Introduction
Peanut (Arachis hypogaea L.) is an important component of human diets in Ghana and other countries in West Africa (Craufurd et al., 2006; Grosso and Guzman, 1995). Although peanut contributes to food security in Ghana and can serve as a cash crop, presence of Aspergillus flavus and A. parasiticus in raw products can result in aflatoxin contamination in food. Chronic exposure to aflatoxin contributes to poor health and a compromised immune system in vulnerable populations (Gong et al., 2012; Jolly et al., 2006; Kew, 2012; Turner et al., 2003; Williams et al., 2003). The aflatoxins B1, B2, G1, and G2 are classified as Group 1 human carcinogens (Ioannou-Kakouri et al., 1999). Over 25% of the world's food may be contaminated by mycotoxins and peanut is particularly prone to contamination (Eskola et al., 2019).
Aflatoxin contamination can occur and increase at all steps of the peanut supply chain including production in the field, drying, storage, and in food products (Awuah et al., 2006; Guchi, 2015; Malaker et al., 2008; Villers, 2014; Waliyar et al., 2015). The warm and humid environmental conditions in some areas of Africa are ideal for the growth of A. flavus, making aflatoxin contamination of food, including peanut, a consistent challenge (Gordon, 2003; Wagacha and Muthomi, 2008). The majority of smallholder farmers often are not able to prevent or mitigate aflatoxin along the value chain. Emmok (2010) estimated less than 20% of peanut produced in Ghana could be exported because of aflatoxin contamination. Consequently, the majority of peanut is consumed domestically, and is often contaminated with aflatoxin. Export of peanut produced in Africa has been reduced substantially during the past decade compared with the 1960s (Fana, 2010). Estimates are that the African continent loses annually 450-670 million US dollars in potential export revenues due to aflatoxins (IITA, 2013).
Downstream in the supply chain, peanut farmers miss market opportunities because of uncertainty regarding aflatoxin levels and other quality considerations. In addition to lost marketing opportunities, malnutrition and nutritional disorders have been linked to aflatoxin exposure (CDC, 2013). According to Kew (2012), a high percentage of people in sub-Saharan Africa and certain areas in Asia are exposed to chronic levels of aflatoxin.
Current aflatoxin mitigation programs have focused on controlling mold that produces aflatoxin in the field through improved management practices (Jordan et al., 2018; Villers, 2014). However, most post-harvest drying and storage conditions often are inadequate to maintain aflatoxin levels safe for consumers (Villers, 2014). Existing literature mostly has focused on aflatoxin mitigation at each step of the peanut value chain (practices in the field, during drying, and in storage). Comparisons of aflatoxin contamination traced from the field through storage using different mitigation approaches are limited. Comprehensive studies that analyze aflatoxin through each of these steps are important, especially for determining the monetary value of improved aflatoxin mitigation practices. The ability to ensure cool soil temperatures, rapid reduction of seed moisture content upon harvest, and minimizing moisture absorption during storage to maintain desirable organoleptic and physicochemical properties for marketing and final use of peanut are critical in developing optimal mitigation protocols (Bulaong and Dharmaputra, 2002; Ellis et al., 1991; Ramesh et al., 2013; Saleemullah et al., 2006). To determine the most vulnerable steps of aflatoxin contamination and compare improved mitigation practices with currently used practices by smallholder farmers, research was conducted in two rural villages in Ghana during 2016 and 2017.
Materials and Methods
The experiments were conducted near Drobonso (-1.121° W, 7.064°N) in the Sekyere Afram Plains District and near Ejura (-1.367° W, 7.383°N) in Ejura-Sekyedumasi district, both in the Ashanti Region of Ghana. Initially, the goal was to conduct the experiment during both the major season (April-July) and minor season (August-October) in both villages. Generally, bimodal rainfall is common in this region of Ghana and farmers often plant two crops of peanut each year. Major and minor seasons would have served as a treatment variable. However, rainfall at Ejura during the major season and at Drobonso during the minor season was inadequate for peanut production during 2016. Therefore, rather than considering major and minor seasons as a treatment variable, each run of the experiment was considered as a separate environment regardless of location or season. This resulted in having six experiments (Table 1). The cultivar Konkoma, grown by most farmers in this region of Ghana, was used in all experiments (Owusu-Akyaw et al., 2019).
Treatments consisted of different combinations of traditional farmer practices and the improved practices during the growing cycle up to harvest, after drying peanut pods to 10%, and storing peanut for 4 months. The improved practice included one additional hand weeding than the traditional farmer practice at 6 weeks after planting (WAP), the application of a locally-derived potash soap at 3 WAP (e.g. at initiation of flowering), and the application of ground oyster shells at the base of peanut plants at 180 kg/ha at 4 WAP. The farmer practice included only one hand weeding 3 WAP and no calcium or soap sprays. The improved practice for drying included drying peanut pods on a polyethylene tarp compared with the farmer practice of drying peanut on the soil surface. Farmers stored peanut in traditional poly bags (farmer practice) or in hermetically-sealed plastic bags (GrainPro, Inc., Boston, MA) for the improved practice. Each farmer served as a replication and 10 to 12 farmers were randomly selected in each experiment. Plot size was 20 rows with a length of 20 m and spaced 30 cm apart. Within each farmer's field, a plot with the farmer practice and a plot with the improved practice were included. Pod yield was determined from the center eight rows of each plot and final yield adjusted to 10% moisture. Pods from five plants randomly selected from each of five sections within a plot (total of 25 plants) were used for aflatoxin determination at harvest (Mahuku et al., 2010). Approximately 20 kg of unshelled pods were placed on tarps (Kotap America LTD, Lawrence, NY) and on soil for drying to 10% or less moisture prior to placing in poly bags or sealed bags for 4 months. Using sampling protocols by Codex Alimentarius Standard (2001), 2 kg of unshelled pods were collected by aggregating incremental weights of 150 g for aflatoxin determination at harvest, after drying, and then again after storage. Samples were transported in plastic zip lock bags on ice until placed in a freezer at -20°C until analysis.
Prior to preparation of samples to determine aflatoxin contamination after 4 months of storage, 100 g of kernels was removed to determine the percentage of kernels considered good for marketing. The inclusion criteria for good kernels were the following: free of shrivel, visible mold, and discoloration. This was achieved by dividing the weight of good kernels by the total weight of the sample (100 g). The final yield of good kernels/ha was then calculated as the product of pod yield and the fraction of good kernels.
Analysis of Aflatoxin Contamination
The entire sample of shelled peanut was used in the aflatoxin extraction procedure regardless of quality. Extraction and quantification of aflatoxin were based on the USDA-GIPSA 2013-041 protocol (USDA-GIPSA, 2015) using RevealQ+ aflatoxin lateral flow strips (Neogen Corp., Lansing, MI) for quantitative test with Mobile Diagnostic Reader (mReader™) (Mobile Assay Inc., Boulder, CO). A 2-kg sample of shelled peanut kernels was milled at mid to high speed using a blender (Preethi Mixer-Blender, Sholinganallur, Chennai, India) after which 10 g was weighed into a 50 ml extraction tube. Thirty ml of 65% ethanol was added to the sample and vortexed for 3 min. The mixture was allowed to settle and then filtered using a 0.45 μm micro filter paper. A volume of 500 μl of Reveal Q+ sample diluent was pipetted into a dilution cup after which 100 μl of sample extract was added. The resulting solution was mixed by pipetting up and down 5 times. A volume of 100 μl of diluted sample extract was pipetted into a new clear sample cup. The test strip was placed into the sample cup to make contact with the solution and allowed to wick. The strip was removed after 6 min and the level of toxin quantified using the Mobile Detection Reader. Aflatoxin levels greater than 50 μg/kg (threshold determination level for the lateral flow strips) were diluted and re-analyzed. Aflatoxin concentration was also determined using High Performance Liquid Chromatography (HPLC) based on the AOAC (Association of Official Agricultural Chemists Method 2005.08 (AOAC International, 2006). The minimal detection levels for the Mobile Diagnostic Reader and HPLC were 2 μg/kg and 0.5 μg/kg, respectively.
Financial Analysis
Base cost of production was set at $140/ha and included costs for land preparation, seed, planting, and one hand weeding. Additional cost for the improved practice during the growing cycle prior to harvest included the local soap for aphid and rosette suppression, one additional hand weeding, and oyster shells was set at $52/ha. Cost of tarps to dry peanut was set at $8/ha. The cost of a poly bag for the farmer practice storage was $0.5. Cost for a hermetically-sealed bag for the improved storage was $1.6. Total cost of storage was the product of individual bag cost and the number of bags required depending on yield. The two types of bags used on this experiment could hold 18 kg of unshelled peanut. The hermetically-sealed GrainPro bags are not available in Ghana and were provided from the US when the study was initiated. Therefore, the cost of sealed bags was based on locally-available PICS storage bags (Purdue Improved Crop Storage bags, West Lafayette, IN). Ibrahim et al. (2013) reported that both GrainPro and PICS bags performed similarly for cowpea (Vigna unguiculata). Data are either not available in the literature or experiments comparing effectiveness of these bags have not been conducted with peanut. Additional research is needed to support or refute our assumption of efficacy of these bags. Cost of removing pods from vines was set at $0.075/kg farmer stock. Shelling cost was set at $0.075/kg shelled peanut.
Peanut price was set at $1.2/kg for whole kernels that were not damaged (referred to as good kernels). The percentage of good kernels was determined based on an estimated 65% shell out from yield of unshelled pods. The percentage of good kernels was measured after storage when aflatoxin concentration was determined. Estimated financial returns were determined for each combination of the improved and farmer practices during the growing cycle prior to harvest and following both drying and storing (8 combinations) by subtracting the gross return (product of unshelled yield in the field, a 65% shell out rate, and percentage of kernels classified as good quality) minus costs of each combination of practices.
Peanut pod yield from plots were considerably higher than the national average and may reflect cooperators selected or land resources and site selection compared to constraints and capacities of most smallholder farmers (Mochiah, B., personal communication). Because the improved practices used in this research are considered expensive and generally fixed (tarps and bags), in a second financial analysis, yield potential was adjusted down by 50% to more effectively determine the monetary value of improved practices to the broader audience of farmers with lower yield compared with traditional practices employed by smallholder farmers.
Statistical Analysis
Data for pod yield, percentage of good kernels, aflatoxin concentration, yield of good kernels, and estimated financial return were subjected to the GLIMMIX Procedure in SAS (SAS, Cary, NC) considering the 2 by 2 by 2 factorial treatment arrangement (2 practices in the growing cycle prior to harvest by 2 drying practices by 2 storing practices). Experiment and replication within experiments were considered random effects and treatments at each step in the supply chain were considered fixed effects. Pooled data are presented with least square means separated using Fisher's Protected LSD test (𝜶 = 0.05). Data for aflatoxin concentration was transformed to natural logs prior to statistical analysis with mean separations based on the transformed data used to separate means for the non-transformed data. Data for the interaction of practices during the growing cycle and drying and the interaction of practices at all three steps in the supply chain are presented. These comparisons allow practitioners to compare and ultimately select various combinations of practices based on availability of inputs and resource constraints. Pearson correlation coefficients were determined for percentage of good kernels, aflatoxin concentration after storage, and estimated financial return.
Results and Discussion
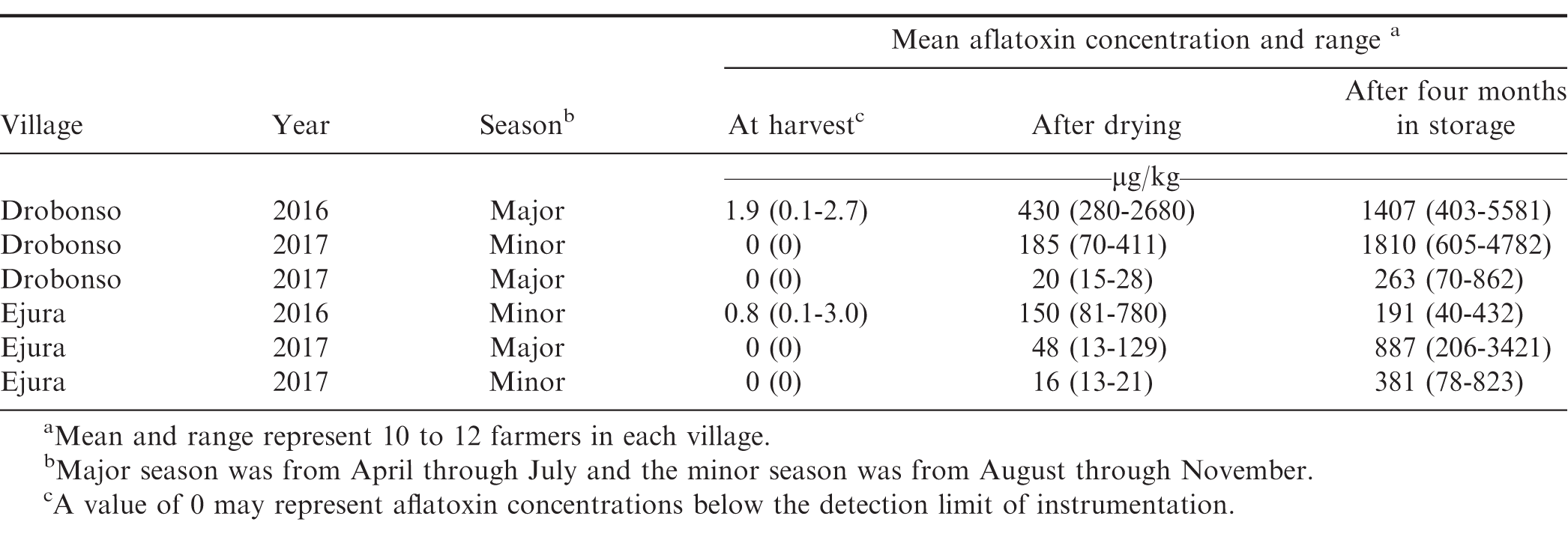
At both Drobonso and Ejura in 2016, low levels of aflatoxin were detected in kernels collected at harvest when peanut was grown using only the farmer practices (Table 1). In 2017, aflatoxin was not detected regardless of location or planting season. Although local weather data were not available from these locations and years, rainfall was generally abundant during 2017 and considered adequate for peanut production in the region (Mochiah, M., personal communication). In contrast, rainfall during both major and minor seasons was much lower in 2016. Rainfall is a major indicator of aflatoxin contamination for peanut at harvest (Craufurd et al., 2006). This most likely explains the difference in detection of aflatoxin from samples collected in the field during 2016 compared with no detection in 2017. Specific information related to temperature and rainfall would have been more informative.
A 226-fold (Drobonso) and 188-fold (Ejura) increase in aflatoxin concentration was observed when comparing concentrations in the field to concentrations after drying on the ground in 2016 (Table 1). Although aflatoxin was not detected in the field sampling in the other four experiments, between 16 and 185 μg/kg of aflatoxin was observed after drying on the soil. A further increase in aflatoxin concentration after four months of storage in traditional poly bags was observed at all locations (Table 1). The change in aflatoxin during storage in poly bags was characterized by a 3.3 to 13.2-fold increase. At Ejura, the increase in aflatoxin concentration during storage was 1.3 to 23.8-fold. These data provide an indication of the scope of aflatoxin contamination from the field through storage under typical management practices used by smallholder farmers in the Ashanti region of Ghana during these two years and growing seasons. The levels of aflatoxin contamination following storage were well above concentrations considered acceptable for human consumption without significant health consequences (Knutsen et al., 2018). These data provide a baseline for comparing efficacy of improved management practices designed to minimize aflatoxin contamination before and after storage.
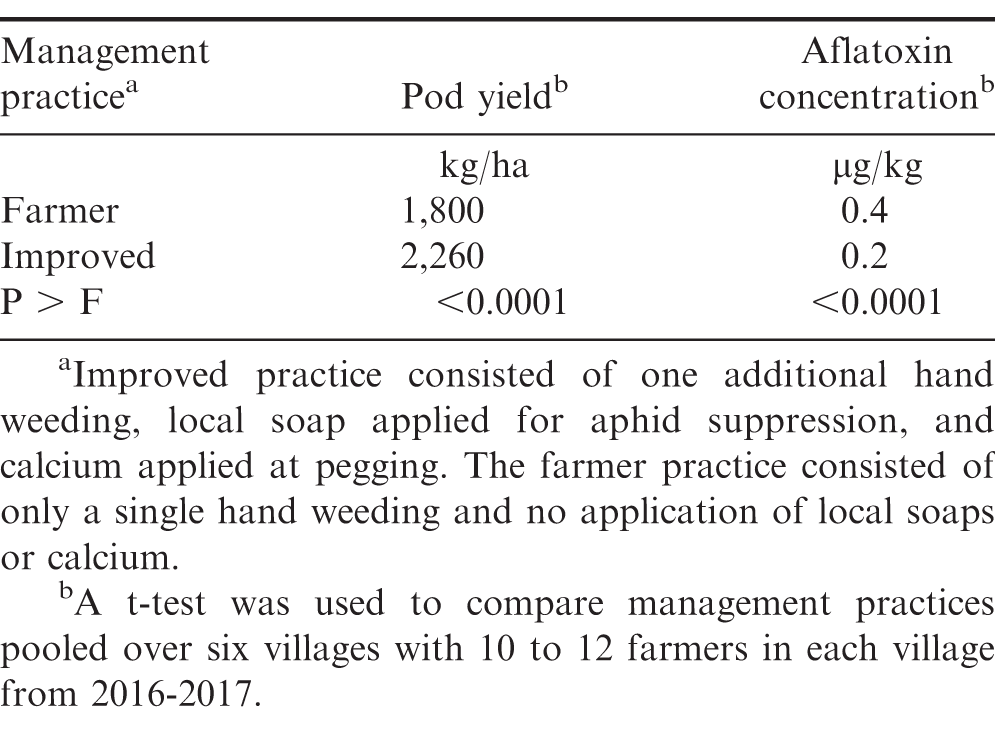
Greater peanut pod yield and lower aflatoxin were observed when one additional weeding occurred, calcium was applied at pegging, and local soap was applied to suppress aphids and rosette (Table 2). Previous research (Curkovic 2016; Mochiah et al., 2011; Nutsugah et al., 2007) demonstrated that local soaps can result in modest reductions of aphid populations and rosette virus. Greater calcium nutrition can also increase yield and decrease aflatoxin contamination (Davidson et al., 1983; Helper, 2005; Waliyar et al., 2008; White and Broadley, 2003). Weed control can also increase yield (Abudulai et al., 2018; Dzomeku et al., 2018). This experiment was not designed to determine the most effective component within the improved production package during the growing cycle prior to harvest. These data suggest that combinations of practices that reduce pest damage and improve plant nutrition likely will increase peanut yield and decrease aflatoxin contamination.
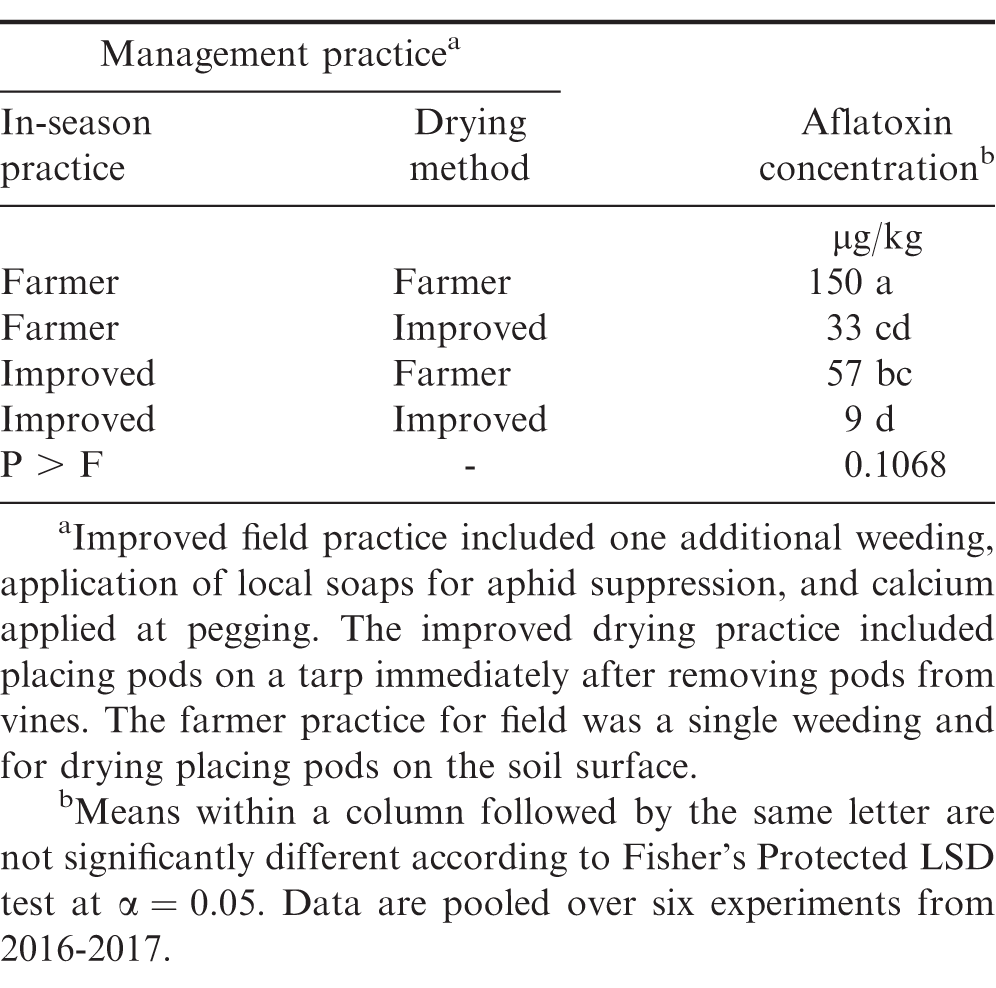
When pooled over experiments, the highest concentration of aflatoxin after drying was observed when farmer practices were employed in the field and during drying (Table 3). Including the improved practice during the growing season (e.g., extra weeding, aphid and rosette suppression, and calcium) and drying on tarps rather than on the ground resulted in lower aflatoxin concentrations after drying. The improved field practice alone or the improved drying practice alone had similar levels of aflatoxin, but lower than farmer practice at both steps. Using improved practices at both steps in the supply chain resulted in lower aflatoxin concentrations compared with the improved growing season practice with farmer drying practice. Including the improved practices at both steps resulted in aflatoxin levels that were similar to using a tarp for drying when the farmer practice was used in the field. It is suspected that drying peanut on tarps minimizes movement of spores from the soil surface onto pods, especially if rain occurs during the drying period. Additionally, if rain occurs during drying, farmers can cover peanut with a portion of the tarp or more easily move peanut under shelter if peanut is placed on a tarp for drying.
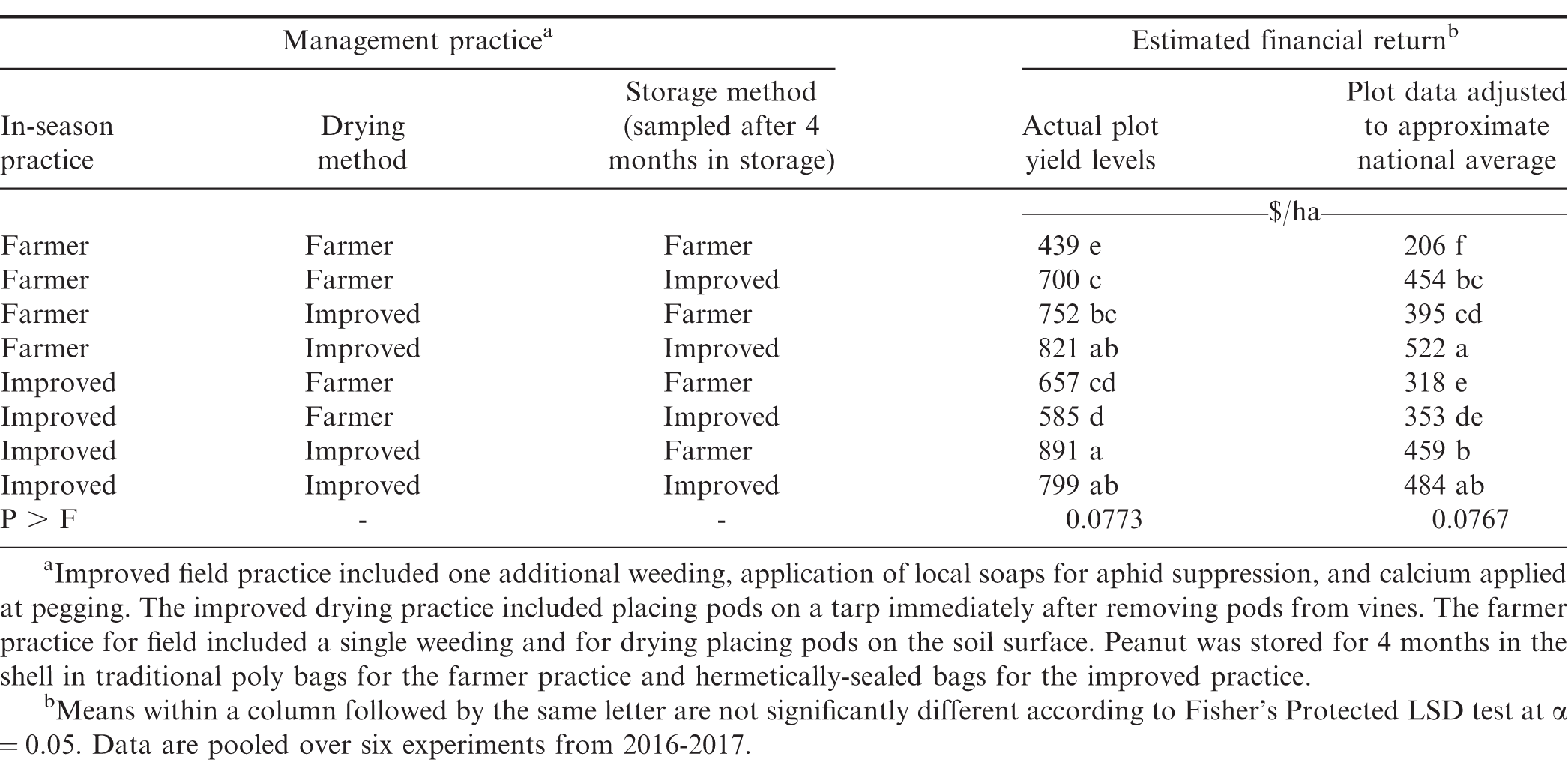
A limited quantity of peanut is sold immediately after harvest in Ghana and most are stored for household use or sale when prices strengthen later in the season. When comparing all possible combinations of improved and farmer practices, the highest aflatoxin concentration was noted when farmer practices were implemented at all steps in the supply chain (Table 4). When comparing a single improved practice, storing peanut in hermetically-sealed bags was the most effective practice to minimize aflatoxin contamination. Improving peanut production practices prior to harvest and drying on tarps resulted in similar concentrations of aflatoxin after storage. Drying on tarps and storing in hermetically-sealed bags resulted in lower aflatoxin after storage than improving management in the field (e.g., additional weeding and application of calcium and local soap) and either drying peanut on a tarp or storing peanut in sealed bags. The lowest concentration of aflatoxin in kernels was noted when improved practices were implemented at all three steps of the supply chain.
These data indicate that implementing improved practices during the growing season, while drying, and when storing peanut will decrease aflatoxin contamination compared with practices currently being implemented by smallholder farmers. The primary goal of this research was to determine the relative importance of practices at each step in the supply chain in minimizing aflatoxin contamination. While smallholder farmers may not be able to implement improved practices at all three steps, it is possible that they could invest in one or two practices that lower the risk of aflatoxin contamination. For example, tarps might be commercially available whereas hermetically-sealed bags may not be an option. While the components (e.g., additional weeding, calcium, and local soaps) of the improved strategy during the growing season were not dissected in a manner that indicates which has the greatest value, implementing one or two of the practices based on labor availability (weeding) or product availability (calcium and local soap and their application) would likely contribute positively to plant health and increase yield and quality of peanut at some level.
Aflatoxin in kernels was negatively correlated with the percentage of good kernels (p < 0.0001, R = -0.79). For example, when farmer practices were implemented at all three steps in the supply chain, the highest average aflatoxin concentration was noted (828 μg/kg) and the lowest percentage of good kernels (59%) and yield of good kernels (680 kg/ha) (Table 4). In contrast, when all three steps in the supply chain incorporated improved practices the lowest concentration of aflatoxin was observed (12 μg/kg) along with the highest percentage of good kernels (95%) and the highest numerical yield of good kernels (1,180 kg/ha). Aflatoxin contamination is often associated with damaged kernels of poor quality with limited market value (Awuah and Ellis, 2002; Awuah et al., 2006; Whitaker et al., 1997).
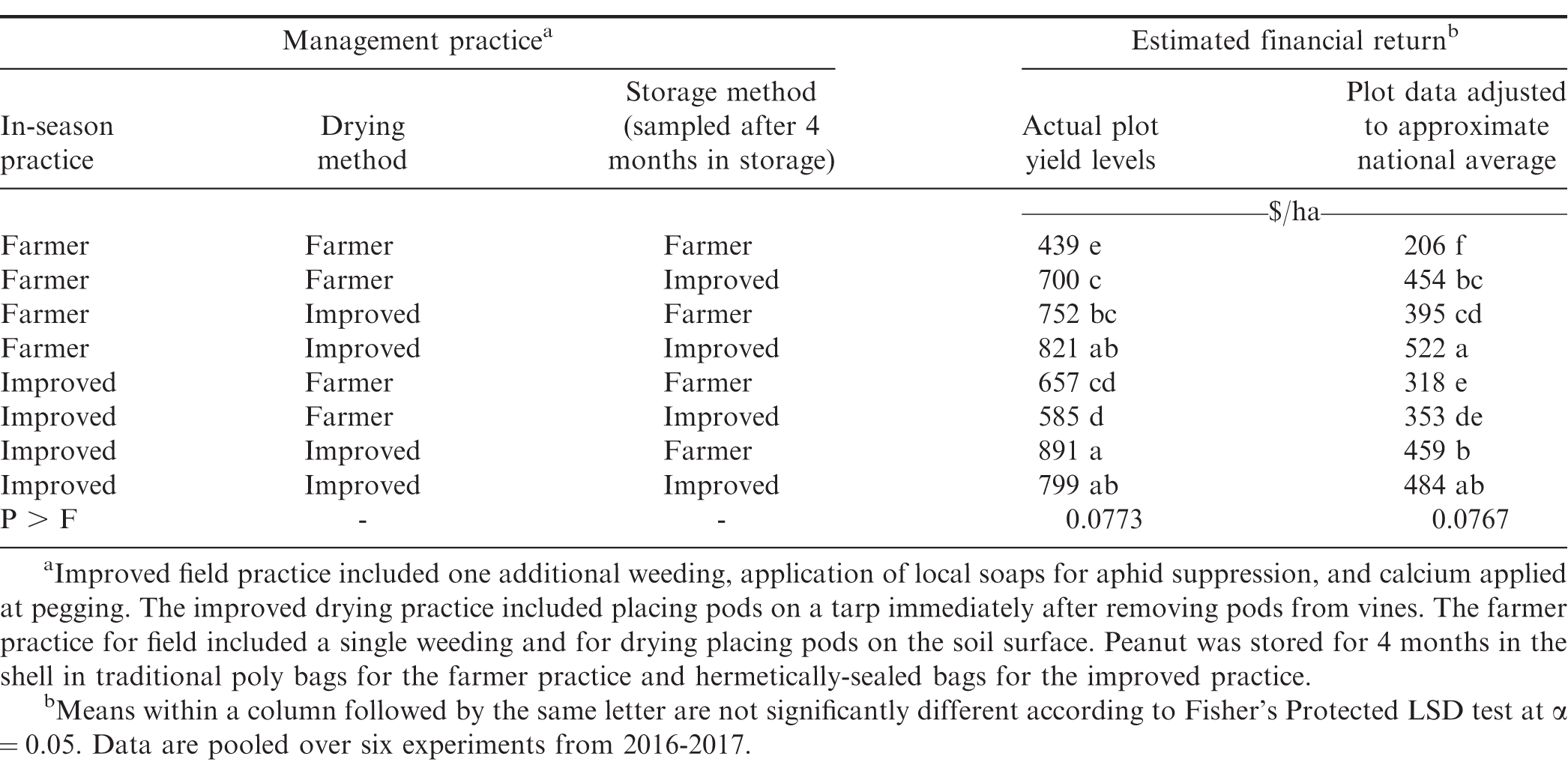
Financial return was derived based on yield, cost of inputs, and quality of kernels that would be marketed after storage. Analyses in Table 5 includes actual farmer stock pod yield (kg/ha) from the experiment and yield from plots adjusted downward to reflect more accurately the financial returns that most smallholder farmers might experience. Yields obtained when farmer practices or improved practices were used were substantially higher than yields estimated to reflect typical smallholder farmer conditions. Because costs for tarps and hermetically-sealed bags are fixed and relatively high, the lower yield scenarios could impact the recommendations on use compared with plot yield that was higher than what most smallholder farmers likely experience.
While some shifts in ranking of financial returns were noted when comparing the two yield structures, differences among combinations of improved and farmer practices were often similar (Table 5). In both instances the lowest returns were noted when the farmer practice was used at all three steps in the supply chain. When actual plot data were used in the analysis, the greatest returns were observed when improved practices were used at each step or when two improved practices were used at steps including drying and storing or prior to harvest and drying. The least effective combination of two improved practices was the combination of practices during the growing cycle and drying. Even though cost was lower when only one improved practice was used, financial return was generally higher when the more expensive approaches at two steps in the supply chain were implemented. For example, financial return for the more expensive approach of including improved practices at all three steps exceeded financial returns when the improved practice was used during the growing cycle followed farmer practices during drying and storage or including the improved practice during storage but not during drying (Table 5). When yield more closely approximated smallholder yields in Ghana, the greatest return was noted when the improved practice was implemented at drying and storage or when improved practices were implemented at all three steps in the supply chain.
The correlation of aflatoxin concentration after storage with estimated financial return for both yield scenarios was significant (p < 0.0001) but not highly correlated (R = -0.21 to -0.24). Similarly, the percentage of good kernels and estimated return were weakly correlated (p < 0.0001, R = 0.20). While kernel quality had an impact, yield in the field also contributed to financial return.
Collectively, the data presented above provide insight into the levels of aflatoxin in peanut in rural villages in Ghana where resources are limited for incorporation of improved practices that could impact yield and quality of peanut and reduce aflatoxin. While there are numerous economic pressures on smallholder farmers, results indicate that the increase in revenue from greater yields and higher quality in the form of non-damaged kernels entering the market more than pay for the costs of improved practices during the growing cycle prior to harvest as well as using tarps for drying and hermetically-sealed bags for storage. However, a major challenge for smallholder farmers is acquiring adequate credit that would enable the purchase of components of the improved practices at each step in the village supply chain (Hong and Hanson, 2016). A further limitation is that even when smallholder farmers have access to credit, they may not have access to inputs or labor that could increase yield and improve quality. None-the-less, these data can be used to assist farmers and their advisors as well as buyers in developing production approaches and post-harvest strategies that could increase the amount and quality of peanut going into the supply chain beyond the village level. This research is the first published information in the peer-reviewed literature that documents aflatoxin contamination prior to harvest through drying and then storage contrasting traditional farmer practices with improved practices at each step along the village supply chain.
Acknowledgements
This publication was made possible through support provided by the Office of Agriculture, Research and Policy, Bureau of Food Security, U.S. Agency for International Development, under the terms of Award No. AID-ECG-A-00-07-0001 to The University of Georgia as management entity for U.S. Feed the Future Innovation Lab on Peanut Productivity and Mycotoxin Control (2012-2017). The opinions expressed herein are those of the authors and do not necessarily reflect the views of the U.S. Agency for International Development. Appreciation is expressed to technical staff and farmers for assistance with this research.
Literature Cited
M., Abudulai, J Naab, S. S Seini, I Dzomeku, K Boote, R Brandenburg, and D Jordan (2018). Peanut (Arachis hypogaea) response to weed and disease management in northern Ghana. , Int. J. Pest Manag. 64 ((3)): 204- 209.
AOAC International [Association of Official Agricultural Chemist International] 2006 Aflatoxins in corn, raw peanuts and peanut butter: liquid chromatography with post-column photochemical derivatization Official Method 2005.08, 49.2.18A.
R. T., Awuah, and W. O Ellis (2002). Effects of some groundnut packaging methods and protection with Ocimum and Syzygium powders on kernel infection by fungi. Mycopathologia , 154: 29- 26.
R. T., Awuah, S. C Fialor, A. D Binns, J Kagochi, and C. M Jolly (2006). Factors influencing market participant decision to sort groundnuts along the marketing chain in Ghana. Peanut Sci 36: 68- 76.
S. S. P., Bulaong, and O. S Dharmaputra, (2002). Fungal population, aflatoxin and free fatty acid contents of peanuts packed in different bag types. Biotropia , 19: 1- 25.
CDC [Centers for Disease Control and Prevention] 2013 Aflatoxin Centers for Disease Control and Prevention (updated 13 January 2012) http://www.cdc.gov/nceh/hsb/chemicals/aflatoxin.htm [Accessed July 28, 2017].
CAS [Codex Alimentarius Standards]. Maximum level and sampling plan for total aflatoxins in peanuts intended for further processing. CODEX STAN 209-1999, Rev. 1-2001., http://www.iss.it/binary/iupa/cont/MaxLevelSamplingAFsPeanut_2001.pdf .
P. Q., Craufurd, P. V Prasad, F Waliyar, and A Taheri (2006). Drought, pod yield, pre- harvest Aspergillus infection and aflatoxin contamination on peanut in Niger. Field Crops Res 98: 20- 29.
Curkovic, S. T 2016 Detergents and soaps as tools for IPM in agriculture Integrated Pest Management (IPM): Environmentally Sound Pest Management, Harsimran Kaur Gill and Gaurav Goyal, IntechOpen, DOI: 10.5772/64343. Available at: https://www.intechopen.com/books/integrated-pest-management-ipm-environmentally-sound-pest-management/detergents-and-soaps-as-tools-for-ipm-in-agriculture .
Davidson, J. I., Jr, P. D Blankenship, and T. H Sanders 1983 Effect of gypsum and calcium carbonate on plants http.//www.fao.org/doCrep/t032e/to32e05.htm .
Dzomeku, I. K., S Baba, M Abudulai, A. M Mohammed, and A. L Abdulai, 2019 Groundnut (Arachis hypogaea L.) response to phosphorus and weed management in the Guinea Savannah Zone of Ghana Tropicultura 37(1). Available at: https://popups.uliege.be:443/2295-8010/index.php?id=253.
W. O., Ellis, J. P Smith, B. K Simpson, and J. H Oldham (1991). Aflatoxins in food: Occurrence, biosynthesis, effects on organisms, detection, and methods of control. Cri. Rev. Food Sci. Nutri 30 ((4)): 403- 439.
C. P Emmok, (2010). Procurement for Development Forum: Groundnuts Case Study Chatham House. http://www.chathamhouse.org/publications/papers/view/109368 .
Eskola, M., G Kos, C Elliott, J Hajšlová, S Mayar, and R Krska 2019 Wordwide contamination of food-crops with myctoxins: Validity of the widely cited 'FAO estimate' of 25% Cri. Rev. Food Sci. Nutri. DOI 10.1080/10408398.2019.1658570
Fana, S 2010 Revitalization of the groundnut sector in West Africa (Gambia, Guinea Bissau, and Senegal Global Agricultural Information Network Report). Available at: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Revitalization%20of%20the%20Groundnut%20sector%20in%20West%20Africa_Dakar_Senegal_12-3-2010.pdf .
Y. Y., Gong, S Wilson, J. K Mwatha, M. N Routledge, J. M Castelino, B Zhao, G Kimani, H. C Kariuki, B. J Vennervald, D. W Dunne, and C. P Wild (2012). Aflatoxin exposure may contribute to chronic hepatomegaly in Kenyan school children. Environ Health Perspect 120 ((6)): 893- 896.
S. S Gordon, (2003). Aflatoxin and food safety: Recent African perspectives. Promec Unit, Medical Research Council, Tygerberg, South Africa. J. Toxicol. Toxin Rev 22: 264- 268.
N. R., Grosso, and C. A Guzman (1995). Chemical composition of aboriginal peanut (A. hypogaea) seeds from Peru. J. Agric. Food Chem 43: 102- 105
E Guchi, (2015). Implication of aflatoxin contamination in agricultural products. Am. J. Food Nutri 3 ((1)): 12- 20.
P. K Helper, (2005). Calcium: A central regulator of plant growth and development. Plant Cell 17: 2142- 2155.
Hong, D., and S Hanson 2016 Scaling up agricultural credit in Africa In Frontier Issues Brief submitted to the Brookings Institution's Ending Rural Hunger project. Available at: https://www.endingruralhunger.org/. Accessed January 9, 2020 .
Ibrahim, B., L Amadou, J Lowenberg-DeBoer, and L Murdock 2013 Side by side comparison of GrainPro and PICS bags for postharvest preservation of cowpea grain in Niger J. Stored Products Res., Vol. 54. DOI:10.1016/j.jspr2013.03.003 .
IITA [International Institute of Tropical Agriculture] 2013 Tackling killer aflatoxins in African food crops European Initiative on Agriculture Research for Development (EIARD). Available at: https://www.iita.org/news-item/iita-partners-launch-initiative-tackle-killer-aflatoxin-african-crops/.
L., Ioannou-Kakouri, A Aletrali, E Christou, A Hadjioannou-Ralli, A, Koliou and D Akkelidou, (1999). Surveillance and control of aflatoxins B1, B2, G1, G2, and M1 in foodstuffs in the Republic of Cyprus: 1992-1996. J. AOAC. Int 82: 883- 892.
P., Jolly, Y Jianga, W Ellis, R Awuah, O Nnedua, T Phillips, J Wange, E Afriyie-Gyawu, L Tange, S Person, J Williams, and C Jolly (2006). Determinants of aflatoxin levels in Ghanaians: Sociodemographic factors, knowledge of aflatoxin and food handling and consumption practices. Int. J. Hyg. Environ. Health 209: 345- 358.
Jordan, D., R Brandenburg, G Payne, D Hoisington, N Magnan, J Rhoads, M Abudulai, K Adhikari, J Chen, R Akromah, W Appaw, W Ellis, M Balota, K Mallikarjunan, K Boote, G MacDonald, K Bowen, B Bravo-Ureta, J Jelliffe, A Budu, H Chalwe, A Mweetwa, M Ngulube, A Dankyi, B Mochia, V Hoffman, A Muitia, A Mwangwela, S Njoroge, D Okello, and N Opoko 2018 Preventing mycotoxin contamination in groundnut cultivation Pages 181- 214 in Sivasankar, S Berguinson, D Gaur, P Kumar, S Beebe, S and Tamó, M eds., Achieving Sustainable Cultivation of Grain Legumes; Improving Cultivation of Particular Grain Legumes. Volume 2 Burleigh Dodds Series in Agricultural Science, Burleigh Dodds Science Publishing, Cambridge, UK.
M. C Kew, (2012). Hepatocellular carcinoma in developing countries: Prevention, diagnosis and treatment. World J. Hepatol 4 ((3)): 99- 104.
H. K., Knutsen, J Alexander, L Barregård, M Bignami, B Brüschweiler, S Ceccatelli, B Cottrill, M Dinovi, L Edler, B Grasl-Kraupp, C Hogstrand, L. R Hoogenboom, C. S Nebbia, I. P Oswald, M Rose, A. C Roudot, T Schwerdtle, C Vleminckx, G Vollmer, H Wallace, P Fürst, K Baert, J. Cortiñas Abrahantes B Dujardin, K Ferrini, and A Petersen (2018). Statement on the effect on public health of a possible increase of the maximum level for 'aflatoxin total' from 4 to 10 μg/kg in peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs. EFSA Journal 16 ((2)): 5175.
Mahuku, G., H. S Nzioki, F Waliyar, B Diarra, and O Kodio 2010 Aflatoxin prevalence data collection: sampling framework and methodology Internal Food Policy Research Institute (IFPRI), Working Paper 1. Available at: https://www.ifpri.org/publication/aflatoxin-prevalence-data-collection .
P. K., Malaker, I. H Mian, K. A Bhuiyah, A. M Akanda, and M. M. A Reza (2008). Effect of storage containers and time on seed quality of wheat. Bangladesh J. Agri. Res., 33: 469- 477.
M. B., Mochiah B Banful, K. N. Fening B. W. Amoabeng, K. Offei Bonsu, S Ekyem, H Braimah, and M Owusu-Akyaw (2011). Botanicals for the management of insect pests in organic vegetable production. J. Entomol. Nematol 3 ((6)): 85- 97.
S. K., Nutsugah, M Abudulai, C Oti-Boateng, R. L Brandenburg, and D Jordan (2007). Management of leaf spot diseases of peanut with fungicides and local detergents in Ghana. Plant Pathol 6: 248- 253.
M., Owusu-Akyaw, M. B Mochiah, J. Y Asibuo, K Osei, A Ibrahim, G. Bolfrey Arku, J. N. L Lamptey, A. A Danyi, A Oppong, J. K Addo, M. K Boateng, H. K Adu-Dapaah, S Addy, S Amoah, S Osei-Yeboah, M Abudulai, N Denwar, J Naab, G Mahama, R Akroma, R. L Brandenburg, J. E Bailey, D. L Jordan, T. H Williams, D Hoisington, and J Rhoads (2019). Evaluation and release of two peanut cultivars: a case study of partnerships in Ghana. Peanut Sci 46: 37- 41.
J., Ramesh, G Sarathchandra, and V Sureshkumar (2013). Survey of market samples of food grains and grain flour for aflatoxin B1 contamination. Int. J. Curr. Microbiol. Appl. Sci 2 ((5)): 184- 188.
A., Saleemullah, I. A Iqbal, and H Shah (2006). Aflatoxin contents of stored and artificially inoculated cereals and nuts. Food Chem 98 ((4)): 699- 703.
P. C., Turner, S. E Moore, A. J Hall, A. M Prentice, and C. P Wild (2003). Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ Health Perspect 111 ((2)): 217- 20.
USDA-GIPSA [United States Department of Agriculture Grain Inspection, Packers and Stockyards Administration Technology and Science Division] 2015 Test Kit Instructions: 8085 AS III and Pro. Neogen Reveal Q+ for aflatoxin using Accusan III and Accuscan Pro Readers. Pages 1-11. Available at: https://www.gipsa.usda.gov/fgis/metheqp/instructions/8085%20AS%20III%20and%20Pro%20Revision%200.pdf .
P Villers, (2014). Aflatoxins and safe storage. Frontiers Microbiol 5 ((158)): 1- 6.
J. M., Wagacha, and J. T Muthomi (2008). Mycotoxin problem in Africa: Current status, implications to food safety and health and possible management strategies. Intl. J. Food Microbiol., 124: 1- 12.
Waliyar, F., P. L Kumar, A Traore, B. R Ntare, B Diarra, and O Kodio 2008 Pre and post-harvest management of aflatoxin contamination in peanuts Pages 209- 218 in J. F Leslie, R Bandyopadhyay, and A Visconti, eds Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade CAB International.
F., Waliyar, V. C Umeh, A Traore, M Osiru, B. R Ntare, B Diarra, O Kodio, K. V. K Kumar, and H Sudini (2015). Prevalence and distribution of aflatoxin contamination in groundnut (Arachis hypogaea L.) in Mali, West Africa. Crop Prot 70 ((1)): 1- 7.
T. B., Whitaker W. M Hagler, F. G Giesbrecht, J. W Dorner, F. E Dowell, and R. J Cole (1997). Estimating aflatoxin in farmers' stock peanut lots by measuring aflatoxin in various peanut-grade components. Journal of AOAC International 81: 61- 67.
P. J., White, and M. R Broadly (2003). Calcium in plants. Ann. Bot 92: 487- 511.
J., Williams, T. D Phillips, P. E Jolly, J. K Stiles, C. M Jolly, and D Aggarwal (2003). Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutri 80: 1106- 1122.
Notes
- Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. [^]
- Council for Scientific and Industrial Research-Crops Research Institute, Kumasi, Ghana. [^]
- Council for Scientific and Industrial Research-Savanna Agricultural Research Institute, Tamale, Ghana. [^]
- Department of Crop and Soil Sciences, North Carolina State University, Raleigh, NC. [^]
- Department of Entomology and Plant Pathology, North Carolina State University, Raleigh, NC. [^]
- University of Connecticut, Storrs, CT. [^]
- Agricultural and Biological Engineering Department, University of Florida, Gainesville, FL. [^]
- Department of Food Science and Technology, University of Georgia, Griffin, GA. [^]
- Department of Food Science and Nutrition, University of Minnesota, St Paul, MN. [^]
- Virginia Polytechnic Institute and State University, Blacksburg, VA. [^]
- Feed the Future Innovation Lab for Peanut, University of Georgia, Athens, GA. [^] Corresponding author: david_jordan@ncsu.edu
Author Affiliations