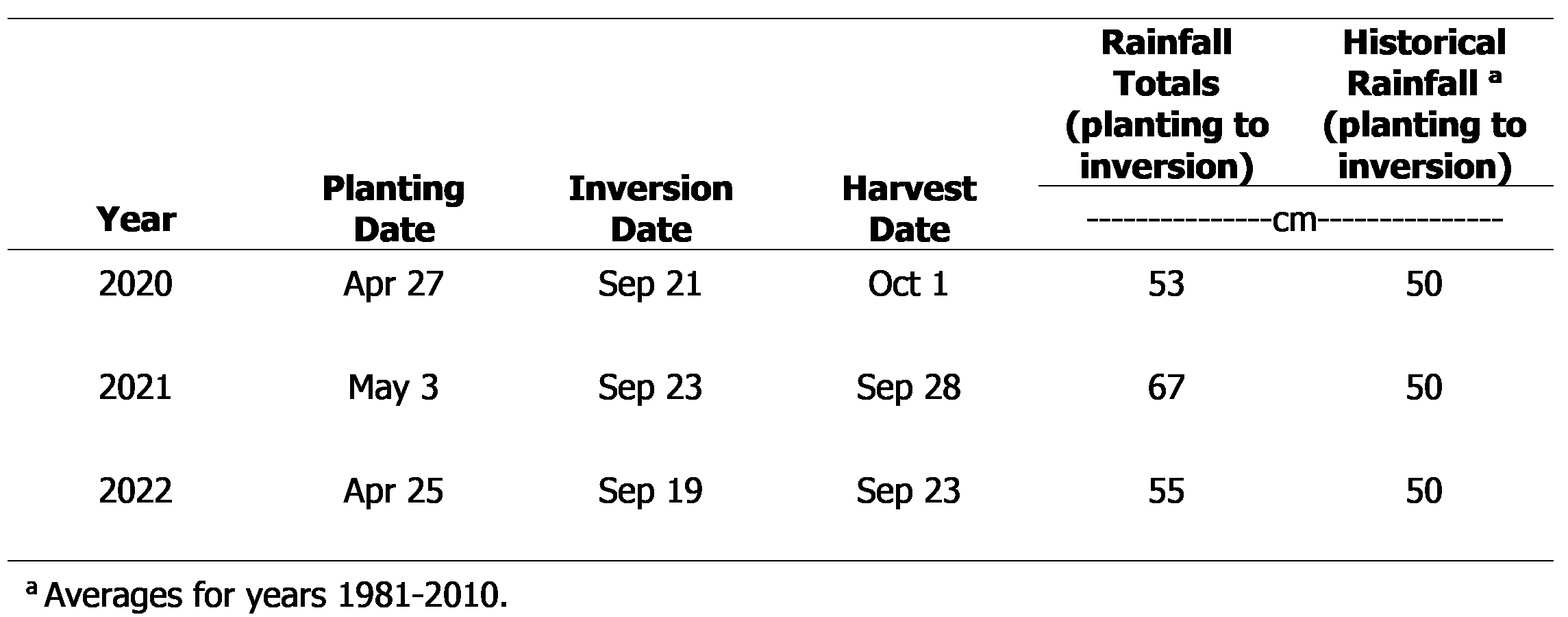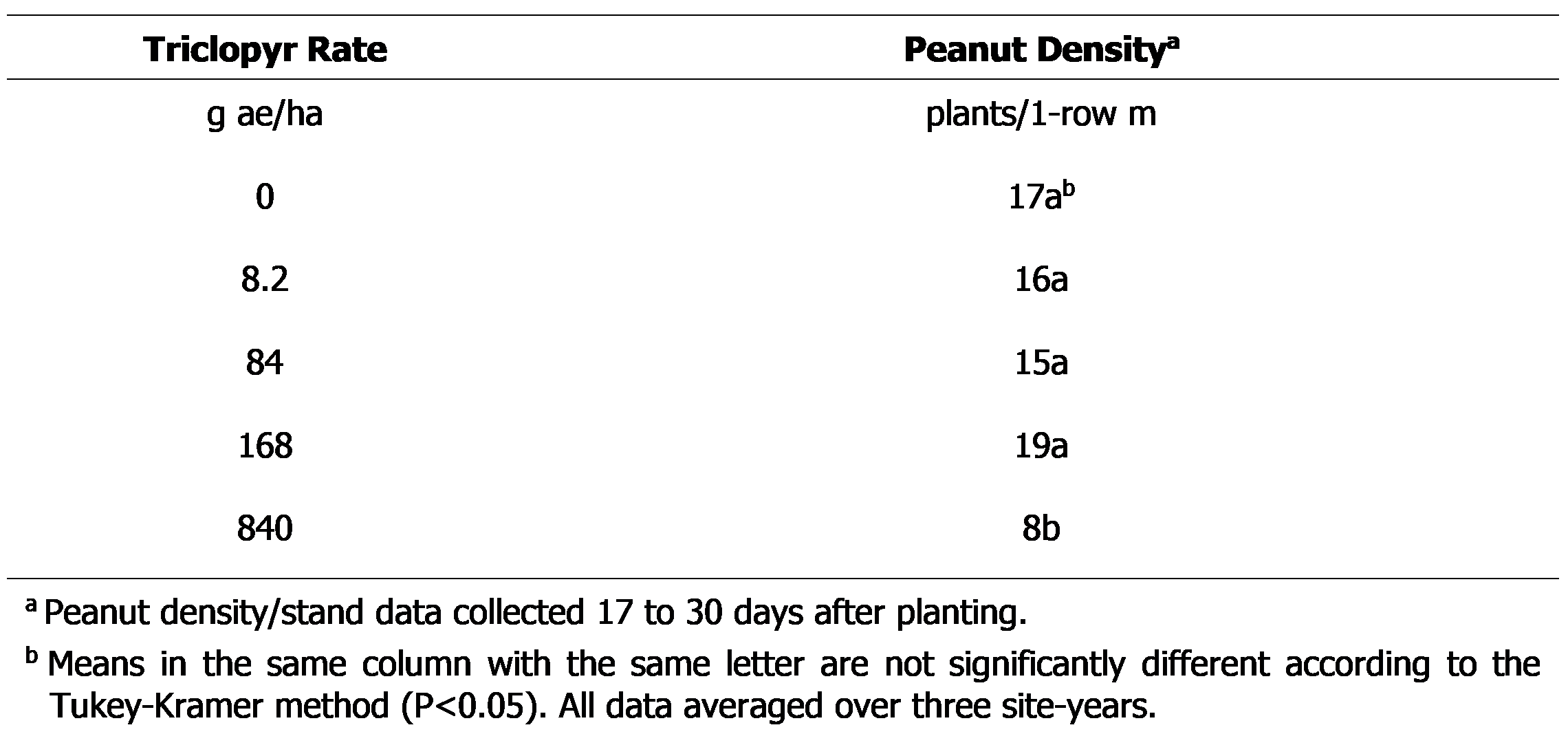INTRODUCTION
Triclopyr 2-[(3,5,6-trichloro-2-pyridinyl)oxy] acetic acid, is an auxin-type herbicide that is a member of the pyridine carboxylic acid family (Johnson et al.,1995; Shaner, 2014). Triclopyr is commercially available in several formulations including a triethylamine salt (TEA), butoxy ethyl ester, pridinyloxyacetic acid, and choline salt and difference in plant response to these formulations have been observed (Dias et al., 2017).
Triclopyr-TEA was first introduced in 1979 for the control of woody plants and broadleaf weeds for non-crop areas and forestry applications (Douglass et al., 2016; US-EPA, 1998). Triclopyr is readily absorbed through foliage and transported through phloem tissues to growing points where it accumulates, but low percentages of triclopyr can accumulate in the roots of certain species, such as horsenettle (Solanum carolinense L.) and honey mesquite (Prosopis juliflora var. glanulosa Torr.) (Bovey and Mayeux, 1980; Shaner, 2014). Triclopyr and other auxin-type herbicides control plants by inhibiting protein synthesis in meristematic regions of plants, along with mimicking indoleacetic acid (IAA) stimulating ethylene evolution which can lead to characteristic epinasty symptoms (twisting and bending of petioles, leaves, and stems) associated with this class of chemistry (Mithila et al., 2011; Shaner, 2014).
Triclopyr is used for both residual activity and postemergence (POST) control of woody plants and broadleaf weeds across a multitude of landscapes including but not limited to forests, rice, home lawns, pastures, rangelands, and rights-of-ways, contributing to a wide window and range of application timings (Sperry et al., 2020; US-EPA, 1998). The average half-life for triclopyr is 10 to 46 d which is influenced by soil type and environmental conditions (Shaner, 2014). The primary breakdown of triclopyr is through microbial degradation in the soil where it is to some extent persistent and mobile; however, photodegradation occurs within two-six hours in water (Shaner, 2014; US-EPA, 1998). Other herbicides in this chemical family such as picloram can persist at phytotoxic levels in certain soils for up to five years depending on the application rate and soil type (Lym and Messersmith, 1988).
To promote successful pine (Pinus spp.) production and reduce or eliminate herbaceous or woody competition, herbicides such as triclopyr-TEA release pines by effectively controlling various types of herbaceous broadleaf weeds and other tree species (Pusino et al.,1994). Forestry herbicides such as triclopyr have a role in helping Georgia’s pine tree production, as forestry is a leading agricultural commodity in Georgia with an economic impact of $44 billion dollars (Georgia Forestry Commission, 2024). Forestry herbicides have effectively changed the way pine silviculture is performed, and Georgia is a direct beneficiary of that as the nation’s leading producer of pine timber volume harvested annually which accounts for 4.49 million ha in production (Bullock, 2011; Georgia Forestry Commission, 2024; Lauer and Quicke, 2022; Minogue, 2021; USDA-CropScape, 2023).
In 2024, Georgia growers harvested 342,105 ha of peanuts (Arachis hypogaea L.) accounting for 50% of the nation’s crop (USDA-NASS, 2025). One of the top peanut producing counties in southwest Georgia (Decatur) produces ~17,140 ha of peanut and maintains ~64,200 ha of pine forest resulting in a ratio of 3.75 ha of pine forest to peanut (USDA-CropScape, 2023). This pine/peanut relationship is typical in many counties in Georgia which increases the risk for off-target movement events from pine forests to peanut fields.
While peanut herbicides are applied by mostly ground application methods, forestry herbicides are applied using ground application methods or aerially via the use of helicopters, which leads to greater risks of off-target movement of herbicides (Bullock, 2011). During a typical planting and growing season, there is a wide range of times when peanut fields could be subjected to off-target movement of triclopyr due to the surrounding pine stand’s age. Therefore, a peanut field prior to planting or up to harvest is at risk of off-target movement due to the variability of timing and aerial application method of triclopyr on neighboring pine forests.
Previous research has been conducted on peanut to determine the effects of other auxin-like herbicides including 2,4-D, dicamba, and picloram (Blanchett et al., 2017; Carter and Prostko, 2020; Leon et al., 2014; Prostko et al., 2011). However, no information has been published about the potential effects of triclopyr on peanut. The effects of triclopyr on rhizoma perennial peanut (RPP) (Arachis glabrata Benth.) has been documented in previous research (Martin et al., 2021; Valencia et al., 1999). Results from these studies suggest that the tolerance of RPP to triclopyr is a function of rate and cultivar.
The objective of this research was to determine the effects of triclopyr applied PRE or POST on peanut growth and yield.
Materials and Methods
Field trials were conducted at the Ponder Research Farm in Ty Ty, Georgia (31.507654̊ N, 83.658395̊ W) from 2020-2022 (three site-years) to determine the effects of direct triclopyr applications to peanut. Soil type was a Tifton sand with 94% sand, 0% silt, 6% clay, 0.91% organic matter, and a pH of 6.0. Conventional tillage practices were used and peanut (cv. Georgia-06G) (Branch, 2007) was planted using a vacuum planter calibrated to deliver 18 peanut seed/m at a depth of 5 cm (Monosem Precision Planters, 1001 Blake St., Edwardsville, KS). Peanuts were planted in twin rows spaced 23 cm apart on a 91 cm center. Plots were 1.8 m (two sets of twin rows) wide and 7.6 m in length.
Treatments were arranged in a randomized complete block design with a three (timing) by five (rate) factorial arrangement with three to four replications. Triclopyr-TEA (Garlon® 3A, Corteva Agriscience, Indianapolis, IN) timings were PRE 1 day after planting (DAP), 30 DAP, and 60 DAP with herbicide rates of 0, 8.4 (1/100X), 84 (1/10X), 168 (1/5X), and 840 g ae/ha (1X, manufacturers suggested use rate). Herbicide treatments were applied using a CO2-pressurized backpack sprayer calibrated to deliver 140 L/ha at 5.3 km/hr. Peanut growth stages at 30 DAP or 60 DAP were R1 (beginning bloom) and R3-R4 (beginning pod to full pod), respectively (Boote, 1982). Plots were maintained weed-free throughout the season using a herbicide program recommended by the University of Georgia Extension and hand-weeding (Prostko, 2024). Supplemental overhead irrigation was applied as needed to maintain optimum peanut yields (Porter, 2022). Production, and pest management practices other than specific treatments were held constant over the entire experiment to optimize peanut growth and development (Monfort, 2022).
Data collected included peanut density (stand) at 17 to 30 DAP, visual subjective estimates of peanut injury (stunting and epinasty), plant height/width, and yield. Peanut density was obtained by counting the number of emerged plants per 1-row m. Visual estimates of peanut injury were obtained at 65 DAP using a scale of 0 to 100 (0=no injury; 100=plant death). Plant height and width data were collected at 110 DAP by measuring five plants/plot at random. Plant height measurements were recorded from the soil line to the top of the highest point of the main terminal, and plant width measurements were recorded from lateral branch to lateral branch within a set of twin-rows. Peanut yield data were obtained using commercial harvesting equipment. Yields were adjusted to 10% moisture. A complete summary of planting dates, harvesting dates, and rainfall totals can be found in Table 1.
Data were subjected to ANOVA using PROC GLIMMIX in SAS, version 9.4 (SAS Institute, Cary, NC). Peanut density, injury (stunting, epinasty), plant height and width, and yield were set as the response variables with year and replication within year included in the model as random factors. All data were pooled over years. All P-values for tests of differences between least-square means were compared and separated using the Tukey-Kramer method (P<0.05).
Results and Discussion
Peanut Density
Triclopyr applied PRE at 840 g ae/ha reduced peanut density by 52%. Lower rates of triclopyr had no influence on final plant density (Table 2). POST applications of triclopyr did not influence plant density (data not shown). Although there is no other research evaluating the influence of triclopyr on peanut density or establishment, PRE applications of picloram + 2,4-D at rates ranging from 1/300X to 1/10X did not reduce peanut density (Carter and Prostko, 2020).
Peanut Injury
For both stunting and epinasty at 65 DAP, a significant interaction between timing and rate was observed (P<0.0001) (Table 3). Stunting was heavily influenced by rate with the 840 g ae/ha rate applied PRE, 30 DAP, or 60 DAP resulting in 32, 58, and 9% stunting, respectively. The only other timing where significant stunting (16%) was observed occurred when 168 g ae/ha was applied at 30 DAP. In regards to epinasty (leaf cupping/curl), the only application where this symptomology was observed was when 840 g ae/ha was applied 60 DAP (5 days after treatment). While epinasty symptoms were visible shortly after this application, injury was transient and not evident at harvest. Leon et al. (2014) observed minor injury symptoms of the peanut canopy from POST applications of 2,4-D at 70 to 280 g ae/ha, but peanut yield was reduced up to 20%. Thus, epinasty symptoms caused by auxin herbicides may not correlate well to peanut yield loss.
Peanut Plant Height and Width
A 29 and 46% reduction in peanut heights was observed when triclopyr at 840 g ae/ha (1X) was applied at 30 or 60 DAP, respectively (Table 3). Additionally, 168 g ae/ha (1/5X) reduced plant height 17% when applied at 60 DAP. Peanut plant width was less responsive to triclopyr with only a 9% width reduction when treated 60 DAP with the 840 g ae/ha rate. Triclopyr at 84 g ae/ha (1/10X) and 8.4 g ae/ha (1/100X) had no effect on peanut height and width. In contrast, picloram plus 2,4-D applied at 1/10X and 1/100X rates reduced peanut plant heights by 9% and 4%, respectively (Carter and Prostko, 2020).
Martin et al. (2021) reported that triclopyr at 450 to 990 g ae/ha applied to RPP would result in a 20% biomass reduction. However, this level of injury was considered acceptable to RPP producers since limited weed management options are available. In other studies, the dry matter yield of RPP was reduced 50% to 59% when triclopyr was applied at 560 or 1120 g ae/ha (Valencia et al., 1999).
Peanut Yield
Peanut yield was significantly reduced, regardless of timing, when treated with triclopyr at 840 g ae/ha (P<0.0001) (Table 3). This rate resulted in yield losses of 21, 42, and 62% when applied PRE, 30 DAP, and 60 DAP, respectively. Applications of triclopyr at 168 g ae/ha at 60 DAP also resulted in a yield reduction of 24%. No other treatment influenced yield. It has been previously documented that various auxin herbicides can have negative impacts on peanut pod yield although results have been variable. Applications of picloram plus 2,4-D resulted in an 11% reduction in peanut yield when treated with a 0.18 + 0.67 kg ai/ha rate (1/10X), regardless of timing (Carter and Prostko, 2020). Multi-state research also determined that peanut was more susceptible to dicamba when treated at 30 and 60 DAP with yield losses up to 29% when treated with a rate of 40 g ae/ha (Prostko et al., 2011). Additionally, peanut exhibited a 65% yield loss with dicamba at 560 g ae/ha and a 41% yield loss with 2,4-D applied at 1120 g ae/ha when applied to peanut between the 8 to 10 leaf and pegging stages of growth (Leon et al., 2014).
Summary and Conclusions
Peanut density (stand) was only reduced by 840 g ae/ha of triclopyr applied PRE resulting in a 52% reduction in stand and ultimately a 21% reduction in yield. Assuming normal temperature, rainfall patterns, and supplemental irrigation programs, this research suggests peanut could be planted following triclopyr at 840 g ae/ha after approximately three field half-lives (~138 d) have occurred in coarse-textured soils. Triclopyr applied POST at 30 DAP with rates ≤ 168 g ae/ha (1/5X) did not influence canopy growth or yield. Peanut stunting only reached 16% with 168 g ae/ha. Triclopyr applied POST at 60 DAP with rates ≥ 168 g ae/ha (1/5X) resulted in significant plant height reductions (17% to 46%) and yield losses (24% to 62%). Thus, it is unlikely that lower off-target or drift rates will cause peanut yield losses. Previous research reported that spray particle drift from ground applications was one to eight percent and 20 to 35% from aerial applications depending upon nozzle type and wind speed (Maybank et al., 1978). This would equate to a range of 1/100X to 1/3X rates of triclopyr. It has also been reported that vapor drift rates are equivalent to 1/1000X rate (Egan and Mortensen 2012). In peanut fields without supplemental irrigation or with inadequate fertility, greater yield losses could be observed than reported herein. It is also possible that peanut could respond differently to the other formulations of triclopyr (Dias et al., 2017) warranting further investigation.
Acknowledgements
This research could not have been conducted without the technical support of Charlie Hilton, Tim Richards, and Dewayne Dales. The contributions of A.S. Culpepper, W.S. Monfort, M.A. Abney, and C.J. Bryant were also greatly appreciated.
Literature Cited
Blanchett B.H., Grey T.L., Prostko E.P., Vencill W.K., and Webster T.M.. 2017. The effect of 2,4-dichlorophenoxyacetic acid (2,4-D) on peanut when applied during vegetative growth stages. Peanut Sci. 44:53-59.
Boote K.J. 1982. Growth stages of peanut (Arachis hypogaea L.). Peanut Sci. 9:35-40.
Bovey R.W., and Mayeux, Jr. H.S. 1980. Effectiveness and distribution of 2,4,5-T, triclopyr, picloram, and 3,6-dichloropicolinic acid in honey mesquite (Prosopis juliflora var. glanulosa). Weed Sci. 28(6):666-670.
Branch W.D. 2007. Registration of ‘Georgia-06G’ peanut. J. Plant Registrations 1:120.
Bullock F.D. 2011. Understanding, selecting, and applying herbicides for vegetation management in Tennessee forestry. Tennessee State University Agricultural and Natural Resources Fact Sheet ANR PB-NO.1. Available: https://www.tnstate.edu/extension/documents/ANR%20P1.
Carter O.W., and Prostko E.P.. 2020. The effect of picloram plus 2,4-dichlorphenoxyacetic acid on peanut growth and yield. Peanut Sci. 47:111-114.
Dias J.L.C.S., Banu A., Sperry B.P., Enloe F., Ferrell J.A., and Sellers B. A.. 2017. Relative activity of four triclopyr formulations. Weed Tech. 31:928-934.
Douglass C.H., Nissen S.J., Meiman P.J., and Kniss A.R.. 2016. Impacts of imazapyr and triclopyr soil residues on the growth of several restoration species. Rangeland Ecol. and Mgt. 69:199-205.
Egan J.F. and Mortensen D.A.. 2012. Quantifying vapor drift of dicamba herbicides applied to soybean. Environ. Toxicol. Chem. 31:1023-1031.
Georgia Forestry Commission. 2024. Sustainability Report for Georgia’s Forests: January 2024. Available online at: https://gatrees.org/wp-content/uploads/2020/01/ Sustainability-Report-2024-WebVer.pdf.
Johnson W.G., Lavy T.L., and Gbur E.E.. 1995. Sorption, mobility and degradation of triclopyr and 2,4-D on four soils. Weed Sci. 43:678-684.
Lauer D.K., and Quicke H.E.. 2022. Harmonized site preparation and post plant herbaceous weed control for establishment of southern pine plantations on coastal bedded sites. Weed Technol. 36:214-228.
Leon R.G., Ferrell J.A., and Brecke B.J.. 2014. Impact of exposure to 2,4-D and dicamba on peanut injury and yield. Weed Tech. 28:465-470.
Lym R.G., and Messersmith C.G.. 1988. Survey for picloram in North Dakota groundwater. Weed Tech. 2:217-222.
Martin L.J., Sellers B.A., Devkota P., Ferrell J.A. Leon R.G., Vendramini J.M.B.. 2021. Tolerance of rhizoma perennial peanut to glyphosate and triclopyr. Weed Technol. 35: 525–531.
Maybank J., Yoshida K., and Grover R.. 1978. Spray drift from agricultural pesticide applications. Air Pollut. Control. Assoc. J. 28:1009-1014.
Minogue P. 2021. 2021 Forest herbicide workshop: Advances in herbicide technology for pine management. UF IFAS. Available on-line at: https://programs.ifas.ufl.edu/media/ programsifasufledu/florida-land-steward/events-calendar/Minogue-2021-Forest-Herbicide-Webinar-Pine-Mgmt-FINAL.pdf.
Mithila J., Hall J.C., Johnson W.G., Kelley K.B., and Riechers D.E.. 2011. Evolution of resistance to auxinic herbicides: historical perspectives, mechanisms of resistance, and implications for broadleaf weed management in agronomic crops. Weed Sci. 59:445-457.
Monfort W.S. (editor). 2022. Peanut production guide. University of Georgia Extension Bulletin 1146. 179 pp. Available on-line at: https://extension.uga.edu/publications/detail.html?number=B1146.
Porter W.M. 2022. Water use and relationships in peanut production. UGA Peanut Production Guide. University of Georgia Extension. Chap. 10:77-86.
Prostko E.P. 2024. in Georgia Pest Management Handbook. University of Georgia Extension Special Bulletin 28. Commercial Edition vol 1:217-236. Available on-line at: https://secure.caes.uga.edu/extension/publications/files/pdf/SB%2028-24_2.PDF.
Prostko E.P., Grey T.L., Marshall M.W., Ferrell J.A., Dotray P.A., Jordan D.L., Grichar W.J., Brecke B.J., and Davis J.W.. 2011. Peanut yield response to dicamba. Peanut Sci. 38:61-65.
Pusino A., Liu W., and Gessa C.. 1994. Adsorption of triclopyr on soil and some of its components. J. Agric. Food Chem. 42:1026-1029.
Shaner D.L. 2014. Herbicide Handbook. 10th Edition. Weed Science Society of America, Champaign, IL. 513 pp.
Sperry B.P., Dias J.L.C.S., Prince C.M., Ferrell J.A., and Sellers B.A.. 2020. Relative activity comparison of aminocyclopyrachlor to pyridine carboxylic acid herbicides. Weed Tech. 402-407.
USDA-CropScape. 2023. 2023 Cropland data layer statistics for Georgia. Available on-line at: https://nassgeodata.gmu.edu/CropScape/.
USDA-NASS. 2025. Crop Production-2024 Summary. Available on-line at: https://downloads.usda.library.cornell.edu/usda-esmis/files/k3569432s/ nk324887m/qn59s0097/cropan25.pdf.
US-EPA. 1998. Triclopyr Re-Registration Eligibility Decision (RED) Fact Sheet. 8p. Available on-line at: https://archive.epa.gov/pesticides/reregistration/web/ pdf/2710fact.pdf.
Valencia E., Williams M.J., Sollenberger L.E.. 1999. Yield and botanical composition of rhizoma peanut-grass swards treated with herbicides. Agron. J. 91:956-961.
Notes
- First and second authors: Graduate Research Assistants; and third author: Professor/Extension Weed Specialist, Dept. of Crop & Soil Sciences, The University of Georgia, Tifton, GA 31793. [^] Corresponding author’s E-mail: eprostko@uga.edu