Introduction
Almost all peanuts are consumed after some type of thermal processing, which is used to improve food safety and food quality. Dry roasting is the most common thermal process used in the peanut industry. The controlled application of heat in a low moisture environment inhibits food borne pathogens and improves palatability (Perren and Escher 2013; Poirier et al. 2014; Shi et al. 2017). This type of roasting converts raw peanuts into a product with textures, flavors, and colors optimized to the industry buyer’s needs (van Boekel et al. 2010; Davis & Dean 2016). Proper roasting can provide additional benefits, such as inactivating natural toxins and enzymes, improving digestibility and the bioavailability of nutrients, and enhancing health-promoting antioxidants (van Boekel et al. 2010). However, if not properly controlled, negative outcomes of thermal processing can include the formation of carcinogens such as acrylamide, the loss of nutrients, and the formation of undesirable flavors. In industry, it is a standard practice to roast peanut seeds to a specified color (Hunter L value), as these measurements are rapid, non-destructive, and there is an association between color and roasted flavors and aromas (Mason et al. 1966; Pattee et al. 1991).
A better understanding of the variation of individual free amino acids with roasting times in peanuts is needed. Previous research has reported that asparagine, glutamic acid, glutamine, alanine, aspartic acid, histidine, and phenylalanine were the primary precursors for typical peanut flavors (Newell et al. 1967). This was inferred from the presence of those free amino acids which made up most of the total free amino acids found. Rodriguez and others (1989) reported that raw peanut seeds had significantly more amino nitrogen than their roasted counterparts. This study attributed this to the Maillard reaction that occurs during roasting between amino acids and sugars, or due to breakdown of the amino acids into non-amino acid forms. The Maillard browning reaction is responsible for the formation of various compounds, including volatile heterocyclic compounds such as pyrazines, pyrroles, pyridines, oxalines, and oxazoles, which are known to contribute to the characteristic flavor and aroma of the peanut (Schirack et al. 2006; Purlis 2010). The Maillard reaction occurs between the carbonyl group of a reducing sugar and the free amino group of an amino acid (Newell et al. 1967). Roasting also reduces the moisture content and impacts the microstructure of peanuts, which creates the desired crunchy texture of roasted peanuts (Lee and Resurreccion 2006).
In the peanut industry, the final application of the roasted product dictates the specific end color used, but the chemical and physical changes within the peanuts change over the course of the entire roast. These changes include moisture content, sugar content, color, free amino acid content, and flavor, and are attributed to or correlated with browning reactions. The Maillard reaction is the predominant browning reaction that occurs during the roasting of peanuts and is considered responsible for the formation of characteristic flavors, aromas, colors and textures in the peanut (Buckholz et al. 1980; Davies and Labuza 1997; Purlis 2010). While the differences between raw and roasted peanuts are understood, the objective of this research was to evaluate how the chemical and physical attributes change at varying roast time durations. Runner peanuts were dry roasted, and evaluated for color, macronutrient content, moisture content, and sensory attributes. The comprehensive findings can aid in optimizing peanut properties during large scale roasting processes.
Materials and Methods
Materials Used
Jumbo-grade sized peanuts of the GA-06G cultivar, large-seed, runner-type variety, were obtained from the most recent U.S. peanut harvest from the USDA ARS National Peanut Research Lab in Dawson, Georgia. Prior to acquiring the peanuts, they had been grown, harvested, cured, shelled, sized, and stored according to standard industry practices. Upon delivery to the USDA ARS Food Science and Market Quality and Handling Research Unit at North Carolina State University in Raleigh, North Carolina, the peanuts were stored in sealed and refrigerated containers until sample analysis and roasting was performed. Chemical reagents utilized in analyses were obtained from Thermo Fisher (Thermo Fisher, Waltham, MA) unless otherwise stated.
Sample Separation
Unblanched peanut seeds (12,800 g) were separated into 128 coded aliquots of 100 grams each. This was done with an inhouse fabricated riffle divider, which divides dry materials in equal halves with a series of chutes that discharge from a divided hopper. Samples were stored in individual plastic tubes at 15 C until analysis.
Seed Sizing
Approximately 75 grams of raw seeds were counted with an Old Mill seed counter, Model 850-3 (International Marketing and Design Corp, San Antonio, TX, USA). The seeds were collected and weighed. The average seed size was calculated by dividing the total weight by number of seeds. Each subsample lot was measured. The mean g/seed was 0.66 ± 0.01 (max 0.71, min 0.64). The mean seed per g was calculated to be 1.50 ± 0.02.
Roasting
A 75 g sample of each peanut subsample was roasted in a lab scale oven (Despatch Industries, Minneapolis, MN, USA) at 177 C for a predetermined time between 4 - 24 minutes in replicates of seven (Table 1). Shi and others (2017) identified 177 C as optimal from five different temperatures tested. This temperature has also been used for research studies in the past (Sanders et al. 1989; Greene et al. 2008; McDaniel et al. 2012). Immediately after roasting, peanuts were cooled to ambient temperature (~21 C) using forced air. The skins were manually removed during the cooling stage.
Total Oil Content
Total oil content was determined in the raw and roasted peanuts by time domain nuclear magnetic resonance (NMR), using a Minispec MQ One Seed Analyzer (Bruker Corporation, Billerica, MA, USA). This method is a standard method for the determination of the oil content in seed (ISO10565) The instrument was first calibrated with a curve composed of varying amounts of peanut oil over a range of 0 to 100 % according to the manufacturer’s instructions and specifications. Each sample aliquot was analysed using a 10 to 11 g sample of whole seeds in triplicate, using different peanuts for each measurement.
Moisture Content
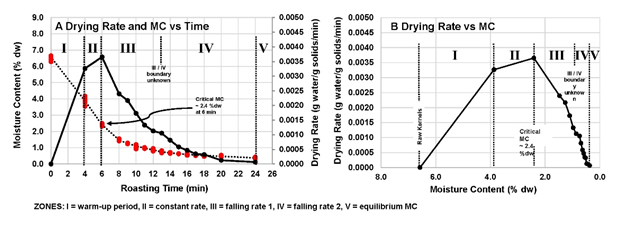
The moisture content of both raw and roasted peanut seeds was measured as described by Young and others (1982). In brief, 6 g samples of whole peanut were loaded into pre-weighed metal weighing dishes (Heathrow Scientific LLC, Vernon Hills, IL, USA). The samples were dried for 6 hours at 130 C in a forced air oven (LXD Series, Despatch Industries, Minneapolis, MN, USA). The drying rate was determined as discussed in the results section and drying curves were constructed (Figure 2).
Color
The L, a, b color values of roasted samples were measured as whole seeds, in five replicates using a HunterLab D25L DP 9000 colorimeter (Hunter Associates Lab Incorporated, Reston, VA, USA). The instrument was standardized every four hours of analysis time using standard color plates sourced from the manufacturer. The samples were loaded as a single layer into glass petri dishes and the measurement taken. The samples were then ground into pastes with a Blixer-3 food processor (Robot Coupe, Jackson, MS, USA). The whole seeds were added into the processor and then ground for 30 seconds, allowed to cool, with the walls of the grinder vessel scraped down into the bottom, and ground again. The process was repeated until a smooth paste was achieved and the temperature was not allowed to rise above 32 C to avoid loss of volatiles (Sanders et al., 1989). The pastes were loaded into glass petri dishes to the rim, smoothed with a plastic knife and then reassessed for color, in three replicates. The pasted samples were stored at -5 C until descriptive sensory analysis was performed.
Analysis of Sugars
The raw and roasted peanut samples were analysed for carbohydrate content following the method of Pattee et al. (2000a). Whole seed samples were ground by pulsing in a coffee grinder (Cuisinart, East Windsor, NJ, USA) to achieve a consistency of a fine meal. After grinding, the samples were defatted by rinsing with hexane at room temperature. The hexane was poured off and the sample was allowed to air dry. In brief, the sugars were extracted with a mixture of methanol/chloroform/water (60/25/150 v/v/v). After the solvent addition, the samples were vortexed and sonicated in screw capped glass tubes using a Model Quantrex 140H sonicator (L&R Ultrasonics, Kearny, NJ, USA). The tubes were centrifuged to separate the insoluble material using an IEC Model K centrifuge (Block Scientific, Inc., Bellport, NY, USA). The supernatant was evaporated using vacuum. The dried residue was dissolved in an aqueous solution of lactose (Sigma Chemical Corp., St. Louis, MO, USA) and cellobiose (Sigma Chemical Corp.) as internal standards. After an additional dilution with water, the samples were filtered through Dionex OnGuard-II H filters (Dionex Corp., Sunnyvale, CA, USA) to remove free amino acids that interfere with carbohydrate analysis. The samples were analysed for carbohydrates using a Dionex BioLC (Dionex Corp.) fitted with an ion exchange column (Dionex Carbopac PA-1, 250 mm length, 4.7 mm interior diameter). The mobile phase was 200 mM NaOH with a flow rate of 1.0 mL/min at 30 C. The peak detection was by Pulsed Amperometry using the waveform recommended by the instrument manufacturer. An aqueous standard solution was prepared containing myo-inositol, glucose, raffinose, stachyose (Sigma Chemical Corp.), sucrose and fructose (Thermo Fisher Scientific, Waltham, MA, USA), in addition to the internal standards and run with the samples. Each sample was extracted and analysed four times. The content of each sugar was calculated using the response factors of the individual sugars to the internal standards.
Analysis of Free Amino Acids
The analysis of the free amino acids was performed on the extracts prepared for sugars (Grimm et al. 1996). The sample extracts were purified using centrifugal filter units (Durapore® Ultrafree-MC-GV, Merck Millipore, Cork IRL) fitted into Eppendorf type tubes. The tubes were centrifuged for ten minutes in an Eppendorf centrifuge (Eppendorf Biotech Co., Hamburg, Germany) at14,000 g. The supernatants were analysed using a Hitachi Model L-8900 Analyzer (Hitachi High Technologies, Dallas, TX, USA). The instrument was fitted with an ion exchange column (Hitachi #2622SC PF, 40 mm length, 6.0 mm interior diameter). The amino acid separation was achieved using a quaternary gradient of borate buffers (PF type, Hitachi High Technologies) with a temperature gradient of 30 C to 70 C, as described by the instrument manufacturer’s instructions. Hitachi High Technologies technical support provides a gradient program for each individual column when sold. Technical support adjusts the gradient recommendation for the needs of each customer. Baseline separation of the peaks was achieved. In the case here, 27 timesteps with varying amounts of the buffers and column temperatures were needed. The actual gradient program would not be applicable to another column and thus is not provided here. Post column derivatization of the amino acids present was performed using ninhydrin. Detection was done using UV at wavelengths of 570 nm and 440 nm. Standard curves of amino acids were created with serial dilutions of Pierce H amino acid standard mixture (Thermo Scientific, Rockford, IL, USA) with the addition of asparagine, glutamine, and tryptophan (Sigma Chemical Corp.) over a range of 1.0 to 100 mcg AA/mL and run with the samples. Each of the raw and roasted samples was extracted and analysed four times.
Descriptive Sensory Analysis
A trained descriptive sensory panel of six panelists (2 male, 4 female) including students and staff from the USDA Agricultural Research Service, Food Science and Market Quality and Handling Research Unit, and the Department of Food, Bioprocessing and Nutrition Sciences at North Carolina State University, Raleigh, NC, USA evaluated the roasted and pasted samples. The panel evaluated the seven replicates of the 15 roasting treatments using the Spectrum™ universal 15-point intensity scale (Meilgaard et al. 1999). A peanut specific lexicon developed by Johnsen et al. (1988) and modified by Sanders et al. (1989) was utilized and additional flavor attributes determined by the panel was used. The samples were tempered to room temperature (~21 C) before sensory analysis. The samples were evaluated in a randomized order for each evaluation. The panellists evaluated a reference peanut paste as a warm-up, cleansed their palates with unsalted crackers and rinsed their mouths with water between each sample. The sensory descriptors and their definitions are listed in Supplementary Table 1. All panelists were made aware of issues with microbial safety of under roasted samples and given the option to reject tasting samples roasted for less than 5 minutes.
Statistical Analysis
Statistical analysis was performed using XLStat version 9.4 for WindowsTM (Addinsoft, Paris, France). An analysis of variance (ANOVA) was employed to determine the significance of roasting time on peanut chemical and physical attributes. Pairwise comparisons were used to find Tukey’s (HSD) groupings of least squares means at a p < 0.05 significance level.
Results and Discussion
Oil Content
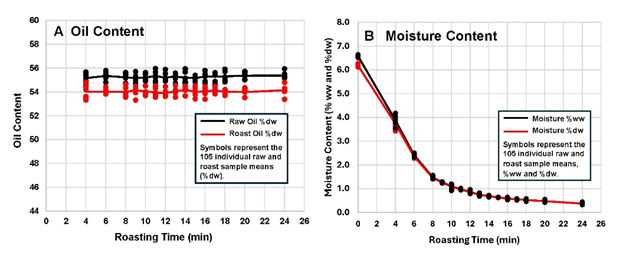
The moisture content in the samples changed greatly as discussed in the next section, but the oil content was not expected to change under these normal dry roasting conditions. The oil content data did provide information on the physical condition of the peanut kernels and enabled the correction of the chemical measurements to a fresh weight basis (FW). The oil contents of the paired raw and roasted measurements remained constant in the raw measurements at 54.0% (DW) and 55.3% (DW) (Figure 1A).
Moisture Content
Moisture content (MC) is known to affect food quality, flavor, shelf-life, and texture. MC affects chemical reaction rates, which can influence the free amino acid and sugar composition of roasted peanuts (Pattee et al. 1981a, Pattee et al. 1982a). The mean raw MC was 6.47% (DW) and ranged from 6.29 to 6.65% (DW) (Figure 1B). Roasting caused MC to drop steadily to a low of 0.37% (DW) at 24 min, consistent with previous studies (McDaniel et al. 2012; Shi et al. 2017). The changes in MC (Figure 2A) exhibited the expected drying curve behaviour (Vijayan et al. 2017).
The Zone I/Zone II interface was possibly less than 4 min in the study, but no lower time points were measured for this report. This did not allow for more than an estimate of the start of Zone II, but the end of Zone II is defined as the CMC, and it is clearly occurring at 6-min.
In Zones I and II, the temperatures between the evaporation front and the kernel surface theoretically remain lower than the oven set temperature due to the large amounts of latent energy within escaping vaporized water molecules. The internal temperatures ahead of this evaporation front are expected to be lower and moisture levels were higher, and similar to the raw kernels. At any given timepoint, the bulk moisture content was therefore partly composed of kernel material that had already seen the passage of the free water loss zone and partly of kernel material that was essentially still raw. Figure 2A allowed determination of the CMC time and thus the Zone II/II boundary time. Comparison to the MC curve in Figure 2A, shows that the CMC occurred at 6 minutes and at a bulk MC of 2.4 % (DW). Although no temperature data was determined in this study, Zone III is defined as beginning when the CMC is reached and is typically where internal temperatures begin to rise above 100 C toward the oven set point. Internal temperatures will lag kernel surface temperatures which reach oven setpoint first (Figure 2A). The Zone III/IV boundary is not well-defined mathematically (Figure 2B). In Zone IV, the drying rate dropped even further until the equilibrium MC was reached at Zone V around 24-min. At equilibrium, the water is tightly adsorbed at the molecular interfaces and is very difficult to remove without a temperature increase.
Color Measurements
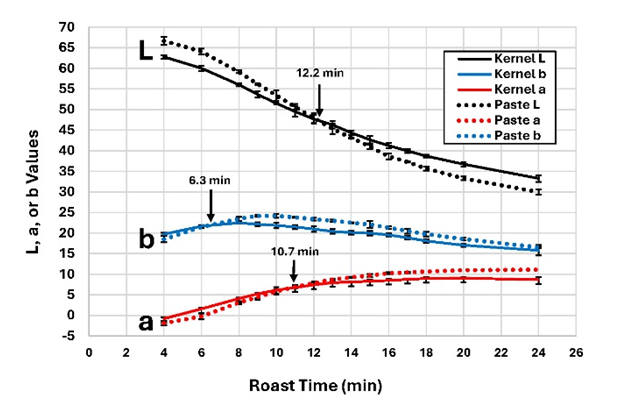
During roasting, the color of the kernel surfaces and the pastes made from the roasted kernels both darkened (Figure 3). The Hunter L value (the lightness-darkness measure) declined from 62.7 in the raw seed to 33.2 at the kernel surfaces and from 66.6 to 29.9 for the paste samples in L value units in 24 min. Longer roasting times promoted the color enhancement that occurred during roasting (Ćosović et al. 2010). The color changes were consistent with previous studies (Moss and Otten 1989; Shi et al. 2017). Initially, seed surface L values were darker than the pasted sample L values at the same roast time because the surfaces of the peanut seeds were darker than the interior as reported previously (Dincer & Genceli 1995; Fadai et al. 2017). When plotted against roasting time, the kernel and paste L-values lines crossed at 12.2 min as indicated by the arrow in the Figure 3. The paste L value curve paste line clearly became steeper than the surface L value color line at 6 min (Figure 3). The Hunter a values for the paste rose (increased reddish chroma) from -1.8 to 11.1 units. The kernel surface Hunter a values rose from -0.8 to 8.6 units. The kernel and paste a-value line crossed at 10.7 min. The kernel Hunter b values rose (more yellow chroma) from 19.7 units, peaked at 22.4 at 8 min, and then fell to 15.8 at 24 min. The paste Hunter b values rose from 18.5 units, peaked at 24.1 at 9 min, and fell to 16.5 at 24 min. The kernel and paste b-value lines crossed at 6.3 min. The crossing of the kernel surface and paste color lines indicated that the color forming reactions differed somehow in the kernel interior compared to the kernel surface. The kernel surface and paste colors dropped in parallel until 6 min (Figure 3). At the time of the CMC, with much less water exiting the kernels, the surface and internal temperatures could begin to rise. At the higher temperatures, more complex reactions can occur leading to browning reactions and flavor formation. Mailliard reactions occur from 140 C to 165 C (Davies and Labuza, 1997), caramelization for most sugars in peanuts starts at 150 C to 170 except fructose (105 C), and pyrolysis will initiate between 200 C and 300 C. The oven setpoint in this study (177 C) mostly avoids pyrolysis but allows caramelization and Mailliard reactions. This raises the question as to why the interior browning occurred more rapidly. The physical conditions at kernel surfaces certainly vary from those in the interior during roasting. As the internal temperatures rose, more water moved to the kernel surface. In the constant rate drying period, this water diffused toward the surface where evaporation is occurred. It is proposed that this water inhibited the surface browning and allowed the internal browning to outpace that at the surface. Another possible difference is the pressure at the surface. When water vapor is produced at the surface, it can diffuse away in the atmosphere. Internally, however, as the free water loss front moves inward, water is being heated to near vaporization and begins to diffuse outward to the surface. It is highly likely that a pressure difference between surface and interior which may have favored the increased browning rates in the interior. The differential browning rates could also be due to certain aspects of the structure and the physiology of the peanut kernels. If parts of the peanut kernels toward the middle of the heated volume are chemically different from the regions nearer the surface, then the browning may have occurred faster in the interior due to the greater availability of substrates such as sugars and free amino acids. One common observational defect in roasted peanuts is the presence of brown hearts where the surfaces of the two halves of each kernel that face each other roast darker than the outer kernel surfaces. This defect could be attributed to immaturity, as immature peanuts are known to roast darker than their mature cohorts (Pattee et al. 1981b). The pattern of color changes in this study has been found in other roasting time tests such as with the Uniform Peanut Performance Trials (UPPT) (unpublished data), often will little evidence of dark heart.
Sugar Composition
The Maillard browning reaction utilizes amine groups on the free amino acids present and sugars as a reactant and produced compounds that have dark pigmentation (Lingnert, 1990). Reducing sugars, that is those with a hemiacetal, have been reported to be important in the development of roasted peanut color, flavor, and aroma (Newell et al. 1967; Mason et al. 1969; Basha 1992; Pattee et al. 2000b). Peanuts naturally contain low concentrations of glucose and fructose, which act as precursors in Maillard browning reactions producing pyrazines and carbonyl compounds (Basha 1992). Peanuts have much larger contents of sucrose, a disaccharide, and oligosaccharides in particular, raffinose, and stachyose. Although not reducing sugars, these carbohydrates will breakdown into monosaccharides during sample processing, most prominent among these being the reducing sugars, glucose, and fructose (Newell et al. 1967; Basha 1992).
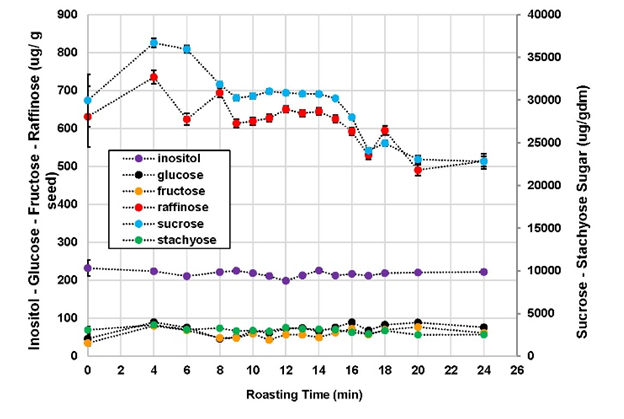
The sugar contents in the raw (time 0) and roasted peanuts are illustrated in Figure 4. The total sugar content describes the combination of glucose, fructose, sucrose, raffinose, inositol, and stachyose. Together, glucose and fructose made up approximately 0.4% of the total sugar in the peanut samples. All individual sugars, aside from inositol, changed significantly with roasting time. As inositol is more closely involved in structural compounds rather than as a source of energy for the seed, it is not unexpected that it would not be involved in forming compounds during roasting (Biffen & Hanke 1991). Sucrose was the most abundant sugar present in both raw and roasted peanuts, contributing to approximately 88% of the total sugar content. All sugar concentrations except inositol rose between 4- and 6-min. After 6 min, sucrose and raffinose content dropped significantly. Inositol changed very little and stachyose dropped slightly after 24 min of roasting. Glucose and fructose rose slightly, most likely due to hydrolysis of sucrose during roasting (Mason et al. 1969). As these are the sugars that are reportedly involved in the Maillard browning reactions, the hydrolyzed products were probably consumed in the formation in those compounds. More discussion of the sugar chemistry follows with the descriptive sensory analysis below.
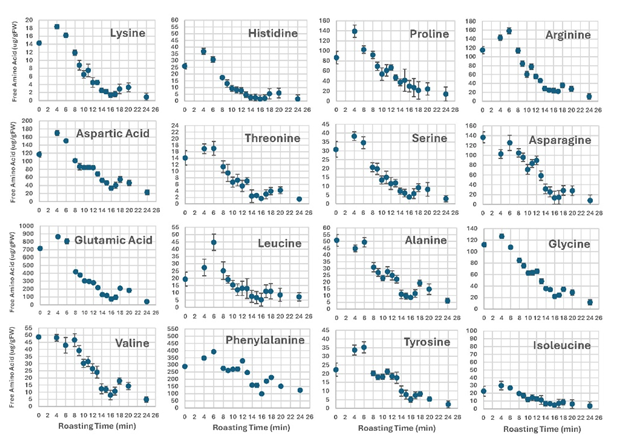
Free Amino Acid Composition
Free amino acids (FAA) are known to be precursors of roasted peanut flavor due to their involvement in sugar-amine nonenzymatic browning reactions that form pyrazines, aldehydes, and carbonyl compounds (Newell et al. 1967; Oupadissakoon & Young 1984b). Most FAA in this study rose or remained unchanged until between 4- to 6-in (Figure 5). Prior to 4 min, the MC was high and increasing temperature was proposed to have led to more FAA by thermally produced protein hydrolysis (Oupadissakoon & Young 1984a). After 6-min, most amino acids decreased significantly over the remaining roast time. Not shown in Figure 5, are free ammonia and glutamine whose low levels changed little with the roasting time. Cysteine became non-detectable after 4-min. Methionine changed from only 13.1 µg/g FW to 1.0 µg/g FW in a pattern to alanine and tryptophan (Figure 5). These FAA trended downward in concentration with increased roast time. Previous studies have attributed the FAA losses to their involvement in the Maillard reactions (Oupadissakoon & Young 1984a; Rodriguez et al. 1989; Sanders et al. 1995. Discussions of the formation of Maillard browning compounds, especially pyrazines, usually describe the combination of the monosaccharides with free amino groups of FAA that are present due to the seed metabolism and because of protein hydrolysis from thermal processes (Lund and Ray 2017). Lysine is often used to study the mechanism of the formation of pyrazines (Scalone et al. 2015). It maybe however that those with the largest changes, aspartic and glutamic acid are being reduced by metabolism of the seed rather than formation of the flavor compounds (Hashim et al, 1998).
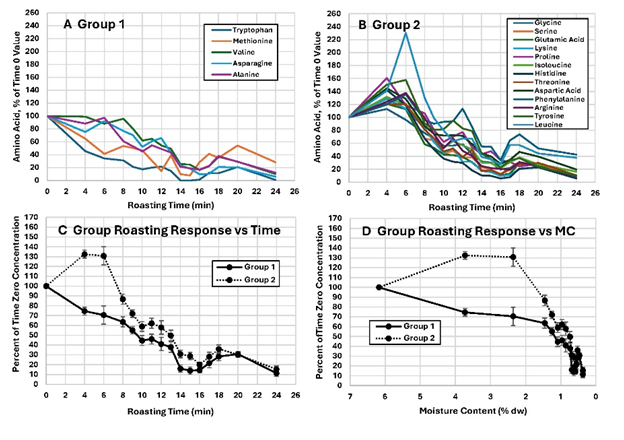
When the FAA were converted to the percent of time 0 value and plotted, all amino acid concentrations (except free ammonia and glutamine) changed in similar fashion (Figures 6A and 6B). The data separated into one of two groups. Group 1 remained level or dropped before 6-min (Figure 6C). Group 2 concentrations rose until 6-min and dropped after the CMC was reached (Figure 6C). All amino acids except cysteine exhibited an increase in concentration at 16 to 18 min and then decreased again afterward (Figures 5, 6A and 6B). After the CMC at 6 min, the surface and internal temperatures were expected to begin rising toward the oven setpoint. This may have induced protein hydrolysis, explaining the small increase at 16 to 18 min, followed later by pyrolytic reactions that occur at even higher temperatures after 18 min of roasting. When the FAA were converted to the percent of time 0 and plotted against MC % (DW), the groups were clearly delineated but both displayed the rapid decrease after the CMC was reached (6 min, 2.4 % (DW) (Figure 6D).
Figure 6. Changes in various free amino acids in whole peanut kernels during roasting expressed as the percentage of time 0 values. A. Free amino acids that remained level or decreased between 0 and 6 min then decreased. B. Free amino acids that increased in concentration from 0 to 6 minutes then decreased. C. Mean Group 1 and Group 2 free amino acid response plotted vs time. C. Mean Group 1 and Group 2 free amino acid response plotted vs. MC % DW.
Descriptive Sensory Analysis
Flavor is the primary driving force behind the consumption of peanut products, and it can be described and quantified through descriptive sensory analysis (DSA) (Sanders et al. 1997; Neta et al. 2010). The characteristic flavor attribute for peanuts is roast peanutty (RP). Another “on” flavor is sweet aromatic (SA). Off notes such as fruity fermented, painty, and cardboardy, along with bitter (BI) and ashy (ASHY) are perceived as negative attributes in peanuts while RP and SA have been reported as generally positive attributes that were associated with contributing pleasant flavor in peanuts (Schirack et al. 2006). Dark roast (DR), raw beany (RB), woody-hulls-skins (WHS), sweet taste (SW), and bitter taste (BI), are always present and are integral parts of the overall peanut flavor profile.
The roast flavor of many foods including coffee, tree nuts, meats, cereals, and roast peanuts has long been attributed to the formation of pyrazines from the free amino acids present in the food and reducing sugars, mainly glucose (Yu et al. 2021). Products of Maillard browning reactions, specifically, volatile heterocyclic nitrogen-containing compounds such as pyrazines and pyrroles (Baker et al. 2003; Neta 2010; Lykomitros et al. 2016), are also linked to color development (Davies & Labuza 1997; Klevorn & Dean 2018). Previous research has drawn strong correlations between roast peanutty and many compounds, including 2-ethyl-6-methylprazine (Buckholz et al. 1980), 2,5- dimethylpyrazine (Baker et al. 2003; Lykomitros et al. 2016), and methylpyrazine (Leunissen et al. 1996). In older studies, many alcohols and aldehydes have also been related (Pattee et al. 1969). Pyrazines in high concentration have been associated with dark roast and bitter attributes (Smyth et al. 1998; McDaniel et al. 2012). 2-acetyl-3-methylpyrazine has been found to correlate with dark roast and 2-ethyl-5-methylpyrazine correlated with dark roast and with woody/hulls/skins. 2-acetyl-3-methylpyrazine possesses a roasted/nutty aroma (Neta 2010). 2-ethyl-5- methylpyrazine has been found in peanuts to comprise a sweet/nutty (Didzbalis et al. 2004), fruity/sweet (Schirack et al. 2006) nutty/roasted (Braddock et al. 1995), and sweet odor (Matsui et al. 1998). Several ketones and aldehydes were identified in these same roasted runner peanuts in another study (Weissburg et al. 2023) and correlated with sweet aromatic sensory attributes, including 2,3-pentanedione, 2-methyl-butanal, and 2,3-butanedione. 2,3-pentanedione has been reported to have a honey-like aroma in almonds (Erten & Cadwallader 2017) and in peanuts (Lykomitros et al. 2016). 2,3- butanedione has also significantly correlated with RP and has a buttery aroma (Erten & Cadwallader 2017).
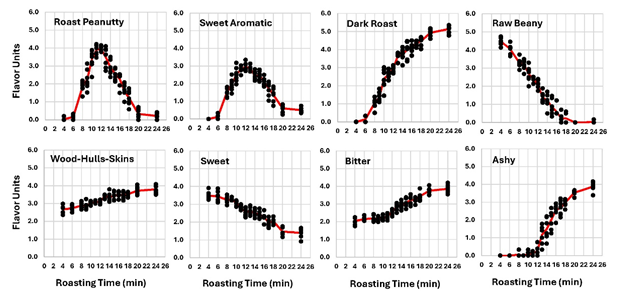
Despite these advances in chemical knowledge, descriptive sensory is still the best way to assess peanut flavor, as it is also how consumers experience the product. Several on-flavor descriptors changed significantly during roasting (Figure 7). Roast peanutty rose dramatically after 6-min and peaked between 11- and 12-min. The maximum RP for these peanuts was 3.9 at 11 min and 4.0 at 12-min, which is low. In the experience of this research unit, excellent peanut samples historically have RP values between 5.0 and 6.0 flavor units. After 12-min, RP dropped back to very low levels by 24-min. Industry empirical experience has long suggested that highest RP levels are obtained when the kernel surface L value is 49.5 to 50.5 units (Pattee et al. 1991). The kernel L value at the maximum RP was between 49.5 at 11-min and 47.6 at 12-min in this study. RP production did not kick in significantly until 6-min. This corresponded to the time of the CMC (Figure 2A) when, in theory, internal temperatures finally rise above 100 C, enabling Mailliard and other reactions to occur (Peterson et al. 1994). Some of the products of these reactions also impart a brown color and this was reflected in the color data. At 6 min, kernel and paste L values began dropping at a faster rate with pastes getting darker faster. Similarly, the b value paste and surface lines were both rising with pastes rising faster than kernel surfaces and crossed at 6.3-min. At 12-min, RP production either shut down, destroyed by rising temperatures or masked by newly formed compounds. During Zone III (falling rate zone), MC decreases more slowly compared to the constant drying rate in Zone II, and internal temperatures rise, in theory. It is possible that RP production shut down at 12 min due to MC < 1.0 % (DW) or to the internal temperatures rising and no longer favoring those particular flavor reactions. It can be seen from this study that conditions after 12-min enhanced flavor loss.
Sweet aromatic (SA) also exhibited the same bell-shaped curved as RP, with a peak at 12 min. The overall dynamic of flavor accumulation followed by flavor loss was similar to RP. Dark roast (DR) rose rapidly, with an inflection point at 12.5 min thereafter rising at a slower rate (Figure 7). Raw beany (RB) was highest in the raw samples and dropped steadily in a linear fashion until becoming non-detectible around 16 min. The ashy (ASHY) off flavor became detectable at 13 min and rose sharply until 18 min, then rose less rapidly. Woody/Hulls/Skins (WHS) rose slowly over 24 min. Sweet (SW) taste dropped steadily over time and was barely detectable by 18 min. Bitter taste rose slowly until 11 min where it began a faster rate of increase. RP, SA, and DR initially became detectable (crossed the threshold of 1.0 units) by the panel between 6 and 7 min near the time of the CMC at 6 min. RP, SA, DR, and bitter all had maximums or inflection points in their curves at 12 min. The first detection of ashy was just after 12 min.
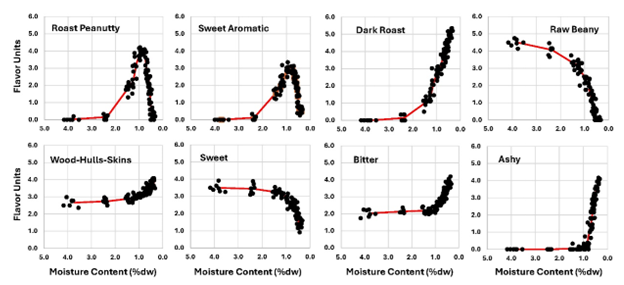
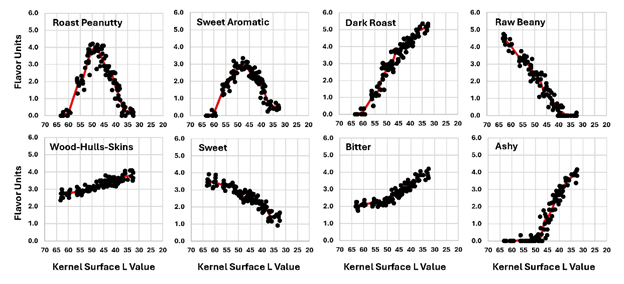
The relationship of flavor responses to MC % (DW) is illustrated in Figure 8. All flavor descriptor vs. MC curves exhibited some kind of inflection point of change when moisture was close to 1.0 % (DW). RP and SA peaked and DR, RB, WHS, SW, BI and ASHY all exhibited inflection points around 1.0 % MC (DW). This suggests that that the chemistry changed significantly after drying advanced to 1.0 % MC (DW). Similar curves were obtained when flavor data were plotted against kernel surface L values (Figure 9). RP and SA peaked at approximately the same color (L=47.5 to 49.5). At L=50, DR formation slowed, SW loss became faster and BI and ASHY rose much faster than at colors lighter than L=50. If other varieties with different physical properties (size, oil content, raw MC, etc.) are tested in this way, they may or may not exhibit the changes shown here at the same times, moisture levels, or surface kernel color. It is logical to assume the timing of various features in the sensory and chemistry plots could altered by changing seed size or raw MC (Pattee et al. 1982b). This would increase the length of the constant drying rate zone and delay the reactions needed to create the flavor and color curves observed here. If it was found that varieties with divergent physical and chemical properties grown in various locations exhibit the same response patterns but shifted in time only, that will constitute evidence that the color and moisture levels associated with the sensory features observed in this study are universal.
Summary and Conclusions
Roasting significantly changes the physical and chemical composition of peanuts. Browning and flavor formation appeared to be associated with moisture movement and subsequent changes in internal kernel temperatures. The drying curve (Figure 2) indicated CMC was at 6 min. At this point, it is theorized that temperatures should begin to rise inside kernels and allow reactions browning reactions which occur from 100 C to 150 C (Koehler et al. 1971). After 6 min, sucrose and raffinose began declining and most of the FAA also began to drop in began to darken more rapidly than the surface color until they crossed at 12 min (Figure 3). At this point, MC was at 0.9% and the RP and SA sensory attributes after having achieved maximum values at 12 min, began to drop rapidly. This may indicate the combination of very low MC and higher temperatures after 12 min had led to caramelization and pyrolysis which occur at higher temperature than the Mailliard reactions (Davies and Labuza 1997). Molecules associated with RP and SA break down and other molecules associated with the sensory attributes of DR, WHS, bitter, and ashy begin to accumulate. The time range in which maximum RP is found is a narrow one. By adjusting either roast time using belt speed in an industrial setting or roast temperature and monitoring kernel color, this maximal flavor impact can be maintained. Allowing for increased dark roasting will significantly reduce RP. When other colors are requested for specific applications of roast peanuts, there is the risk of losing RP flavor impact. For peanut breeders, when selecting for flavor or trying to associate genetic markers with flavor, care must be taken to roast at the correct time to achieve a color that will give the maximum RP. The specific timing of flavor changes may vary from those reported here if the seed size, raw MC, or maturity are different in other lots being tested. Larger size and higher raw MC should increase the time to reach the CMC. Thus, optimal roasting parameters will shift for peanut seeds of another size or MC, but achievement of the stabilized moisture content and the color equivalence between the exterior and the interior of the seed will result in the optimum roasted flavor.
Acknowledgements
This project was funded by USDA ARS CRIS project number 6070-43440-012-00D. The authors declare no conflicts of interest.
Disclaimer
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Literature Cited
Baker G.L., Cornell J.A., Gorbet D.W., O’Keefe S.F., Sims C.A., and Talcott S.T.. 2003. Determination of pyrazines and flavor changes in peanuts during roasting. J. Food Sci. 68(1):394-400.
Basha S.M. 1992. Soluble sugar composition of peanut seed. J. Agric. Food Chem. 40:780-783.
Biffen M., and Hanke D.E.. 1991. Metabolic fate of myo-inositol in soybean callus cells. Plant Sci. 75(2):203-213.
Braddock J.C., Sims C.A., and Okeefe S.F.. 1995. Flavor and oxidative stability of roasted high oleic-acid peanuts. J. Food Sci. 60(3):489-493.
Buckholz L.L., and Lawrence L.. 1980. Influence of roasting time on sensory attributes of fresh roasted peanuts. J. Food Sci. 45:547-554.
Chen P., Chen N., Zhu W., Wang D., Jiang M., Qu C., Li Y., and Zou Z. 2023. A heat and mass transfer model of peanut convective drying based on a two-component structure. Foods. 12(9):1823.
Davies C.G.A., and Labuza T.P. 1997. The Maillard reaction: Application to confectionery products, pp. 35-66. In G.R. Zeigler (ed). Confectionary Science. Pennsylvania University Press, Philadelphia, PA.
Davis J.P., and Dean L.L.. 2016. Peanut composition, flavor, and nutrition, pp. 289-345. In: T.H. Stalker and R.F. Wilson (eds). Peanuts: Genetics, Processing, and Utilization. Academic Press, New York, NY.
Didzbalis J., Ritter K.A., Trail A.C., and Plog F.J.. 2004. Identification of fruity/fermented odorants in high-temperature-cured roasted peanuts. J. Agric. Food Chem. 52(15):4828- 4833.
Dincer I., and Genceli O.F.. 1995. Determination of surface heat transfer coefficients from measured temperature data for spherical and cylindrical bodies during cooling. Heat Mass Trans. 30:215-220.
Erten E.S. and Cadwallader K.R.. 2017. Identification of predominant aroma compounds of raw, dry roasted and oil roasted almonds. Food Chem. 217:244-253.
Fadai N.T., Melrose J., Please C.P., Schulman A. and Van Forder R.A.. 2017. A heat and mass transfer study of coffee bean roasting. Int. J. Heat Mass Trans. 104:787-799.
Greene J. L., Sanders T. H., and Drake M. A.. 2008. Characterization of volatile compounds contributing to naturally occurring fruity fermented flavor in peanuts. J. Agric. Food Chem. 56(17):8096-8102.
Grimm D.T., Sanders T. H., Pattee H. E., Williams D.E., and Sanchez-Dominguez S.. 1996. Chemical composition of Arachis hypogaea Var. hirsute peanuts. Peanut Sci. 23(2):111-116.
Hashim P., Selamat J., Muhammad S.K.S. and Ali A.. 1998. Changes in free amino acid, peptide-N, sugar and pyrazine concentration during cocoa fermentation. J. Sci. Food Agric. 78:535-542.
ISO10565. 2019. Oilseeds. Simultaneous determination of oil and water contents. International Organization for Standardization. Geneva, Switzerland.
Johnsen P.B., Civille G.V., Vercellotti J.R., Sanders T.H., and Dus C.A.. 1988. Development of a lexicon for the descriptors of peanut flavor. J. Sens. Stud. 3(1):9-17.
Klevorn C.M. and Dean L.L.. 2018. A metabolomic-based approach identifies changes in the small molecular weight compounds composition of the metabolome of the peanut as a result of dry-roasting. Food Chem. 240:1193-1200.
Koehler P.E., Mason M.E., and Odell G.V.. 1971. Odor threshold levels of pyrazine compounds and assessment of their role in the flavor of roasted foods. J. Food Sci. 36:816-818.
Lee C.M. and Resurreccion A.V.A . 2006. Predicting sensory attribute intensities and consumer acceptance of stored roasted peanuts using instrumental measurements. J. Food Qual. 29(4):319-338.
Leunissen M., Davidson V.J., and Kakuda Y. . 1996. Analysis of volatile flavor components in roasted peanuts using supercritical fluid extraction and gas chromatography mass spectrometry. J. Agric. Food Chem. 44(9):2694-2699.
Lingnert H. 1990. Development of the Maillard reaction during food processing, pp. 171-185. In: Finot P. (ed.) The Maillard Reaction in Food Processing, Human Nutrition and Physiology. Birkhauser, Basel, Switzerland.
Lund M.N., and Ray C.A.. 2017. Control of Maillard reactions in foods: strategies and chemical mechanisms. J. Agric. Food Chem. 65(23):4537-4552.
Lykomitros D., Fogliano V., and Capuano E.. 2016. Flavor of roasted peanuts (Arachis hypogaea)- part 2: Correlation of volatile compounds to sensory characteristics. Food Res. Int. 89(1):870- 881.
Mason M.E., Johnson B.R., and Hamming M.C.. 1966. Flavor component of roasted peanuts. Some low molecular weight pyrazines and pyrrole. J. Agric. Food Chem. 14:454- 460.
Mason M.E., Newell J.A., Johnson B.R., Koehler P.E., and Waller G.R.. 1969. Nonvolatile flavor components of peanuts. J. Agric. Food Chem. 17(4):728-732.
Matsui T., Guth H., and Grosch W.. 1998. A comparative study of potent odorants in peanut, hazelnut, and pumpkin seed oils on the basis of aroma extract dilution analysis (AEDA) and gas chromatography olfactometry of headspace samples (GCOH). Fett-Lipid 100(2):51-56.
McDaniel K.A., White B.L., Dean L.L., Sanders T.H., and Davis J.P.. 2012. Compositional and mechanical properties of peanuts roasted to equivalent colors using different time/temperature combinations. J. Food Sci. 77:C1292-C1298.
Meilgaard M., Civille C.V., and Carr B.T.. 1999. Sensory Evaluation Techniques. CRC PressBoca Raton, FL.
Moss J.R., and Otten L.. 1989. A relationship between color development and moisture during roasting of peanuts. Can. Inst. Food Sci. Tech. 1:34-39.
Neta E.R., Sanders T.H. , and Drake M.A. . 2010. Understanding peanut flavor: A current review, pp. 985-1022. In Y.H. Hui (ed.) Handbook of Fruit and Vegetable Flavors. John Wiley & Sons, Inc. Hoboken, NJ.
Newell J.A., Mason M.E., and Matlock R.S.. 1967. Precursors of typical and atypical roasted peanut flavour. J. Agric. Food Chem. 15:767-772.
Oupadissakoon C., and Young C.T.. 1984a. Changes in free amino acid and sugars of peanuts during oil roasting. Peanut Sci. 11:6-9.
Oupadissakoon C., and Young C.T.. 1984b. Modeling of roasted peanut flavor for some virginia-type peanuts from amino acid and sugar contents. J. Food Sci. 49:52-58.
Pattee H.E., Singleton J.A., Cobb W.Y. 1969. Volatile components of raw peanuts: Analysis by gas liquid chromatography and mass spectrometry. Journal of Food Science. 34:625.
Pattee H.E., Young C.T., and Giesbrecht F.G.. 1981a. Free amino acids in peanuts as affected by seed size and storage time. Peanut Sci. 8:113-116.
Pattee H.E., Young C.T., and Giesbrecht F.G.. 1981b. Seed size and storage effects on carbohydrates of peanuts. J. Food Sci. 29(4):800-802.
Pattee H.E., Young C.T., Pearson J.L., Singleton J.A., and Giesbrecht F.G.. 1982a. Storage and moisture effects on peanut composition and roasted flavor. Peanut Sci. 9:98-101.
Pattee H.E., Pearson J.L., Young C.T., and Giesbrecht F.G.. 1982b. Changes in roasted peanut flavor and other quality factors with seed size and storage time. J. Food Sci. 47(2):455-456.
Pattee H.E., Giesbrecht F.G., and Young C.T.. 1991. Comparison of peanut butter color determination by CIELab L a b and Hunter color-difference methods and the relationship of roasted peanut color to roasted peanut flavor response. J. Agric. Food Chem. 39(3):519-523.
Pattee H.E., Isleib T.G., Giesbrecht F.G., and McFeeters R.F.. 2000a. Investigations into genotypic variations of peanut carbohydrates. J. Agric. Food Chem. 48:750–756.
Pattee H.E., Isleib T. G., Giesbrecht F.G., and McFeeters R.F.. 2000b. Relationships of sweet, bitter, and roasted peanut sensory attributes with carbohydrate components in peanuts. J. Agric. Food Chem. 48:757–763.
Perren R., and Escher F.E.. 2013. Impact of roasting on nut quality, pp. 173-197. In L.J. Harris (ed.). Improving the Safety and Quality of Nuts. Woodhead Publishing, Cambridge, UK.
Peterson B.I., Tong C.H., Ho C.T., and Welt B.A.. 1994. Effect of moisture content on Maillard browning kinetics of model system during microwave heating. J. Agric. Food Chem.
Poirier D., Sanders T.H., and Davis J.P.. 2014. Salmonella surrogate reduction using industrial peanut dry roasting parameters. Peanut Sci. 41(2):72-84.
Purlis E. 2010. Browning development in bakery products - a review. J. Food Eng. 99(3):239-249.
Rodriguez M.M., Basha S.M., and Sanders T.H.. 1989. Maturity and roasting of peanuts are related to precursors of roasted flavor. J. Agric. Food Chem. 37:760-765.
Sanders T.H., Vercellotti J.R., Crippen K.L., and Civille G.V.. 1989. Effect of maturity on roast color and descriptive flavor of peanuts. J. Food Sci. 54:475-477.
Sanders T.H., Pattee H.E., Vercellotti J.R., and Bett K.L.. 1995. Advances in peanut flavor quality, pp. 528-553. In H.E. Pattee, and H.T. Stalker, (eds.), Advances in Peanut Science. American Peanut Research and Education Society, Stillwater, OK.
Sanders T.H., Vercellotti J.R., Bett K.L., and Greene R.L.. 1997. The role of maturation in quality of stackpole-cured peanuts. Peanut Sci 24:25-31.
Scalone G.L.L., Cucu T., De Kimpe N. and De Meulenaer B.. 2015. Influence of free amino acids, oligopeptides, and polypeptides on the formation of pyrazines in Maillard model systems. J. Agric. Food Chem. 63(22):5364-5372.
Schirack A.V., Drake M.A., Sanders T.H., and Sandeep K.P.. 2006. Characterization of aroma active compounds in microwave blanched peanuts. J. Food Sci. 71(9):C513-C520.
Shi X., Sandeep K.P., Davis J.P., Sanders T.H., and Dean L.L.. 2017. Kinetics of color development of peanuts during dry roasting using a batch roaster. J. Food Proc. Eng. 40: e12498.
Smyth D.A., Macku C., Holloway O.E., Deming D.M., Slade L., and Levine H. . 1998. Evaluation of analytical methods for optimizing peanut roasting for snack foods. Peanut Sci. 25:70-76.
van Boekel M., Fogliano V. , Pellegrin N. , Stanton C. , Scholz G. , Lalljie S. , Somoza V. , Knorr D. , Jasti P.R., and Eisenbrand G. . 2010. A review on the beneficial aspects of food processing. Mol. Nutr. Food Res. 54(9):1215-1247.
Vijayan S., Arjunan T.V., Kumar A. 2017. Fundamental Concepts of Drying. In: Prakash, O., Kumar, A. (eds) Solar Drying Technology. Green Energy and Technology. Springer, Singapore. pp 3-38. https://doi.org/10.1007/978-981-10-3833-4_1.
Weissburg J. R., Johanningsmeier S. D., and Dean L. L.. 2023. Volatile compound profiles of raw and roasted peanut seeds of the runner and virginia market-types. J. Food Res. 12 (3):47-68.
Young J.H., Whitaker T.B., Blankenship P.D., Brusewitz G.H., Troeger J.M., Steele J.L., and Person N. K.. 1982. Effect of oven drying time on peanut moisture determination. Trans. ASAE. 6823:491-496.
Yu H., Zhang R. , Yang F. , Xie Y. , Guo Y. , Yao W. , and Zhou W. . 2021. Control strategies of pyrazine generation from Maillard reaction. Trends Food Sci. Tech. 112:795-807.
Notes
- First author: Graduate Student, Dept. of Food, Bioprocessing and Nutrition Sciences, North Carolina State University Raleigh, NC 27695-7624; Second, Third authors: Research Food Technologist, Food Technologist, Food Science and Market Quality and Handling Research Unit, USDA, ARS, SEA, Raleigh, NC 27695-7624. [^] Corresponding author’s E-mail: Lisa.Dean@usda.gov