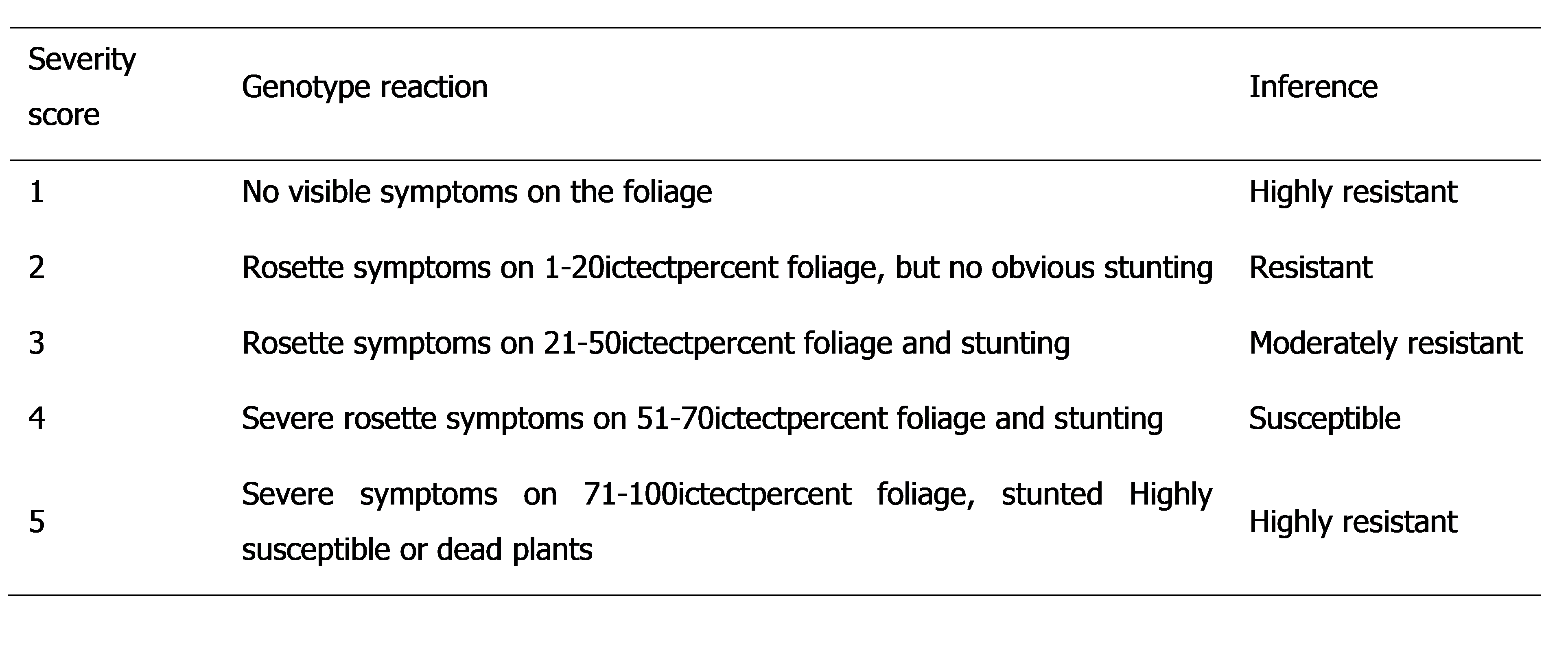INTRODUCTION
Peanut (Arachis hypogaea L.), also known as groundnut, is an important legume for food, feed, nutrition, and income (Ojiewoet al., 2020). Grown throughout the world, peanut is highly nutritious with several health benefits (Mienieet al., 2013;Willettet al., 2019) and an invaluable source of protein, calories, essential fatty acids, vitamins, and minerals (Mienieet al., 2013;Okelloet al., 2013). Although Africa and Asia together account for about 90percent of the global peanut production, a downward production trend has been observed in Africa from 33percent global contribution in 2019 to 30percent in 2021 (FAOSTAT, 2021). Part of this reduction in productivity is attributed to various biotic and abiotic stresses, of which groundnut rosette disease (GRD), early leaf spot (ELS) and late leaf spot (LLS) are among the most important (Naiduet al., 1999;Waliyaret al., 2007;Mohammedet al., 2018).
Groundnut rosette disease (GRD) is considered the most devastating viral disease of peanut in Sub-Saharan Africa, causing yield losses of up to 100percent when the disease attacks the crop before flowering (Okelloet al., 2014). The disease is transmitted by aphids (Aphis craccivoraKoch) (Lynch 1990) and caused by a complex of three viral agents that work synergistically: groundnut rosette assistor luteovirus (GRAV) which encapsidates GRV and its satellite RNA for transmission by the aphids, groundnut rosette umbravirus (GRV) which plays a role in the replication of its satellite RNA (satRNA) and GRV satellite RNA which is responsible for the symptoms observed (Naiduet al., 1999; Deomet al., 2000 ). The presence of all the three disease agents results in acutely stunted and bushy plants with shortened internodes and reduced leaf size (Nigamet al., 2012).
Early leaf spot caused by Passalora arachidicola and late leaf spot caused by Nothopassalora personata are the most devastating and economically important foliar fungal diseases and major yield reducing factor of peanut worldwide with an annual yield losses of 15 to 50 percent (Pandeyet al., 2017;Ancoet al., 2020). The predominance of ELS or LLS varies by location and shifts over time depending on the cultivars grown, weather and cultural practices. Although just one leaf spot pathogen usually predominates in a production region, both leaf spot species are generally found in a single field. Shifts in leaf spot species also have been observed over a period of years. Late leaf spot currently is the predominant leaf spot disease in East Africa, whereas ELS is common in Southern Africa (Subrahmanyam et al., 1997;Okelloet al.,2013).
Late leaf spot results in the reduction of pod yield and the quality of fodder (Okelloet al., 2013) with an estimated $599 million in annual losses (Monyoet al., 2009). The typical symptoms are dark brown or black lesions on the underside of affected leaves (Tshilenge-Lukandaet al., 2012), which result in reduced chlorophyll activity and photosynthesis (Singhet al., 2011). Late leaf spot is soilborne and appears on the plant 3-5 weeks from planting. It starts when mycelium directly producing conidia in the soil from crop debris is dumped on young peanut plant leaves by rain splashes. The disease thrives at temperatures ranging between 25 to 30 C and relatively high humidity (McDonaldet al., 1985).
The use of insecticides against aphids and fungicides against fungal pathogens can result in the pollution of the environment, health risks and higher costs of production for farmers (Kheraet al., 2016). The use of several cultural methods have been useful in reducing LLS inoculum but these approaches are quite labour intensive (Kankamet al., 2022). The development of resistant peanut varieties is the most effective and practical approach to manage the diseases (Nigamet al., 2012;Wankhadeet al., 2021). It is therefore important to develop varieties with dual resistance to GRD and LLS to minimize yield losses from both diseases.
Studies have identified lines resistant to both GRD and LLS (Iwo and Olorunju, 2009;Mohammedet al., 2018;Essandohet al., 2022). However, these lines are of limited and unknown diversity. Core collections which are of known diversity, have been practical for enhanced utilization of germplasm for improvement of various traits in crops and identification of new sources of variation (Upadhyayaet al., 2013). This is because variation from core collections represents the diversity in entire collections but are a smaller set of accessions with minimal repetitiveness (Frankel, 1984). In order to identify sources of variation for any trait, core collections would be the primary point of reference.
The aim of this study was to identify new sources of resistance to GRD and LLS from a diverse GINA core collection. The best performing lines can be used as sources of resistance in breeding programs across Africa to introgress GRD and LLS resistance in farmer preferred lines or tested for their adaptability, yield potential and released to farmers.
MATERIALS AND METHODS
Plant material
Two-hundred and twenty-nine (229) breeding lines from nine African countries (Ghana, Mali, Malawi, Mozambique, Niger, Senegal, Togo, Uganda and Zambia) were used for this study (Figure 1 , Table 1). These lines were part of the GINA core collection (Condeet. al., 2023) which was created on the basis of 116 breeders-preferred lines and extended to 300 genotypes using genotyping data and the core hunter software (DeBeukelaeret al., 2018). The 229 genotypes consisted of the subspecies fastigiata (32 “hybrid” combinations between the Virginia and Spanish botanical types, 111 Spanish, 11 Valencia) and the hypogaea subspecies (75 Virginia) (Table 1). The basis on which the 229 lines were chosen from the “300 core” was the number of seed available for trials across two locations each season. Each trial was limited to a maximum of 200 genotypes each season.
Phenotypic evaluation
Field evaluation of genotypes was carried out in Eastern Uganda at Nakabango and Serere, recognized as GRD and LLS hotspot locations (Okelloet al., 2010). Nakabango lies 33o12'47.588" E and 0o31'26.762" N at 1169m above sea level while Serere lies 33A°26'43.943"E and 1A°31'58.580" N at 1126m above sea level. Across the three seasons, NaSARRI had an average temperature of 24.56 C, relative humidity (RH) of 71.5percent, monthly rainfall of 146.25mm and windspeed of 2.35m/s, while Nakabango had an average temperature of 22.8 C, RH of 78.25percent, monthly rainfall of 167.12 and wind speed of 2.18m/s ( https://power.larc.nasa.gov/data-access-viewer/).
At each location, the 200 lines were planted at a spacing of 45x15cm (between and within rows, respectively), with two 1-meter rows per plot. The trial was planted in a 10 x 20 lattice design, in seasons 2020A, 2020B and 2021B in two replicates across the two locations. Genotypes Ug-43_Oug-RED_BEAUTY_UG and Gh2-54_GhaII-NUMEX_03 were used as susceptible checks, Ug-41_Oug-DOK_1_RED_UG and Ug-194_Oug-ICGV_90099 as resistant checks for GRD while Mz-52_MZG-JL-24 was used as a susceptible and Ug-7_Oug-SERENUT 14R UG as a resistant check for LLS.
Data collection
GRD percentage disease incidence (Table1 ) and disease severity (Table 2 ) ratings (Waliyaret al., 2007) were used to assess the response of the genotypes to GRD while LLS severity (Table 3) (Subrahmanyamet al., 1995) was used for LLS resistance assessment. With reference to the 1-9 LLS severity scale, disease scores were categorized as resistant (1-3), moderately resistant (4–5), susceptible (6–7) and highly susceptible (8-9) (Chaudhariet al., 2019;Pooniyaet al., 2020). GRD percentage disease incidence (PDI) and LLS severity data for both diseases were collected at 4, 8 and 12 weeks after sowing. GRD severity data was collected at 12 weeks. Data at 12 weeks was used to categorize responses of the lines to each disease.
Percentage disease incidence ratings for groundnut rosette disease
- Source: Waliyaret al., 2007 [^]
Notes
Severity scale for field screening of groundnut lines against groundnut rosette disease.
- Source: Waliyaret al., 2007 [^]
Notes
Modified Late leaf spot scale applied for field screening of groundnut.
- Source: Subrahmanyamet al., 1995 [^]
Notes
Data analysis
Groundnut rosette disease percentage disease incidence (GRD PDI) was calculated as:
The Area Under Disease Progress Curve (AUDPC) for GRD PDI and LLS severity data at 4, 8 and 12 weeks was calculated using the formula:
where y iis the GRD PDI/LLS severity at the i thobservation; t i is time (in weeks) at thei th observation and n is the total number of observations (Simko and Piepho, 2012).
For preliminary yield assessment, the average number of pods per genotype was calculated as the total number of mature pods divided by the number of plants analyzed.
Best linear unbiased predictions (BLUPs) and variance components for each environment were generated in the Meta-R software (Alvaradoet al., 2020) while the PBIB test function in the Agricolae package in R software was used to do analysis of variance (R core team, 2021). The Restricted Maximum Likelihood (REML) method was used in both cases with the following model:
where Y ijk is denoted as the k th observation for the i th genotype, µ the grand mean, Gi the genotype effect,Rjthe replication effect, R/Bjk the block effect nested in replicates correspondingly, and εijk the error term associated withY ijk .
The estimated BLUP variance components were used to calculate Broad-sense heritability (BSH) for all the traits using the formula:
where σ 2 g is the genetic variance component, σ 2 e is the residual (error) component, andnr is the number of replications.
The Least Significant Difference (LSD) at 5 percent level of significance was calculated as:
LSD = t (1−0.05,dfErr) × ASED
where t is designated as the accumulative student's t distribution, 0.05 is the chosen α (alpha) level (5 percent), dfErr is the degrees of freedom for the error in the linear mixed model while ASED is the Average Standard Error of the Differences of the means.
The percentage coefficient of variation was generated from the formula:
where MSE is the mean squared error.
The relationship between variables was established by calculating Pearson's correlation coefficients from combined datasets (n equal 229) at the location Nakabango where disease pressure was consistent across seasons. Thecorfunction in R was used (R Core Team, 2021).
Principal component analysis (PCA) was carried out to determine the overall variation between disease variables, average number of pods, market types and countries of origin. It was done using the FactoMineR, factoextra, and GGPlot packages in R software (R Core Team, 2021).
RESULTS AND DISCUSSION
Statistically significant differences (P<0.001) were observed for GRD PDI at Nakabango at 4, 8, 12 weeks and AUDPC (Table4 ). At 12 weeks, Serere had lower GRD pressures for seasons 2020A and 2020B. This is evidenced by the lower grand mean and broad sense heritability estimates; 10.9 and 28percent for Serere 2020B and 11.3 and 23percent for Serere 2021B respectively (Table4 ) making it difficult to identify truly resistant lines. In the case of LLS, moderate to high heritabilities at 8 and 12 weeks and AUDPC (up to 86percent) were observed because the LLS pressure was relatively high and consistent across seasons. High heritability estimates are a good indication that the variation observed is mostly due to genetic rather than environmental factors (Youet al., 2016) thus making selection for the trait possible at early generations of the crop.
Analysis of Variance (ANOVA) and descriptive statistics for groundnut rosette disease, late leaf spot and a yield parameter in the GINA core collection.
- SOV-Source of variation, Df-Degrees of freedom, percent CV-Coefficient of Variation, GM-Grand Mean, BSH-Broad Sense Heritability, PDI 4W-Groundnut Rosette Disease Percentage Disease Incidence (PDI) at 4 weeks, PDI 8W- GRD PDI at 8 weeks, PDI 12W- GRD PDI at 12 weeks, SEV- GRD Severity at 12 weeks, PDI AUDPC-PDI Area Under Disease Progress Curve, LLS 4W-Late Leaf Spot (LLS) at 4 weeks, LLS 8W- LLS at 8 weeks, LLS 12W- LLS at 12 weeks, LLS AUDPC-LLS Area Under Disease Progress Curve, AV POD # -Average number of mature pods per plant [^]
- * Significant at P<0.05 [^]
- ** Significant at P<0.01 [^]
- *** Significant at P<0.001 ns – non-significant [^]
Notes
GRD pressure was lowest in Serere 2020B and 2021B and yet higher in Serere 2020A where wind speed was lower (2.15 m/s). Similar lower wind speed values were observed at Nakabango across seasons (2.2, 2.17 and 2.18 m/s for seasons 2020A, 2020B and 2021B respectively) where disease pressure was high and consistent. Lower disease pressure in Serere 2020B and 2021B may be partly attributed to the reduction in the aphid populations in Serere resulting from an increase in wind speed observed in Serere 2020B (2.3 m/s) and 2021B (2.6 m/s). An increase in windspeed affects GRD incidence negatively since the aphids populations are not allowed to accumulate on the plants (Mugisaet al., 2016).
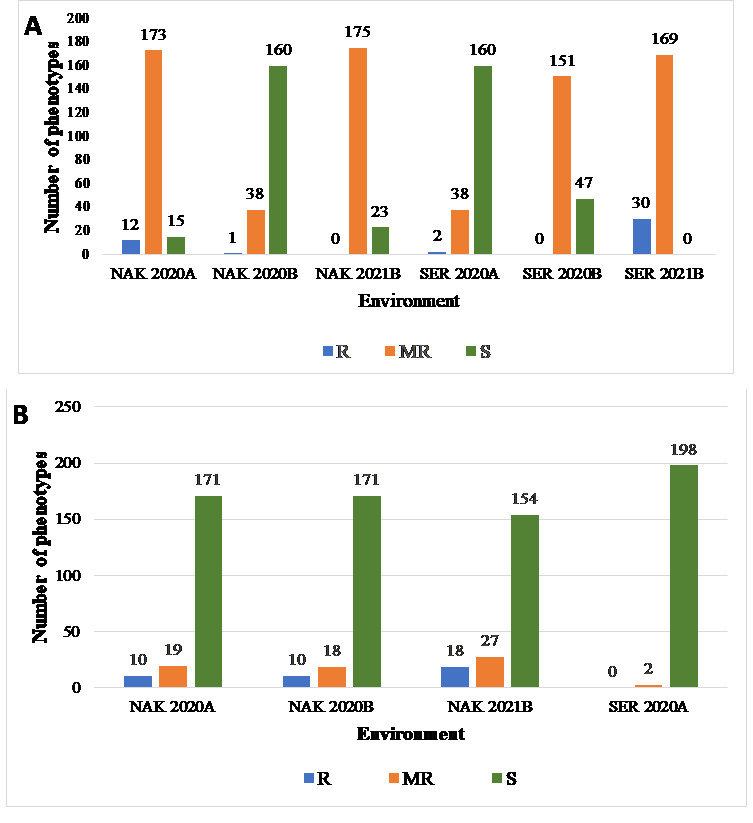
Across the three seasons at Nakabango, the largest proportion of the genotypes evaluated were either moderately resistant or susceptible to LLS and GRD (Figures2 A and B). Across the seasons, thirty of the lines in the GINA core collection were resistant or moderately resistant to GRD. Of these, 67percent were of the Virginia market class, 26percent from the Spanish and 7percent of hybrid market class. None of the resistant or moderately resistant lines were from the Valencia market class. 43percent percent of the lines were from Uganda, while 17 percent were from Malawi. The remainder (40percent) were spread across all other countries, except for Mali that had no resistant lines.
In the case of LLS, eighty-six (86) lines were resistant to moderately resistant across seasons. Of these, 66percent were of the Virginia market class, 24percent Spanish, 6percent hybrid and 4percent Valencia. Of these, Uganda contributed 27percent, Mozambique 5percent, Ghana 24percent, Malawi 15percent, Zambia 9percent, Senegal 8percent, Togo 5percent, Mali 5percent, and Niger 2percent.
Across the three seasons, fifteen lines were either resistant or moderately resistant to both GRD and LLS. (Table5 ). The highest average pod number across lines was 16.25 for line Ug-121_Oug-ICGV SM 15583. Two lines Ug-5_Oug-SERENUT_9T_UG and Ug-164_Oug- ICGV_SM_06518 with average pod number 12.24 and 12.88 respectively (Table5 ) harboured all five favourable haplotypes identified for GRD resistance byAcholaet al. (2023). The only lines from the Spanish market class with resistance to both GRD and LLS were Ug-41_Oug-DOK 1 RED UG and Sn-42_Sen-DOK IT (Table5 ). However, these lines with one or two seasons of disease screening may require more testing to enable confident reporting of these data.
BLUP values and disease ratings for GRD and LLS showing resistant and moderately resistant genotypes with their corresponding average pod number per plant, number of seasons planted and market type
- PDI 12W- GRD PDI at 12 weeks, LLS 12W- LLS severity at 12 weeks, R-Resistant, MR-Moderately resistant, AV POD # -Average number of mature pods per plant, # Seasons -Number of seasons for which line appeared for field screening. [^]
Notes
Across all the seasons, 87percent of the lines in the GINA core collection were susceptible to GRD and 62percent susceptible to LLS suggesting that the collection has limited sources of resistance for GRD and LLS even though it was generally considered diverse based on genotypic data. This indicates the need to develop trait-based collections in addition to utilizing the genotypic data. A core collection evaluated by ICRISAT also revealed limited sources of resistance to LLS (Sudiniet al., 2015). Interestingly, 49percent of the lines in the GINA core collection were lines developed at ICRISAT (India and Malawi). Alleles from the wild-type gene pool can confer immunity to GRD and LLS (Subrahmanyamet al., 2001;Stalker, 2017). However, reproductive and hybridization barricades (Kumariet al., 2014) have hampered their use in breeding programs. Improvement of cultivated peanut using synthetic tetraploids for GRD and LLS has been on-going (Leal-Bertioliet al., 2009;Foncekaet al., 2012;Wankhadeet al., 2021;Moretzsohnet al., 2023) with the best performing lines reported as either moderately resistant or susceptible to both diseases. Consequently, more populations with wild alleles need to be developed and continually tested to expand the diversity for GRD and LLS.
Studies using similar lines utilized in the GINA core collection for LLS (Aliduet al., 2019;Kankamet al., 2020) and GRD (Appiahet al., 2016;Kankamet al., 2020) showed variable performances for LLS and GRD across West Africa and Uganda.. The differences in the performance of these lines for GRD may be attributed to variability of the causal agents of GRD which make the GRD complex more virulent in certain areas as compared to others (Wangaiet al., 2007;Joneset al., 2019;Mabeleet al., 2019, 2021). It is therefore essential to invest in understanding the variability of the GRD virus complex across areas where GRD is predominant and all germplasm contributing countries which will give insights into the stable performance of germplasm across countries. The same applies to LLS where the ELS pathogen is more predominant in some areas such as in West and Southern Africa, while LLS is more predominant in East Africa. Similar effort should be geared towards understanding the LLS pathotypes across peanut growing areas in Africa.
PCA analysis using phenotypic data showed clustering of genotypes according to market type and botanical groups (Figure3 A), GRD (Figure3 B) and LLS (Figure3 C) groups based on levels of resistance and number of pods. The PCA graphs showed genotypes in GINA core collection to have unclear distinctions between Spanish, Virginia, Valencia and mixed groups although the Virginia class was easily clustered towards higher average pod number, resistance to GRD and resistant/moderate resistance to LLS (Figure3 A). The Spanish botanical group was clustered as lines susceptible to LLS. The lines that clustered as susceptible to GRD consisted of Spanish, Virginia and Valencia (Figure3 A) market types with Spanish contributing the highest number of genotypes. No distinguishing clusters were observed according to country of origin for both LLS and GRD resistance groups (Figure3 D).
Clusters in PCA analysis revealed that the lines resistant to GRD and LLS were all from the Virginia botanical/market class and from Uganda. Notably, the lines were evaluated in Uganda (a regional evaluation nursery) where selection during breeding favoured lines which are well adapted to both GRD and LLS. The Virginia market class which is mostly mid-late maturing were also observed to be associated with lower GRD and LLS scores as compared to early maturing Valencia and Spanish groups (Ijazet al., 2019;Acholaet al., 2023).
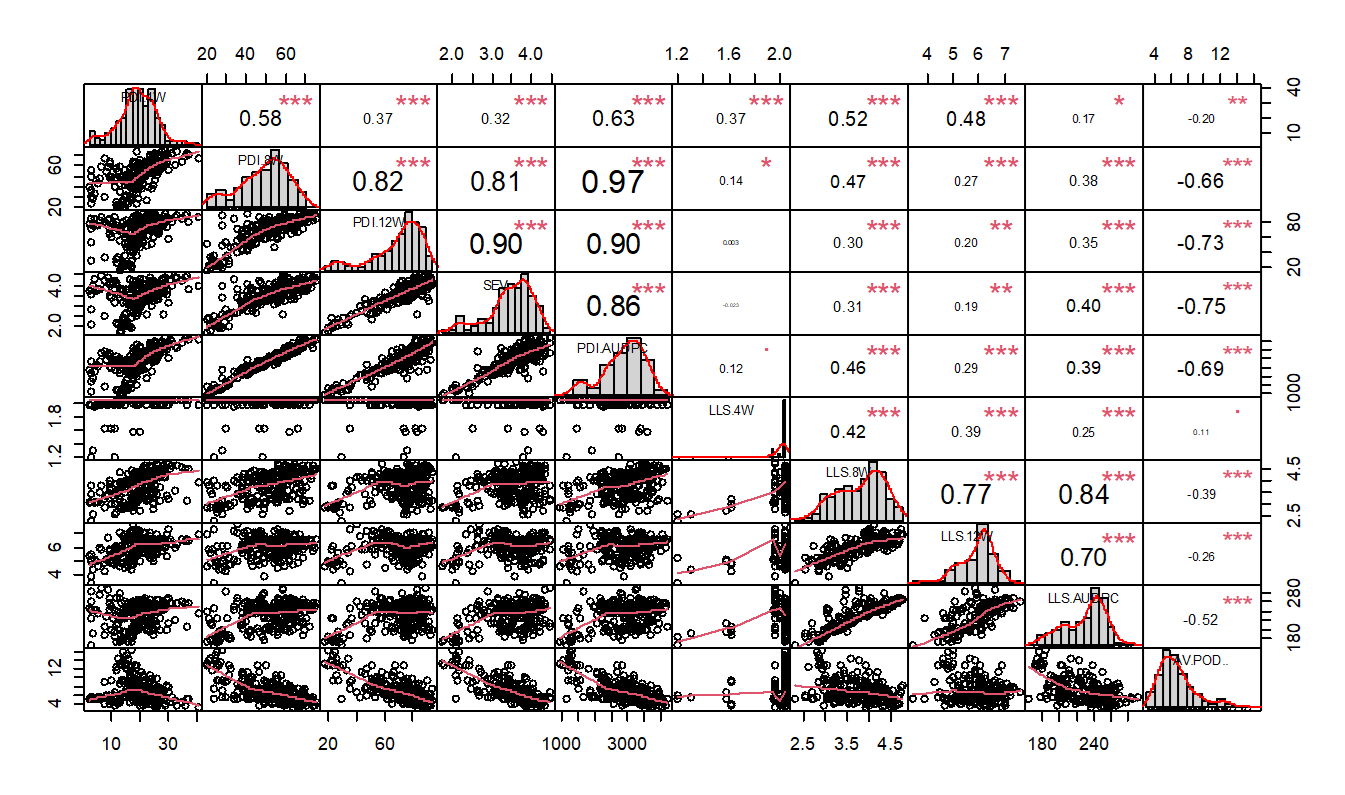
The strongest and highly significant correlation coefficients were observed between GRD PDI at 8 weeks versus PDI AUDPC (0.97, P<0.001), PDI at 12 weeks (0.82, P<0.001) and GRD severity at harvest (0.81, P<0.001), PDI at 12 weeks and severity at harvest (0.90, P<0.001), PDI at 12 weeks and PDI AUDPC (0.90, P <0.001) (Figure4 ). In the case of LLS, positive correlations were observed between LLS at 8 weeks versus LLS at 12 weeks (0.77, P<0.001), LLS AUDPC (0.84, P<0.001), LLS at 12 weeks and LLS AUDPC (0.70, P<0.001). The strong, positive and significant correlations observed shows that as one variable increased so did the other in the same direction. A high correlation coefficient also suggests that any of the two variables can be used to predict one another when assessing disease damage in the presence of adequate disease pressure. However, since disease pressure is variable for GRD and may be unreliable across locations, the use of molecular markers as early as 2 weeks after planting will reduce the number of cycles in breeding for a cultivar (Xu et al., 2017).Achola et al. (2023)identified molecular markers and haplotypes for resistance to GRD which can be developed into routine marker assays for deployment in breeding programs. Following validation of molecular markers identified and development of marker assays, selection for GRD resistance can be done as early as fourteen days after planting.
The negative correlation between GRD, LLS scores and average pod number per plant indicates that the increase in GRD, LLS diseases contributed to reduction in the number of pods. These findings were contrary to those inEssandohet al. (2022)who reported a positive correlation coefficient between LLS scores implying that LLS had no effect on yield. However, to be able to clearly establish the individual contribution of each disease to the amount of yield lost, experiments with and without controls for GRD, ELS and LLS need to be done to focus on each disease separately for a given crop cycle. In addition, since LLS tends to overshadow ELS as is the case in Uganda, there is need for separate screening to identify resistance to the two diseases. Several molecular markers have been identified for both ELS and LLS (Ahmadet al., 2020;Chuet al., 2019;Clevengeret al., 2018;Pandeyet al., 2017,Shobaet al., 2013;Zhanget al., 2020;Zhouet al., 2016;Zongoet al., 2017) in both bi-parental and diverse populations. However, Quantitative Trait Loci (QTLs) for both ELS and LLS are largely affected by environment and the genetic material utilized for the study. AlthoughOteng-Frimponget al. (2023)identified QTLs on the same GINA core collection in Ghana, it is highly probable that the QTLs identified in Uganda may differ due to varying pathotypes causing the extent of disease severity. QTLs for LLS resistance specific to Ugandan environments need to be identified to develop precise molecular markers for selection.
SUMMARY AND CONCLUSIONS
We utilized the GINA core collection to identify sources of resistance to GRD and LLS using phenotypic data. Lines identified for resistance to both diseases should be deployed in breeding programs as parents for improving susceptible lines/varieties or evaluated for yield trials and released as improved varieties for farmers across Africa. Limited diversity for the traits in the GINA core collection which resulted in fewer resistant and high yielding lines being selected can be improved by harnessing alleles from the wildtype gene pool, more nominations, and landraces collections. In order to ensure that phenotypic selection is improved, especially at Serere, the disease pressure needs to be enhanced by the use of inoculated infector rows or screening of germplasm in the screenhouses with artificial inoculation. Moreover, it would be important to separate resistance based on the various viral agents forming the GRD complex. The use of molecular techniques to identify each viral agent and quantify the amount of viral load in the plant would give a better idea of the “level of resistance”. Similarly, the use of molecular techniques to identify ELS and LLS pathogens separately would enable confident identification of lines resistant to ELS and/or LLS since LLS tends to mask ELS during phenotypic screening. An understanding of the pathotypes for both LLS and GRD across Africa will enable identification of resistance specific to given pathotypes which will pave the way for gene pyramiding and confer broader resistance to the diseases. The availability of modern high throughput phenotyping and genotyping platforms and tools will enable more accurate and speedy selection for resistant lines.
This work estimated the average number of pods per plant, one of several yield parameters. Future work needs to focus on other yield parameters such as dry pod yield, seed weight, 100 seed weight and haulm weight to give a clearer picture of yield estimates. Larger plot sizes which give a fairly accurate estimate of yield should be utilized to avoid erroneous data or negligible weights from small plots.
Acknowledgements
We acknowledge the contribution of Daniel Fonceka, Josh Paul Clevenger, Peggy OziasAkins and JeanFrançois Rami towards multiplying seed, genotyping and the development of the GINA Core Collection. We also acknowledge Essohouna Modom Banla, Dramane Sako, Issa Faye, Amade Muitia, Justus Chintu, Lutangu Makweti, Richard Oteng-Frimpong, James Asibuo and Adama Mamadou Coulibaly for contributing germplasm to the GINA Core Collection.
This work was supported through the generous support of the American people through the United States Agency for International Development (USAID) through Cooperative Agreement No. 7200AA 18CA00003 to the University of Georgia as management entity for U.S. Feed the Future Innovation Lab for Peanut (2018-2023). The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government.
Literature Cited
Achola E.,Wasswa P.,Fonceka D.,Clevenger J.P.,Bajaj P.,Ozias-Akins P.,Rami J.F.,Deom C.M.,Hoisington D.A.,Edema R.,Odeny D.A., andOkello D.K. . 2023.Genome-Wide Association Studies Reveal Novel Loci for Resistance to Groundnut Rosette Disease in the African Core Groundnut Collection.Theoretical and Applied Genetics. 136(3):1–20. doi: [: 10.1007/s00122-023-04259-4].
Ahmad S.,Nawade B.,Sangh C.,Mishra G.P.,Bosamia T.C.,Radhakrishnan T.,Kumar N.,Dobaria J.R., andGajera H.P.. 2020.Identification of Novel QTLs for Late Leaf Spot Resistance and Validation of a Major Rust QTL in Peanut (Arachis Hypogaea L.). 3 Biotech 10: 1–13. doi:10.1007/s13205-020-02446-4.
Alidu M.S.,Abukari S., andAbudulai M. . 2019.Screening Groundnut (Arachis Hypogaea. L) Genotypes for Resistance to Early and Late Leaf Spot Diseases.Journal of Experimental Agriculture International. 37(4):1–9. doi: [: 10.9734/jeai/2019/v37i430274].
Alvarado G.,Rodríguez F.M.,Pacheco A.,Burgueño J.,Crossa J.,Vargas M.,Pérez-Rodríguez P., andLopez-Cruz M.A. . 2020.META-R: A Software to Analyze Data from Multi-Environment Plant Breeding Trials.Crop Journal. 8(5):745–56. doi: [: 10.1016/j.cj.2020.03.010].
Anco D.J.,Thomas J.S.,Jordan D.L.,Shew B.B.,Monfort W.S.,Mehl H.L.,Small I.M.,Wright D.L.,Tillman B.L.,Dufault N.S.,Hagan A.K., andCampbell H.L. . 2020.Peanut Yield Loss in the Presence of Defoliation Caused by Late or Early Leaf Spot. Plant Disease. 104(5):1390–99. doi: [: 10.1094/PDIS-11-19-2286-RE].
Appiah A.S.,Offei S.K.,Tegg R.S., andWilson C.R. . 2016.Varietal Response to Groundnut Rosette Disease and the First Report of Groundnut Ringspot Virus in Ghana. Plant Disease. 100(5):946–52.
Beukelaer H.D.,Davenport G.F., andFack V. . 2018.Core Hunter 3: Flexible Core Subset Selection.BMC Bioinformatics. 19(1).1-12. doi:10.1186/s12859-018-2209-z.
Chaudhari S.,Khare D.,Patil S.C.,Sundravadana S.,Variath M.T.,Sudini H.K.,Manohar S.S.,Bhat R.S., andPasupuleti J.. 2019.Genotype × Environment Studies on Resistance to Late Leaf Spot and Rust in Genomic Selection Training Population of Peanut (Arachis Hypogaea L.).Frontiers in Plant Science. 10. doi:10.3389/fpls.2019.01338.
Chu Y.,Chee P.,Culbreath A.,Isleib T.G.,Holbrook C.C., andOzias-Akins P. . 2019.Major QTLs for Resistance to Early and Late Leaf Spot Diseases Are Identified on Chromosomes 3 and 5 in Peanut (Arachis Hypogaea).Frontiers in Plant Science. 10:1–13. doi: [: 10.3389/fpls.2019.00883].
Clevenger J.,Chu Y.,Chavarro C.,Botton S.,Culbreath A.,Isleib T.G.,Holbrook C. C., andOzias-Akins P. . 2018.Mapping Late Leaf Spot Resistance in Peanut (Arachis Hypogaea) Using QTL-Seq Reveals Markers for Marker-Assisted Selection.Frontiers in Plant Science. 9:1–10. doi: [: 10.3389/fpls.2018.00083].
Conde S.,Rami J.F.,Okello D.K.,Sambou A.,Muitia A.,Oteng-Frimpong R.,Makweti L.,Sako D.,Faye I.,Chintu J.,Coulibaly A.M.,Miningou A.,Asibuo J.Y.,Konate M.,Banla E.M.,Seye M.,Djiboune Y.R.,Tossim H.A.,Sylla S.N.,Djiboune Y.R.,Tossim H.A.,Sylla S.N.,Hoisington D.,Clevenger J.,Fonceka D. . 2023.The Groundnut Improvement Network for Africa (GINA) germplasm collection: a unique genetic resource for breeding and gene discovery. G3: Genes, Genomes, Genetics, 14(1): 1–12.https://doi.org/10.1093/g3journal/jkad244.
Essandoh D.A.,Odong T.,Okello D.K.,Fonceka D.,Nguepjop J.,Sambou A.,Ball C.,Chavarro C.,Bertioli D.J., andLeal S.C.M. -bertioli. 2022. Quantitative Trait Analysis Shows the Potential for Alleles from the Wild Species Arachis Batizocoi and A . Duranensis to Improve Groundnut Disease Resistance and Yield in East Africa. Agronomy. 12. https://doi.org/10.3390/ agronomy12092202.
Fonceka D.,Tossim H.A.,Rivallan R.,Vignes H.,Faye I.,Ndoye O.,Moretzsohn M.C.,Ndoye O.,Moretzsohn M.C.,Bertioli D.J.,Glaszmann J.C.,Courtois B., andRami J.F.. 2012.Fostered and Left behind Alleles in Peanut: Interspecific QTL Mapping Reveals Footprints of Domestication and Useful Natural Variation for Breeding. BMC Plant Biology. 12. doi:10.1186/1471-2229-12-26.
Ijaz M.,Rashad S.,Shah A.,Izhar M. -ul-haq, and A. Afzal. 2019. Relationship between Components of Resistance to Late Leaf Spot in Groundnut Botanical Genotypes. Pakistan Journal of Agricultural Research. 32(2): 390-397. http://dx.doi.org/10.17582/journal.pjar/2019/32.2.390.397.
Iwo G.A., andOlorunju P.E. . 2009.Yield Stability and Resistance to Leaf Spot Diseases and Rosette in Groundnut.Czech Journal of Genetics and Plant Breeding 45(1):18–25. doi: [: 10.17221/30/2008-CJGPB].
Jones S.,Cowan G.,MacFarlane S.,Mukoye B.,Mangeni B.C.,Were H., andTorrance L. . 2019.RNA Sequence Analysis of Diseased Groundnut (Arachis Hypogaea) Reveals the Full Genome of Groundnut Rosette Assistor Virus (GRAV).Virus Research. 277:197837.
Kankam F.,Akpatsu I.B., andTengey T.K. . 2022.Leaf Spot Disease of Groundnut: A Review of Existing Research on Management Strategies.Cogent Food and Agriculture. 8(1). doi: [: 10.1080/23311932.2022.2118650].
Kankam F.,Kojo K.Y., andAddai I.K. . 2020.Evaluation of Groundnut (Arachis Hypogea L.) Mutant Genotypes for Resistance against Major Diseases of Groundnut.Pakistan Journal of Phytopathology. 32(1):61–69. doi: [: 10.33866/phytopathol.032.01.0554].
Kumari V.,Gowda M.V.C.,Tasiwal V.,Pandey M.K.,Bhat R.S.,Mallikarjuna N.,Upadhyaya H.D., andVarshney R.K. . 2014.Diversification of Primary Gene Pool through Introgression of Resistance to Foliar Diseases from Synthetic Amphidiploids to Cultivated Groundnut (Arachis Hypogaea L.).Crop Journal. 2:110–119. doi: [: 10.1016/j.cj.2014.03.002].
Leal-Bertioli S.C.,José A.C.V.,Alves-Freitas D.M.,Moretzsohn C.,Guimarães P.M.,Nielen S.,Vidigal B.S.,Pereira R.W.,Pike J.,Fávero A.P.,Parniske M.,Varshney R.K., andBertioli D.J. . 2009.Identification of Candidate Genome Regions Controlling Disease Resistance in Arachis. BMC Plant Biology 9:1–12. doi: [: 10.1186/1471-2229-9-112].
Mabele A.S.,Were H.K.,Florence M., andNdong O. 'a. 2019. Distribution, Molecular Detection and Host Range of Groundnut Rosette Assistor Virus in Western Kenya. Journal of Plant Sciences. 7(5):100-105.
Mabele A.S.,Were H.K.,Florence M.,Ndong O. ’a, and B. Mukoye. 2021. Occurrence and Genetic Diversity of Groundnut Rosette Assistor Virus in Western Kenya. Crop Protection. 139(2021): 105381. doi:10.1016/j.cropro.2020.105381.
McDonald D.,Subrahmanyam P.,Gibbons R.W., andSmith D.H. . 1985.Early and Late Leaf Spots of Groundnut. Information Bulletin 21: 19. ICRISAT.http://oar.icrisat.org/id/eprint/821.
Mienie M.S.C., andPretorius E.A. . 2013.Application of Marker-Assisted Selection for AhFAD2A and AhFAD2B Genes Governing the High-Oleic Acid Trait in South African Groundnut Cultivars (Arachis Hypogaea L.).African Journal of Biotechnology 12(27):4283–89. doi: [: 10.5897/ajb2012.2976].
Mohammed K.E.,Afutu E.,Odong T.L.,Okello D.K.,Nuwamanya E.,Grigon O.,Rubaihayo P.R., andOkori P. . 2018.Assessment of Groundnut (Arachis Hypogaea L .) Genotypes for Yield and Resistance to Late Leaf Spot and Rosette Diseases.Journal of Experimental Agriculture International. 21(5):1–13. doi: [: 10.9734/JEAI/2018/39912].
Monyo E.S.,Osiru M.O.,Kadyampakeni D.,Mponda O., andChinyamunyamu B.. 2009.Improving Food Security and Nutrition in Malawi and Tanzania through Research on Edible Legumes. Malawi and Tanzania through Research on Edible Legumes. Proceedings of Stakeholder Workshops on Groundnut Production in Malawi and Tanzania. www.icrisat.org.
Moretzsohn M.,Santos J.F.,Moraes A.R.,Custódio A.R.,Michelotto M.D.,Mahrajan N.,Leal-Bertioli S.C.M.,Godoy I.J., andBertioli D.J.. 2023.Marker-Assisted Introgression of Wild Chromosome Segments Conferring Resistance to Fungal Foliar Diseases into Peanut (Arachis Hypogaea L.).Frontiers in Plant Science. 14(March): 1–16. doi:10.3389/fpls.2023.1139361.
Mugisa I.O.,Karungi J.,Akello B.,Ochwo M.K.N. -ssemakula, M. Biruma, D.K. Okello, and G. Otim. 2016. Determinants of Groundnut Rosette Virus Disease Occurrence in Uganda. Crop Protection. 79:117–23. doi:10.1016/j.cropro.2015.10.019.
Naidu R.A.,Kimmins F.M.,Deom M.C.,Subrahmanyam P.,Chiyembekeza J.A., andVan der Merwe P. . 1999.Groundnut Rosette: A Virus Affecting Groundnut Production In Sub-Saharan Africa.Plant Disease. 83(8):700–709.
Nigam S.N.,Rao P.,Bhatnagar-Mathur P., andSharma K.K. . 2012.Genetic Management of Virus Diseases in Peanut. Plant Breeding Reviews. 36(1):293–356. doi: [: 10.1002/9781118358566.ch4].
Ojiewo C.O.,Pasupuleti J..,Bhatnagar-Mathur P.,Pandey M.K.,Desmae H.,Okori P.,Mwololo J.,Ajeigbe H.,Njuguna-Mungai E.,Muricho G.,Akpo E.,Gichohi-Wainaina W.N.,Variath M.T.,Radhakrishnan T.,Dobariya K.L.,Bera S.K.,Rathnakumar A.L.,Manivannan N.,Vasanthi R.P.,Kumar M.V.N., andVarshney R.K.. 2020.Advances in Crop Improvement and Delivery Research for Nutritional Quality and Health Benefits of Groundnut (Arachis Hypogaea L.) .Frontiers in Plant Science. 11(February):1–15. doi:10.3389/fpls.2020.00029.
Okello D.K.,Akello L.B.,Tukamuhabwa P.,Ochwo-Ssemakula M.,Adriko J., andDeom C.M. . 2014.Groundnut Rosette Disease Symptoms Types Distribution and Management of the Disease in Uganda. African Journal of Plant Science. 8(3):153–63. doi: [: 10.5897/ajps2014.1164].
Okello D.K.,Biruma M., andDeom C.M. . 2010.Overview of Groundnuts Research in Uganda : Past , Present and Future.African Journal of Biotechnology. 9(39):6448–6459.
Okello D.K.,Monyo E.,Deom C.M.,Ininda J., andOloka H.K.. 2013.Groundnut Production Guide for Uganda: Recommended Practices for Farmers. Entebbe: National Agricultural Research Organisation.
Oteng-Frimpong R.,Karikari B.,Ko E.,Kassim Y.B.,Puozaa D.K.,Rasheed M.A.,Fonceka D.,Okello D.K.,Balota M.,Burow M., andOzias-Akins P. . 2023.Association Studies Reveal Genomic Regions and Putative Candidate Genes Associated with Leaf Spot Diseases in African Groundnut (Arachis Hypogaea L.) Germplasm.Frontiers in Plant Science. 13(2022):1–16. doi: [: 10.3389/fpls.2022.1076744].
Pandey M.K.,Khan A.W.,Singh V.K.,Vishwakarma M.K.,Shasidhar Y.,Kumar V.,Garg V.,Bhat R.S.,Chitikineni A.,Pasupuleti J.,Baozhu G., andVarshney R.K. . 2017.QTL-Seq Approach Identified Genomic Regions and Diagnostic Markers for Rust and Late Leaf Spot Resistance in Groundnut (Arachis Hypogaea L.).Plant Biotechnology Journal. 15(8):927–41. doi: [: 10.1111/pbi.12686].
Pandey M.K.,Wang H.,Khera P.,Vishwakarma M.K.,Kale S.M.,Culbreath A.K.,Holbrook C.C.,Wang X.,Varshney R.K., andBaozhu G.. 2017.Genetic Dissection of Novel QTLs for Resistance to Leaf Spots and Tomato Spotted Wilt Virus in Peanut (Arachis Hypogaea L.).Frontiers in Plant Science 8(January). doi:10.3389/fpls.2017.00025.
Pooniya S. K.,Yadav S..,Rathore M.S.,Tiwari S.,Sikarwar R.S., andTripathi M.K. . 2020.Field Evaluation of Early and Late Leaf Spot Diseases in Advanced Breeding Lines of Groundnut (Arachis Hypogaea L.).International Journal of Current Microbiology and Applied Sciences. 9(7):3910–19. doi: [: 10.20546/ijcmas.2020.907.458].
Shoba D.,Manivannan N.,Vindhiyavarman P., andNigam S.N. . 2013.Identification of Quantitative Trait Loci (QTL) for Late Leaf Spot Disease Resistance in Groundnut (Arachis Hypogaea L.).Legume Research. 36(5):467–72.
Singh M.P.,Erickson J.E.,Boote K.J.,Tillman B.L.,Bruggen A.H.C., andJones J.W. . 2011.Photosynthetic Consequences of Late Leaf Spot Differ between Two Peanut Cultivars with Variable Levels of Resistance. Crop Science. 51(6):2741–48. doi: [: 10.2135/cropsci2011.03.0144].
Stalker H.T. 2017.Utilizing Wild Species for Peanut Improvement. Crop Science. 57(3):1102–1120. doi: [: 10.2135/cropsci2016.09.0824].
Subrahmanyam P.,McDonald D.,Waliyar F.,Reddy L.J.,Nigam S.N.,Gibbons R.W.,Rao V.R.,Singh A.K.,Pande S.,Reddy P.M, andRao P.S. . 1995.Screening Methods and Sources of Resistance to Rust and Late Leaf Spot of Groundnut.Information Bulletin, no. 47: 21 pp. ICRISAT.
Subrahmanyam P.,Anaidu R.,Reddy L.J.,Kumar P.L., andFerguson M.E. . 2001.Resistance to Groundnut Rosette Disease in WildArachisSpecies.Annals of Applied Biology. 139(1):45–50. doi: [: 10.1111/j.1744-7348.2001.tb00129.x].
Sudini H.,Upadhyaya H.D.,Reddy S.V.,Mangala U.N.,Rathore A., andKumar K.V.K. . 2015.Resistance to Late Leaf Spot and Rust Diseases in ICRISAT’s Mini Core Collection of Peanut (Arachis Hypogaea L.).Australasian Plant Pathology. 44(5):557–66. doi: [: 10.1007/s13313-015-0368-1].
Tshilenge-Lukanda L.,Nkongolo K.K.C.,Kalonji-Mbuyi A., andKizungu R.V. . 2012.Epidemiology of the Groundnut (Arachis Hypogaea L.) Leaf Spot Disease: Genetic Analysis and Developmental Cycles.American Journal of Plant Sciences. 3(5):582–588. doi: [: 10.4236/ajps.2012.35070].
Upadhyaya H.D.,Dronavalli N.,Dwivedi S.L.,Kashiwagi J.,Krishnamurthy L.,Pande S.,Sharma H.C. . 2013.Mini Core Collection as a Resource to Identify New Sources of Variation. Crop Science. 53(6):2506–17. doi: [: 10.2135/cropsci2013.04.0259].
Waliyar F.,Kumar P.L.,Ntare B.R.,Monyo E.,Nigam S.N.,Reddy A.S.,Osiru M., andDiallo A.T. . 2007.A Century of Research on Groundnut Rosette Disease and Its Management. International Crops Research Institute for Semi-Arid Tropics Information Bulletin no. 75:44pp.
Wangai A.W.,Pappu S.S.,Pappu H.R.,Deom C.M., andNaidu R.A. . 2007.Distribution and Characteristics of Groundnut Rosette Disease in Kenya. Plant Disease. 85(5):470–74. doi: [: 10.1094/pdis.2001.85.5.470].
Wankhade A.P.,Kadirimangalam S.R.,Viswanatha K.P.,Deshmukh M.P.,Shinde V.S.,Deshmukh D.B., andPasupuleti J. . 2021.Variability and Trait Association Studies for Late Leaf Spot Resistance in a Groundnut Magic Population. Agronomy. 11(11):1–13. doi: [: 10.3390/agronomy11112193].
Willett W.,Rockström J.,Loken B.,Springmann M.,Lang T.,Vermeulen S.,Garnett T.,Tilman D., andDeclerck F. . 2019.Food in the Anthropocene : The EAT – Lancet Commission on Healthy Diets from Sustainable Food Systems.Lancet. 393:447–92. doi:10.1016/S0140-6736(18)31788-4.
You M.F.,Gaofeng J.,Sylvie C.,Helen M.B.,Scott D.D., andKhalid Y.R. . 2016.A Method of Estimating Broad-Sense Heritability for Quantitative Traits in the Type 2 Modified Augmented Design.Journal of Plant Breeding and Crop Science. 8(11):257–72. doi: [: 10.5897/jpbcs2016.0614].
Zhang H.,Chu Y.,Dang P.,Tang Y.,Jiang T.,Clevenger J.P.,Ozias-Akins P.,Holbrook C.,Wang M.L.,Campbell H.,Hagan A., andChen C. . 2020.Identification of QTLs for Resistance to Leaf Spots in Cultivated Peanut (Arachis Hypogaea L.) through GWAS Analysis.Theoretical and Applied Genetics. 133(7): 2051–61. doi: [: 10.1007/s00122-020-03576-2].
Zhou X.,Xia Y.,Liao J.,Liu K.,Li Q.,Dong Y.,Ren X.,Chen Y.,Huang L.,Liao B.,Lei Y.,Yan L., andJiang H.. 2016.Quantitative Trait Locus Analysis of Late Leaf Spot Resistance and Plant-Type-Related Traits in Cultivated Peanut (Arachis Hypogaea L.) under Multi-Environments. PLos ONE. 11(11):e0166873. doi:10.1371/journal.pone.0166873.
Zongo A.,Khera P.,Sawadogo M.,Shasidhar Y.,Sriswathi M.,Vishwakarma M.K.,Sankara P.,Ntare B.R.,Varshney R.K.,Pandey M.K., andDesmae H.. 2017.SSR Markers Associated to Early Leaf Spot Disease Resistance through Selective Genotyping and Single Marker Analysis in Groundnut (Arachis Hypogaea L.).Biotechnology Reports. 15(May):132–137. doi:10.1016/j.btre.2017.07.005.
Notes
- Department of Agricultural Production, College of Agricultural and Environmental Sciences, Makerere University, P.O. Box 7062, Kampala, Uganda [^]
- International Crops Research Institute for the Semi-Arid Tropics, PO Box 39063 - 00623 Nairobi, Kenya [^]
- Feed the Future Innovation Lab for Peanut, University of Georgia, Athens, GA 30602. [^]
- National Semi-Arid Resources Research Institute-Serere, P.O. Box 56, Uganda [^] Corresponding author: Esther Achola:esterjocelyn@gmail.com