, particularly those peanut genotypes that are highly water use efficient. However, increased water use efficiency must accompany the maintenance of high yield to have any utility in agricultural systems. The intrinsic physiology of peanut matches this criteria; peanut has the potential to have very high photosynthetic capacity accompanied by low stomatal conductance levels, translating into high water use efficiency without sacrificing carbon assimilation and possibly yield (Wright et al., 1993).
Physiological water use efficiency (WUE) is defined as the ratio of photosynthesis to transpiration and is often a limitation to peanut productivity under drought (Nageswara Rao and Wright, 1994). However, determining the variability in WUE among peanut genotypes or pinpointing the production conditions that significantly affect WUE can be extremely problematic due to the complexity and tedious nature of direct measures of WUE (Wright et al., 1993). Traditional procedures of measuring WUE require weighing lysimeters and accurate measurements of water applied throughout the growing season (Wright et al., 1988; Hatfield et al., 1989). In the last two decades, extensive research has identified an accurate surrogate for WUE in peanut through the measurement of natural δ13C composition in plant tissue. The isotopic discrimination of 13C that occurs during the photosynthetic pathway in C3 plants (Farquhar et al., 1982; Farquhar and Richards, 1984) is well correlated with WUE in peanut and provides a long-term measurement of WUE across the season (Wright et al., 1993; Nageswara Rao and Wright, 1994). Water-use efficiency is positively correlated with δ13C composition (i.e. the higher the δ13C composition, the higher the WUE).
While extensive information documenting varietal differences in δ13C content (and thus WUE) have been conducted in Australia, almost nothing is known about the variability in isotopic composition among genotypes utilized in U.S. peanut producing regions. Surveys of the genetic variation in δ13C are needed for U.S. peanut genotypes, and the role that environment and production practices, particularly irrigation, play in changing the expression of these genetic differences needs to be quantified. This type of information is invaluable for developing more water use efficient varieties adapted to the U.S. production environment.
Additional information is needed about the interaction of δ13C with other leaf phenotypic characteristics, particularly δ15N. Theoretical analyses and field data suggest that WUE may interact with nitrogen fixation and other nitrogen nutrition effects (Schulze et al., 1991; Guehl et al., 1998). The overriding pattern appears to be a positive correlation between δ13C and δ15N, such that N2 fixation (low δ15N values) is associated with reduced water-use efficiency (more negative δ13C) (Schulze et al., 1991; Handley et al., 1994; Knight et al., 1993). Therefore, in studies examining δ13C in a leguminous crop, it is important to likewise examine the relationship between δ13C and δ15N and the implications for nitrogen fixation. Lastly, the relationship of δ13C with the leaf characteristics SPAD chlorophyll content and specific leaf area (SLA) would be important to evaluate because the characteristics are being used in other breeding programs and could potentially be screening tools for WUE (Nageswara Rao and Wright, 1994; Nageswara Rao et al., 1995). None of this information is available for U.S. peanut cultivars or developing breeding lines.
This experiment was conducted in order to document genetic variation and the effect of irrigation environment on δ13C, δ15N, and other leaf characteristics in U.S. grown peanut genotypes and to quantify the relationship between δ13C, SLA, SPAD, and δ15N. The specific experimental objectives were: 1) to determine if genetic variability existed in δ13C, and δ15N SLA, SPAD among three commonly grown U.S. peanut genotypes; 2) to determine if differing irrigation levels affected the pattern of variability; and 3) to quantify the relationship between δ13C and several leaf phenotypic characteristics.
Materials and Methods
Field Site
The experiment was conducted during 2001 and 2002 at the USDA-ARS Multi-crop Research Farm in Shellman, Georgia, U.S.A. The soil was a Greenville fine sandy loam (fine, kaolinitic, thermic Rhodic Kandiudults) with 0-2% slope. Conventional tillage practices were followed and included: disking, subsoiling, moldboard plowing, field cultivating, rototilling, and planting. Two side by side experimental fields were established at this research farm, one ca. 146 m × 329 m with overhead (OH) irrigation treatments, and one ca. 49 m × 329 m as the non-irrigated (NI) treatment. Each field was divided into 16.5 m × 36.6 m plots containing 18 cropping rows and 2 border rows each containing randomized crop rotations and replications. The field was established in 2001 and followed a fallow history; in 2002, the three peanut replications sampled were following planted cotton in 2001.
The lateral overhead irrigation system contained three equal length spans with approximately 13 nozzles each. To apply differential levels of water to each treatment plot, different sized sprinkler heads were placed on each drop nozzle within a span, such that one span applied a full rate of desired irrigation (100%), the second span applied 66% of this amount, and the third span applied 33% of this amount. Overhead sprinkler irrigation amount and timing were determined by the Irrigator Pro expert system (Lamb et al., 1993; Davidson et al., 1998) based on soil temperature measurements collected in the 100% treatment. Time between irrigation events was typically three to four days during the most active crop growth period of the season. The NI (nonirrigated) block received only rainfall precipitation.
Plant Collection
During the 2001 and 2002 growing seasons, plant samples from three peanut genotypes (cvs. Georgia Green, C99R, and AT201) were taken in four water treatments (100%, 66%, 33%, NI) and three replications. The following traits were measured in both years: δ13C, δ15N, percent carbon (%C), percent nitrogen (%N), SLA, and SPAD chlorophyll content. Leaf samples were collected from six plants spaced along two rows per replication. Sampling was completed in a single day and within the morning hours (800 – 1200). In both years, peanut leaf tissue was collected approximately 90 days after planting. This phenological period is associated with the highest ribulose bisphosphate carboxylase (rubisco) levels and concomitantly the highest photosynthetic levels of the season. Sampling during this time period ensures varietal differences in photosynthesis (and therefore WUE) would be most evident (Nageswara Rao and Wright, 1994; Nageswara Rao et al., 1995). Tissue collection was standardized to second nodal apex leaves that had relatively no insect or disease damage. Standardizing to the second nodal leaf position has been shown to maximize the relationship between chlorophyll content and specific leaf area (Nageswara Rao et al., 2001). Tetrafoliate leaves were excised and chlorophyll content was measured using the Minolta SPAD (Soil-Plant Analyses Development Unit, Minolta Corp., Ramsey, N.J., U.S.A.) chlorophyll meter directly after removal from the plant. The SPAD chlorophyll meter measures absorbance by plant tissues of wavelengths in the visible spectrum and serves as a measure of the relative internal concentration of chlorophylls a and b. One SPAD chlorophyll reading was taken on each of the four leaflets, avoiding the midrib, and then averaged for one chlorophyll reading per plant to correct for possible non-homogeneous distribution of chlorophyll throughout the leaf (Monje and Bugbee, 1992). Tetrafoliate leaves were then placed on ice and refrigerated at 4 C until further analysis.
Leaves were taken back to the laboratory and hydrated in distilled water for at least three hours prior to leaf area measurement in order to bring them all to a standardized turgor level (Nageswara Rao et al., 2001). Leaflets were removed from each petiole and the leaf area of the four leaflets was measured with an LI-3000A leaf area meter (LI-COR Inc., Lincoln, NE, U.S.A.) and summed to give total leaf area. Leaves were then oven dried at 60 C for 72 hours and weighed. Specific leaf area (SLA) was calculated as the ratio of leaf area to leaf dry weight. Leaves were then fine ground using a Braun ® (model KSM2) coffee grinder and analyzed for carbon isotope composition (δ13C), δ15N, %C, and %N.
Corrections for vapor pressure deficit (VPD) on SLA were applied following Nageswara Rao et al. (2001); but by replacing the measurement of “prevailing VPD” by logged VPD and incident radiation (R) measured on 15-minute intervals using HOBO ® (Onset Computer Corporation, Bourne, MA, U.S.A.) dataloggers throughout the day prior to tissue sampling. VPD and R values were averaged between the hours of 900 and 1600 (those hours that correspond with the majority of photosynthetic activity of the peanut plant), and the SLA correction from Nageswara Rao et al. (2001) was applied as follows:
This formula is corrected from the original published value (Nageswara Rao, personal communication).
In order to determine the isotopic composition in the peanut samples, leaf tissue was analyzed at the University of Arkansas Stable Isotope Laboratory in 2001 and at the Colorado Plateau Stable Isotope Laboratory, Department of Biological Sciences, Northern Arizona University in 2002. Samples of the ground leaves (2 mg, +/- 0.2 mg) were weighed, sealed in capsules and, along with standards, loaded into the elemental analyzer autosampler (a “Zero Blank” autosampler from Costech Analytical Technologies in Valencia, CA). Samples and standards were combusted in the elemental analyzer (Carlo Erba NC2500 elemental analyzer coupled with a Thermoquest Finnigan Delta plus isotope ratio mass spectrometer). Lab standards, which were calibrated against internationally distributed isotope standards, were analyzed at regular intervals throughout the sample runs. The resulting N2 and CO2 gases (along with isotopic reference gases for N2 and CO2) were admitted to the mass spectrometer via Finnigan's Conflo II interface. Data were collected and processed by Finnigan's Isodat software. Sample results are based on one analysis per sample (δ13C, δ15N,%N and %C were all determined with the same analysis). Isotope results are reported in delta notation vs. Air (for nitrogen) and vs. PDB (for carbon) in permil. Stable carbon isotope composition was expressed as δ13C where δ13C (‰) = [(R sample/R standard)-1] × 1000, and where R is the 13C/12C ratio. Composition of 13C/12C (δ13C) rather than discrimination of 13C (δ) is reported due to the possible differences in atmospheric components linked to natural or man-made C emissions between seasons.
Harvest and Yield Determination
Peanuts were dug using a two-row peanut inverter (Kelly Manufacturing Co., Inc., Tifton, GA, USA). They were allowed to dry in the windrow for approximately 3 days and then harvested with a two row peanut combine (Amadus Industries, Inc., Suffolk, VA). Samples were returned to the laboratory and dried using air and/or heat to less than 10.5% kernel moisture content and weighed. Net pod weight was calculated by deducting the weight of foreign material and excess moisture beyond 7%.
Statistical Analyses
Statistical analyses were performed using JMP SAS (SAS 1997) and SAS (Version 8). Factorial analysis of variance (ANOVA) was used to determine the effect of year, genotype, irrigation, and all possible interactions; the factors of replication and plant nested within replication were also included in the model. Differences among multiple levels of a given factor were determined using a Tukey's HSD multiple comparisons test. Pearson product-moment correlations were used to determine the relationship between δ13C, yield, and leaf phenotypic characteristics.
A drought susceptibility index (S) was calculated for each measured trait following Fischer and Maurer (1978) as presented in Peleg et al. (2005) and expressed as:
where Ydry and Ywet are the average values of a given peanut genotype under dryland and irrigated (averaged across water levels) treatments, respectively, and Xdry and Xwet are the mean values of all genotypes under these treatments, respectively.
Results
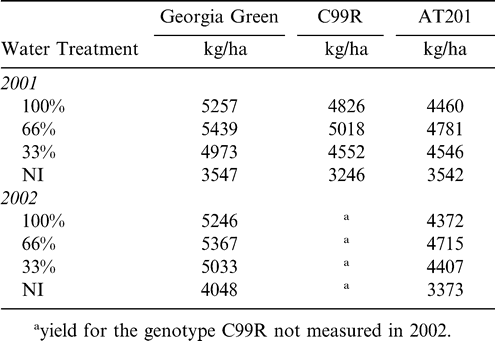
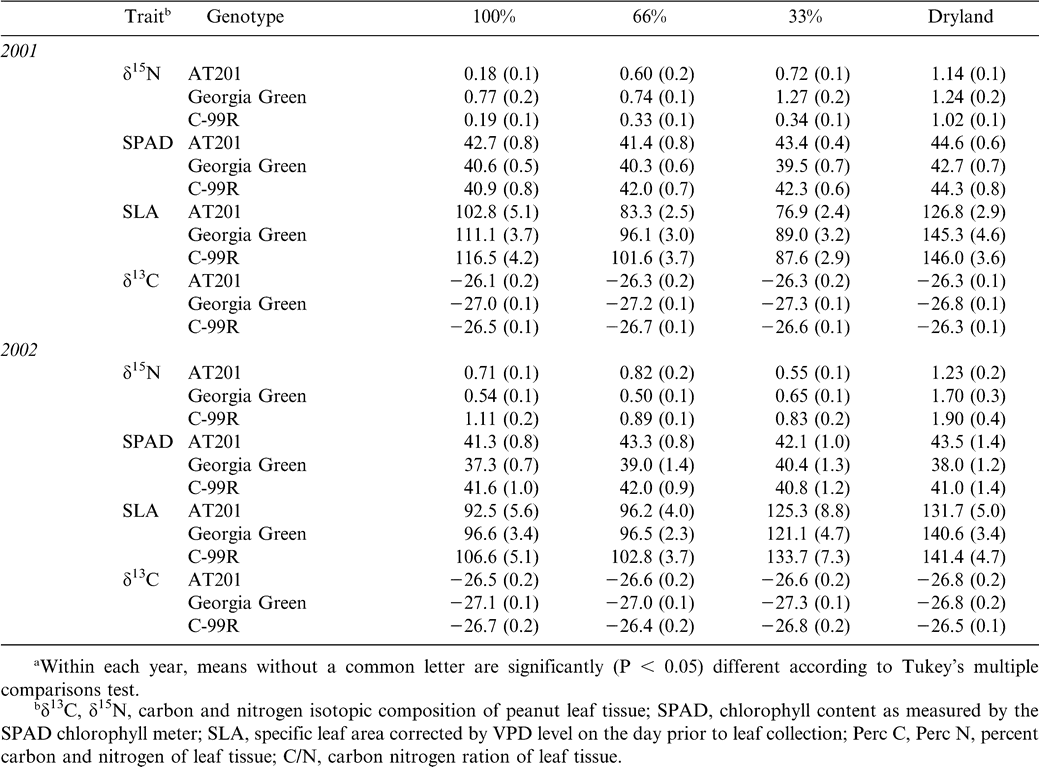
Total water received within each water treatment ranged from 711 and 627 mm ha−1 in 2001 and 2002, respectively, for the 100% irrigation treatment and 528 and 43 mm ha−1 in 2001 and 2002, respectively, for the non-irrigated treatment (Table 1). Water treatment affected peanut yield, with no significant differences between 2001 and 2002. Across years, there were significant differences in yield for peanut genotype (df = 2, F Ratio = 5.9, P value = 0.0048), with Georgia Green having significantly higher yields than the other two genotypes. In addition, irrigation treatment significantly affected yield across genotypes (df = 3, F Ratio = 18.4, P value = 0.0001) such that the NI treatment yields were significantly lower than any of the irrigated treatments (33%, 66%, or 100%; Table 2). In both years the yield in the 100% treatment was numerically lower than the 66% treatment, indicating possible over-watering in the former treatment.
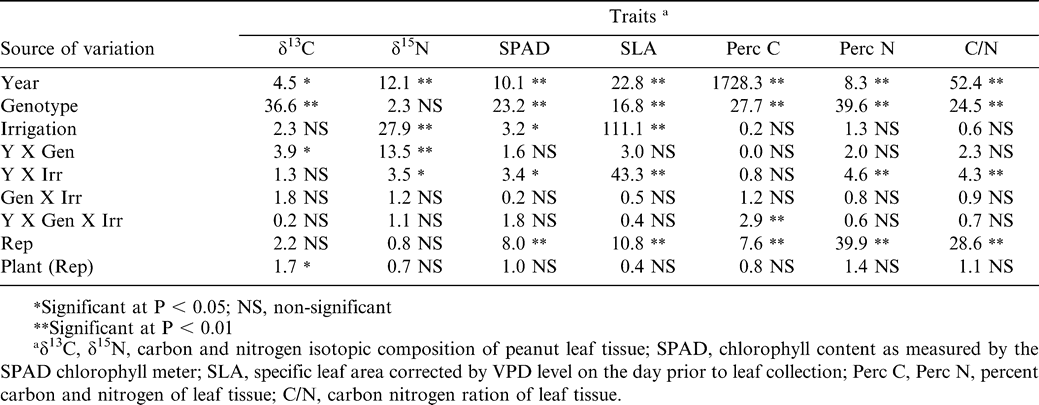
Significant differences in δ13C, δ15N, SPAD chlorophyll content, SLA, %C, %N, and C to N ratio were found between years, and differences existed in all traits except δ15N among genotypes (Table 3). Irrigation effects were less apparent with differences existing only for δ15N, SPAD chlorophyll, and SLA. Year by genotype interactions were significant for both δ13C and δ15N, while year by irrigation interactions were significant for δ15N, SPAD, specific leaf area, %N, and C to N ratio. There was no significant genotype by irrigation effect. The three-way interaction among year, genotype, and irrigation was significant only for %C (Table 3).
Mean δ13C was significantly lower (more negative) in 2002 than in 2001; while mean δ15N, specific leaf area, and %N were greater in 2002 (Table 4). Multiple comparisons tests revealed that the significant differences among genotypes for all traits except δ15N were due to the cultivar Georgia Green having significantly lower δ13C and SPAD chlorophyll content than the other two genotypes and lower %N than the cultivar C99R; while SLA and %C for Georgia Green were significantly greater than for the cultivar AT201. The significant effects of irrigation treatment revealed higher values of δ15N and SLA in the NI treatment as compared to the other three irrigation levels, and higher SPAD chlorophyll content in the NI treatment in comparison to the 100% irrigation level. For mean SLA values, the 66% irrigated treatment had the lowest SLA in comparison to the other three treatments (Table 4).
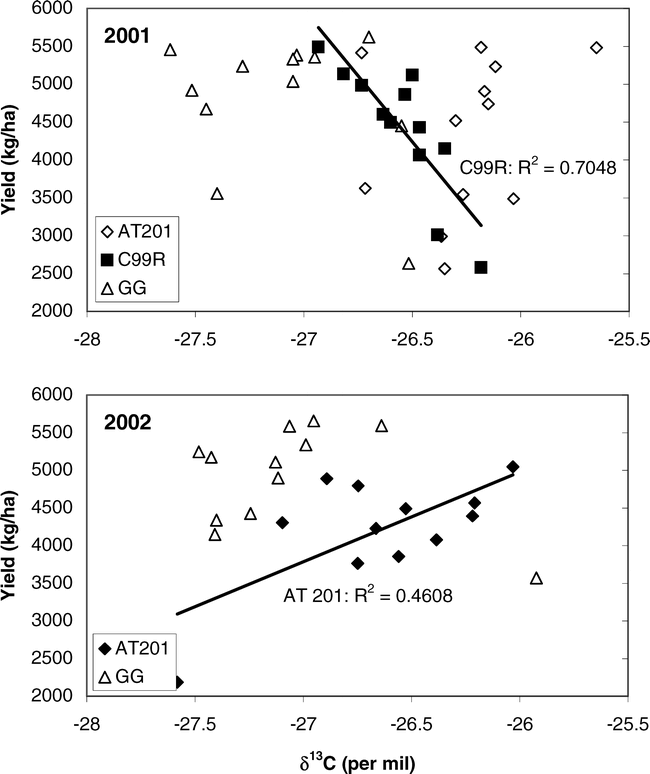
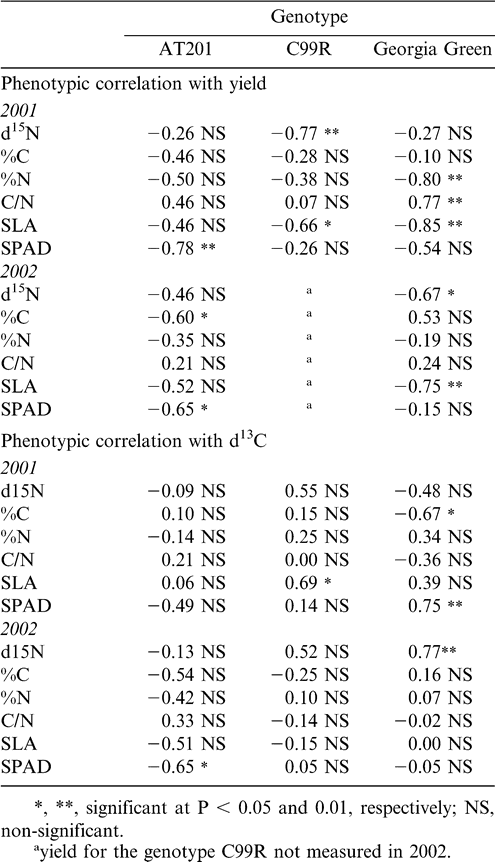
The relationship between δ13C and peanut yield (across irrigation treatments) is shown in Figure 1 for 2001 and 2002. In 2001, the correlation between δ13C and peanut yield was only significant for the cultivar C99R, which showed a negative correlation with yield as δ13C increased (became less negative). In 2002, yield data on C99R was unavailable; however, the correlation between δ13C and yield was significant for the cultivar AT201, but in this case, was a positive relationship such that δ13C increased (became less negative) with increasing yield. There were limited significant correlations with yield for other phenotypic traits (Table 5). For the cultivar AT201, SPAD chlorophyll content in 2001 and 2002 was significantly negatively correlated. None of the other cultivars showed such a relationship. Both C99R and Georgia Green had significant negative correlations of yield with SLA in 2001 and 2002 (with the exception of no measured yield for C99R in 2002). C99R further showed a negative relationship with yield for δ15N; while Georgia Green had a negative and positive relationship with yield for %N and C/N, respectively.
The phenotypic correlations between δ13C and the other measured traits were equally sparse (Table 5). In 2001, significant positive relationships between δ13C and SLA for C99R and SPAD chlorophyll for Georgia Green were noted. For Georgia Green in this same year, %C was negatively associated with δ13C. In 2002, SPAD was negatively correlated with δ13C for AT201, while Georgia Green showed a positive correlation between δ13C and δ15N. There were no significant correlations between δ13C and the other phenotypic traits for the cultivar C99R in this same year.
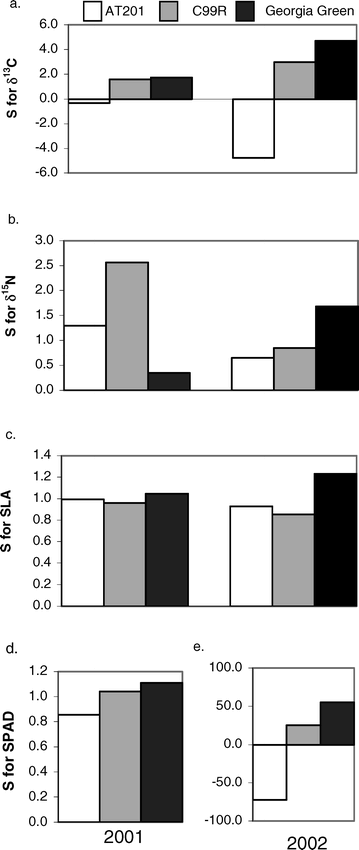
The drought susceptibility index (S) showed interesting differences among cultivars for the various phenotypic traits in both 2001 and 2002 (Figure 2). Overall and with few exceptions, the cultivar AT201 exhibited an advantage (lower values) in the drought susceptibility index in comparison to the other genotypes for δ13C, δ15N, SLA, and SPAD for both years. On the other hand, the cultivar Georgia Green (with the exception of its S value for δ15N in 2001) exhibited a disadvantage in drought tolerance with its consistently higher S values for the four traits measured. The cultivar C99R primarily exhibited intermediate S values between the cultivars AT201 and Georgia Green for the four traits.
Discussion
Significant variation in isotopic composition and related leaf phenotypic characteristics was found for three Arachis genotypes that are currently utilized in the southeastern U.S. production regions. This finding supports the possibility of selecting for U.S. genotypes with increased δ13C (and thus WUE) in programs aimed at increasing the efficiency of peanut production under water scarce environments. These results also concur with previous studies that found genotypic variation in δ13C for peanut in greenhouse (Hubick et al., 1986) and field conditions (Nageswara Rao et al., 1993). In peanut, variation in δ13C appears to be due to variability among genotypes in their photosynthetic capacity and not stomatal factors (Nageswara Rao et al., 1995; Nageswara Rao et al., 2001). The δ13C content of leaf tissue can be controlled either through an intrinsic photosynthetic capacity (capacitance types) or through stomatal diffusive properties (conductance types) (Udayakumar et al., 1998). In conductance type plants, high δ13C concentrations, and thus high WUE, is at the expense of dry matter production and yield. In contrast, peanut is a capacitance type plant that has the ability to concentrate δ13C in its tissues without stomatal closure, thereby making it possible to select for high δ13C and high WUE while maintaining high growth rates and yield (Udayakumar et al., 1998). Mean differences among genotypes in this study revealed that the cv. Georgia Green had significantly lower δ13C, and thus WUE, in comparison to the other two genotypes.
While the current results agree with findings of variability in δ13C among peanut genotypes, they counter many studies examining irrigation effects on δ13C in other crop species. What was surprising in this study was the lack of effect of irrigation on the expression of δ13C, which is contrary to other studies in crops such as wheat, cotton, (Yakir et al., 1990; Saranga et al., 1998; Monneveux et al., 2005; Merah et al., 1999) and even loblolly pine (Choi et al., 2005). In these studies, δ13C was increased under non-irrigated or drought stressed conditions, indicating that WUE increases under water scarcity. In the current study, the only significant environmental effects on δ13C were reflected through the significant effect of year, indicating the climatic variation between 2001 and 2002 had an effect on δ13C. Further, the existence of a significant G X E interaction between genotype and year for δ13C (Table 3) indicated that the genetic effects on δ13C were strong, but environment played a role as well. Evidence for G X E interactions for WUE in peanut is scarce (Hubick, 1990; Nageswara Rao and Wright, 1994). A few previous studies in peanut documented that variation in δ13C was influenced by location and genotype (Nageswara Rao and Wright, 1994; Brown and Byrd, 1996); while Wright et al. (1988) found strong genetic control over δ13C with little effect of the environment in four peanut genotypes when grown either in open or closed canopies.
There were significant genetic and environmental effects on the other phenotypic traits measured. Genetic variation was strong for all the leaf characteristics except δ15N; in addition, significant variation among years was present indicating an environmental component to control of the expression of these traits. But in this instance, irrigation environment also had a significant role in determining the variability in δ15N, SPAD, and SLA. Irrigation, in general, tended to decrease δ15N, SPAD, and SLA. For δ15N, these results are in line with the study of Handley et al. (1999) that found the natural abundance of δ15N in leaf tissue decreased with increasing soil moisture and therefore, was reflective of water availability at a given site. Our results for SLA, however, are contrary to most studies. Decreased SLA under limited irrigation is a common finding in many drought studies (Marcelis et al., 1998), and may be a mechanism of increasing water use efficiency through the concentration of chlorophyll and proteins in thick leaves leading to greater photosynthetic capacity (Liu and Stutzel, 2004). The SPAD chlorophyll content results in this study do show that the highest chlorophyll concentrations are in leaves in the NI treatment, but are concentrated in thinner leaves in comparison to the irrigated treatments. Given the low SLA in the irrigated treatments and the lack of irrigation effect on δ13C, severe drought stress was likely not present in either 2001 or 2002.
The relationship between δ13C and yield was complicated for the genotypes tested and appeared to vary among the cultivars. The cultivar Georgia Green showed no significant correlation with δ13C and yield in either 2001 or 2002. A reduced correlation between δ13C and yield in wheat has been linked to an absence of variability in δ13C. This can be explained by increased photosynthetic capacity being offset by increases in stomatal aperture, leading to lower variation in δ13C and reduced correlation between δ13C and yield (Monneveux et al., 2005). This may indicate that Georgia Green tends to be a conductance physiological type instead of a capacitance type. The high relative drought susceptibility index values (S) for Georgia Green for the trait δ13C also indicate that this genotype is more drought susceptible, and indeed its mean δ13C values signify lower WUE than the other genotypes. The cultivar C99R showed a negative correlation with δ13C and yield, indicating that this genotype may also be acting as a conductance type, where δ13C (and thus WUE) is controlled predominantly through stomatal aperture. In the case of C99R, WUE increases only through stomatal closure and so an increase in WUE brings a concomitant decrease in yield. On the other hand, the genotype AT201 appears to be acting as a capacitance type where increased δ13C (and thus WUE) is correlated positively with yield in 2004, likely due to an increase in photosynthetic capacity. The low drought tolerance index values for δ13C also indicate that AT201 may be more water use efficient than the other genotypes, possibly through increased photosynthetic capacity.
The relationships between δ13C and yield with the other leaf phenotypic characters were extremely limited. Very few traits correlated directly with yield, with the exception of nitrogen related traits (δ15N, and %N) for Georgia Green. Specific leaf area was the most consistent correlation with yield for genotypes and years, indicating that thicker leaves were correlated with higher yields. The relationships with δ13C and leaf phenotypic characters were nearly nonexistent. In 2002, the relationship between δ15N with δ13C was significantly positive for Georgia Green. Very few studies have related δ15N with δ13C, and those that have, show the relationship to be affected by environment or nonexistent (Guehl et al., 1998). The paucity of significant correlations between δ13C and SPAD or SLA is unfortunate. In Australia and India, research has confirmed the relationship between SLA and SPAD, two very easily measured characters, with carbon isotope discrimination (Wright et al., 1993; Nageswara Rao and Wright, 1994; Nageswara Rao et al., 1995). This relationship has been purported as a useful, inexpensive, and easily utilized tool to screen large numbers of breeding lines for water use efficiency. However, this study has failed to show a consistent relationship between the two traits in a field setting for these three peanut cultivars. Nageswara Rao et al. (2001) also showed a link between SLA and SPAD chlorophyll content, and it was assumed that because δ13C and SLA are related, δ13C and SPAD would be related as well. However, in this study, the correlation between δ13C and SPAD was significant only for Georgia Green in 2001 and AT201 in 2002, with the direction of the correlation being inconsistent between cultivars. Overall, it appears that the utility of SLA and SPAD chlorophyll for WUE screening tools in U.S. peanut genotypes in a field environment is limited because of the weak correlations between δ13C and SLA or SPAD.
In conclusion, this study has documented variation in δ13C, δ15N, and other leaf phenotypic characteristics among currently grown U.S. peanut genotypes. Based on the copious number of studies documenting the plasticity of δ13C in other crop species, it is surprising that there was no significant effect of irrigation on the trait in this study. It might be argued that the crop received adequate water through precipitation regardless of supplemental irrigation level in both years, thus negating the effect of irrigation on δ13C. However, the differences in yields among the irrigation levels in both 2003 and 2004 somewhat refute this explanation. Perhaps the explanation lies in a relatively strong genetic control for δ13C in peanut as compared to other crops, making the success of breeding for increased WUE in peanut quite achievable.
Acknowledgements
We thank Kathy Gray who provided invaluable field collection, sample processing, expertise and, in general, made this research possible. We thank Latoya Rucker for sample preparation and Jessie Childre for plot establishment and maintenance.
Literature Cited
Brown R. H. and Byrd G. T. 1996 Transpiration efficiency, specific leaf weight, and mineral concentration in peanut and pearl millet. Crop Science 36 : 475 – 480 .
Choi W. J. , Chang S. X. , Allen H. L. , Kelting D. L. , and Ro H. M. 2005 Irrigation and fertilization effects on foliar and soil carbon and nitrogen isotope ratios in a loblolly pine stand. Forest Ecology and Management 213 : 90 – 101 .
Davidson J. I. , Griffin W. J. , Lamb M. C. , Williams R. G. , and Sullivan G. 1998 Validation of EXNUT for scheduling peanut irrigation in North Carolina. Peanut Science 25 : 50 – 58 .
Farquhar G. D. , O'Leary M. H. , and Berry J. A. 1982 On the relationship between carbon isotope discrimination and intercellular carbon dioxide concentration in leaves. Aust. J. Plant Physiol 9 : 121 – 137 .
Farquhar G. D. and Richards R. A. 1984 Isotopic composition of plant carbon correlates with water-use efficiency of wheat cultivars. Aust. J. Plant Physiol 11 : 539 – 552 .
Fischer R. A. and Maurer R. 1978 Drought resistance in spring wheat cultivars. I. Grain yield responses. Australian Journal of Agriculture Research 29 : 879 – 912 .
Guehl J. M. , Domenach A. M. , Bereau M. , Barigah T. S. , Casabiance H. , Ferhi A. , and Garbaye J. 1998 Functional diversity in an Amazonian rainforest of French Guyana: a dual isotope approach (δ15N and δ13C). Oecologia 116 : 316 – 330 .
Hammons R. O. 1982 Origin and early history of the peanut. 1 – 20 In Pattee H. E. and Young C. T. eds. Peanut Science and Technology American Peanut Research and Education Society Yoakum, TX, USA .
Handley L. L. , Austin A. T. , Robinson D. , Scrimgeour C. M. , Raven J. A. , Heaton T. H. E. , Schmidt S. , and Stewart G. R. 1999 The 15N natural abundance (δ15N) of ecosystem samples reflects measures of water availability. Aust. J. Plant Physiol 26 : 185 – 199 .
Handley L. L. , Odee D. , and Scrimgeour C. M. 1994 δ15N and δ13C patterns in savanna vegetation: dependence on water availability and disturbance. Functional Ecology 8 : 306 – 314 .
Hatfield P. M. , Wright G. C. , and Tapsall W. R. 1989 A large retractable, low cost and relocatable rainout shelter design. Exp. Agric 26 : 57 – 62 .
Hubick K. T. 1990 Effects of nitrogen source and water limiation on growth, transpiration efficiency and carbon-isotope discrimination in peanut cultivars. Aust. J. Plant physiol 17 : 413 – 430 .
Hubick K. T. , Farquhar G. D. , and Shorter R. 1986 Correlation between water-use efficiency and carbon isotope discrimination in diverse peanut (Arachis) germplasm. Australian Journal of Plant Physiology 13 : 803 – 816 .
Knight J. D. , Verhees F. , Van Kessel C. , and Slinkard A. E. 1993 Does carbon isotope discrimination correlate with biological nitrogen fixation? Plant and Soil 153 : 151 – 153 .
Lamb M. C. , Davidson J. I. , and Butts C. L. 1993 Peanut yield decline in the southeast and economically feasible solutions. Peanut Science 20 : 36 – 40 .
Lamb M. C. , Davidson J. I. , Childre J. W. , and Martin N. R. 1997 Comparison of peanut yield, quality, and net returns between nonirrigated and irrigated production. Peanut Science 24 : 97 – 101 .
Liu F. and Stutzel H. 2004 Biomass partitioning, specific leaf area, and water use efficiency of vegetable amaranth (Amaranthus spp.) in response to drought stress. Scientia Horticulturae 102 : 15 – 27 .
Marcelis L. F. M. , Heuvelink E. , and Goudriaan J. 1998 Modeling biomass production and yield of horticultural crops: a review. Scientia Horticulturae 74 : 83 – 111 .
Merah O. , Delèens E. , and Monneveux P. 1999 Grain yield, carbon isotope discrimination, mineral and silicon content in durum wheat under different precipitation regimes. Physiologia Plantarum 107 : 387 – 394 .
Monje O. A. and Bugbee B. 1992 Inherent limitations of nondestructive chlorophyll meters: a comparison of two types of meters. Hortscience 27 : 69 – 71 .
Monneveux P. , Reynolds M. P. , Trethowan R. , Gonzàlez-Santoyo H. , Pena R. J. , and Zapata F. 2005 Relationship between grain yield and carbon isotope discrimination in bread wheat under four water regimes. Europ. J. Agronomy 22 : 231 – 242 .
Nageswara Rao R. C. , Talwar H. S. , and Wright G. C. 2001 Rapid assessment of specific leaf area and leaf nitrogen in peanut (Arachis hypgaea L.) using a chlorophyll meter. J. Agronomy and Crop Science 186 : 175 – 182 .
Nageswara Rao R. C. , Udaykumar M. , Farquhar G. D. , Talwar H. S. , and Prasad T. G. 1995 Variation in carbon isotope discrimination and its relationship to specific leaf area and ribulose-1,5-bisphosphate carboxylase content in groundnut genotypes. Australian Journal of Plant Physiology 22 : 545 – 551 .
Nageswara Rao R. C. , Williams J. H. , Wadia K. D. R. , Hubick K. T. , and Farquhar G. D. 1993 Crop growth, water-use efficiency and carbon isotope discrimination in groundnut (Arachis hypogaea L.) genotypes under end-of season drought conditions. Ann. appl. Biol 122 : 357 – 367 .
Nageswara Rao R. C. and Wright G. C. 1994 Stability of the relationship between specific leaf area and carbon isotope discrimination across environments in peanut. Crop Science 34 : 98 – 103 .
Peleg Z. , Fahima T. , Abbo S. , Krugman T. , Nevo E. , Fakir D. , and Saranga Y. 2005 Genetic diversity for drought resistance in wild emmer wheat and its ecogeographical associations. Plant, Cell and Environment 28 : 176 – 191 .
Saranga Y. , Flash I. , and Yakir D. 1998 Variation in water-use efficiency and its relation to carbon isotope ratio in cotton. Crop Science 38 : 782 – 787 .
SAS 1997 JMP Statistical Discovery Software SAS Institute Inc Cary, NC, USA .
Schulze E-D. , Gebauer G. , Ziegler H. , and Lange O. L. 1991 Estimates of nitrogen fixation by trees on an aridity gradient in Namibia. Oecologia 88 : 451 – 455 .
Udayakumar M. , Sheshshayee M. S. , Nataraj K. N. , Bindu Madhava H. , Devendra R. , Aftab Hussain I. S. , and Prasad T. G. 1998 Why has breeding for water use efficiency not been successful? An analysis and alternate approach to exploit this trait for crop improvement. Current Science 74 : 994 – 1000 .
Wright G. C. , Hubick K. T. , and Farquhar G. D. 1988 Discrimination in carbon isotopes of leaves correlates with water-use efficiency of field-grown peanut cultivars. Aust. J. Plant Physiol 15 : 815 – 825 .
Wright G. C. , Hubick K. T. , Farquhar G. D. , and Nageswara Rao R. C. 1993 Genetic and environmental variation in transpiration efficiency and its correlation with carbon isotope discrimination and specific leaf area in peanut. 247 – 267 In Ehleringer J. R. , Hall A. E. , and Farquhar G. D. eds. Stable Isotopes and Plant Carbon-Water Relations Academic Press, Inc San Diego, CA, USA .
Yakir D. , DeNiro M. J. , and Ephrath J. E. 1990 Effects of water stress on oxygen, hydrogen and carbon isotope ratios in two species of cotton plants. Plant, Cell and Environment 13 : 949 – 955 .
Notes
- 1USDA-ARS, National Peanut Research Laboratory, Dawson, GA 39842; drowland@nprl.usda.gov [^]
- *Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. [^]