INTRODUCTION
The peanut (Arachis hypogaea L.) cultivar Florunner was released by the University of Florida in 1969 (Norden et al. 1969). Florunner soon became the predominant cultivar grown in the southeastern U.S. and the predominant runner-type cultivar grown throughout the U.S. (Gorbet 1999, Sholar et al. 1995). During the late 1970s, Florunner was grown on over 98% of the peanut area in the southeastern U.S. (Gorbet 1999). In 1985, over 84% of the runner peanut production area in the entire U.S. was planted to Florunner (Sholar et al. 1995). In the late 1980s, tomato spotted wilt (TSW), caused by Tomato spotted wilt virus (TSWV) was reported in Alabama, Georgia, and Florida (Hagan et al. 1990, Culbreath et al. 2003). Although the virus was new to the area, tobacco thrips (Frankliniella fusca Hinds) were already endemic in peanut, and proved to be a competent vector for TSWV (Todd and Culbreath 1995, Todd et al. 1995). Florunner was very susceptible to TSWV (Culbreath, et al. 1992a, Culbreath et al. 1992b), and between 1989 and 1997, incidence of TSW and losses to the disease in peanut increased dramatically (Culbreath and Srinivasan 2011). Although TSW was a new problem in peanut in the southeastern U.S., it was discovered that the cultivar Southern Runner (Gorbet et al. 1987) had a moderate level of field resistance to TSWV (Black and Smith 1987). Southern Runner was never planted on a large portion of the peanut hectarage, but it was used as a parent in breeding programs seeking to develop cultivars with resistance to TSWV (Culbreath and Srinivasan 2011). By 1996, the cultivar Georgia Green was released (Branch 1996). Georgia Green had field resistance similar to that of Southern Runner (Culbreath et al. 1996), which was one of its parents (Branch 1996), but was more acceptable for peanut production than Southern Runner. It displaced Florunner as the predominant peanut cultivar in the southeastern U.S. as soon as the seed supply allowed (Culbreath et al. 2003). Georgia Green was the predominant peanut cultivar grown in the southeastern U.S. from 1997 until 2007 (Culbreath and Srinivasan 2011). Georgia Green was a critical component of the TSW management system, but the level of field resistance in Georgia Green is not sufficient to provide adequate control of TSW when the potential for development of epidemics is high (Culbreath and Srinivasan 2011). Thus, it was desirable, if not essential, to use as many other suppressive factors as possible with that cultivar (Culbreath and Srinivasan 2011).
Several new cultivars with higher levels of field resistance to TSWV than Georgia Green have been developed (Culbreath and Srinivasan 2011, Culbreath et al. 2016). Planting these cultivars has improved levels of control of TSW in general, and allowed more flexibility with other factors in the TSW management programs (Culbreath et al. 2008, Culbreath et al. 2010, Culbreath et al. 2012, Culbreath et al. 2013, 2016, Tubbs et al. 2011) than was possible with Georgia Green.
Similarly, these cultivars have allowed more flexibility with insecticides used for control of thrips on the young plants. TSWV is transmitted by thrips, but use of most insecticides for control of tobacco thrips (Frankliniella fusca), the primary thrips species associated with direct damage and spread of TSWV in peanut in the southeastern U.S., generally has not resulted in reductions in incidence of TSW (Culbreath et al. 2003, Culbreath and Srinivasan 2011). Phorate is the only insecticide that has provided suppression of TSW in peanut (Culbreath et al. 2003, Todd et al. 2005, Culbreath and Srinivasan 2011), and it has been an important component of an integrated management system for TSW in peanut. Culbreath and colleagues (2008) reported much lower incidence of TSW in new cultivars Florida-07 (Gorbet and Tillman 2009) and Tifguard (Holbrook et al. 2008) than in Georgia Green, regardless of whether they were treated with phorate. In most cases, there was less response to phorate in those cultivars for suppressing TSW than in Georgia Green (Culbreath et al. 2008). The insecticide imidacloprid is commonly used for thrips control. However, it was reported to increase incidence of spotted wilt in susceptible cultivars (Todd et al., 1994, Culbreath and Srinivasan 2011).
Georgia-16HO is a runner-type cultivar released by the Georgia Agricultural Experiment Station in 2016 (Branch 2017). It has field resistance to TSWV and good yield potential. However, response of new cultivars such as Georgia-16HO to applications of phorate or imidacloprid have not been compared to that of Florunner. The primary objective of this study was to make direct comparison of Georgia-16HO to the previous standard but TSWV-susceptible cultivar Florunner for effects on spotted wilt epidemics, yield, and crop value. This was intended to provide an indication of the value of improved resistance to TSWV in a recently released cultivar compared to the standard cultivar grown before spotted wilt emerged as a problem. Another objective was to compare the effects of in-furrow application of phorate and imidacloprid insecticides on those same variables in Florunner and Georgia-16HO.
MATERIALS AND METHODS
Experimental design, field layout and treatment structure
Field experiments were conducted at the University of Georgia Coastal Plain Experiment Station Lang-Rigdon Farm, Tifton GA in 2019-2020. Soil type in both fields was a Tifton sandy loam (fine-loamy, kaolinic, thermic Plinthic Kandiudult). The fields used in both years had a history of severe epidemics of TSW in previous years when peanut had been grown.
In both experiments, experimental design was a randomized complete block with six replications. In each year, six treatments consisted of two cultivars, Florunner and Georgia-16HO in factorial arrangement with: i) in-furrow application of phorate (Thimet 20 G, AMVAC Chemical Corporation, Los Angeles, CA) at 1.12 kg ai/ha; ii) in-furrow application of imidacloprid (Admire Pro 4.6, Bayer, Research Triangle Park, NC) at 0.40 kg ai/ha; and iii) nontreated control. Planting dates were 16 April 2019 and 8 May 2020. Plots were 1.8 m wide and contained two single rows 91.4 cm apart. Plot length was 8.8 m in 2019 and 9.1 m in 2020. Seeding rates were 14.8 seed/m of row in each of the two single rows.
Plots were maintained according to University of Georgia Extension recommendations. Calcium sulfate was applied as gypsum at 1570 kg/ha 75 days after planting (DAP) in 2019 and 2240 kg/ha 73 DAP in 2020. Fungicides were applied at approximate 14-day intervals throughout each season for control of foliar and soilborne fungal diseases.
Inoculum and thrips vectors
Development of TSW epidemics was reliant upon inoculation by resident viruliferous thrips vectors (Frankliniella fusca and Frankliniella occidentalis Pergande). The immediate source of virus and vector was not identified.
Thrips injury assessment
Injury caused by feeding of tobacco thrips larvae was assessed for each plot using an ordinal scale of 0 to 10 adapted from Herbert and colleagues (2007) where: 0 = no injury, 1 = 10% leaves injured, 2 = 20% leaves injured, 3 = 30% leaves injured, 4 = 40% leaves injured, 5 = >50% of leaves injured and < 5% terminals injured, 6 = > 50% of leaves injured and < 25% terminals injured, 7 = >50% of leaves injured and < 50% terminals injured, 8 = >50% of leaves injured and < 75% terminals injured, 9 = >50% of leaves injured and < 90% terminals injured, and 10 = dead plants. Injury ratings were taken 20 DAP in 2019 and 22 DAP in 2020.
Disease assessment
Spotted wilt was evaluated for each plot at 62, 83, 112, and 125 DAP in 2019, and 61, 75, 99, and 119 DAP in 2020. Incidence of TSW was determined by counting the number of 0.3-m portions of row containing severely stunted, chlorotic, wilted or dead plants for each plot and converting that number to a percentage of total row length (Culbreath et al. 1997). Area under the disease progress curve (AUDPC) for incidence of TSW was calculated for each plot as described by Shaner and Finney (1977).
Pod yield, grade and value
In 2019, due to severity of late season spotted wilt and severe vine decline, plots of Florunner were inverted early, 133 DAP, whereas plots of Georgia-16HO were inverted 142 DAP. Plots of both cultivars were inverted 136 DAP in 2020. Pods were harvested mechanically 5 to 10 days after plants were inverted, and were dried. Yields were adjusted to 10% wt/wt moisture.
Grades were determined using commercial grading equipment according to official Federal-State Inspection Service methods (USDA-FSA 2019). A sample of 1000 g of harvested pods was collected from each plot. Loose shelled kernels and non-pod materials (foreign material) were removed from the sample and weighed. Percent foreign material and loose shelled kernels were calculated for each 1000 g sample. One 500 g sample was taken from the cleaned pods of each sample. The pods were shelled using a grade sheller, and kernels were classified as sound mature kernels, other kernels and damaged kernels. Pod grades were defined as percent total sound mature kernels (TSMK).
Seed costs
Since seed are sold on a per weight basis, the price per hectare for seed costs is greater for larger seeded cultivars even if the price/kg of seed is the same. Seed cost (γ) was computed using the following equation:
γ = (164,444 ÷ α) x β
Where:
γ is the seed cost in $/ha,
α is the number of seed/kg, and
β is the seed price/kg.
Estimates of the number of seed/kg were 1628.11 and 1726.22 for Georgia-16HO and Florunner, respectively. Estimates of seed weights were 101.00 kg/ha and 95.26 kg/ha for Georgia-16HO and Florunner, respectively, using row spacing of 0.9 m and 14.8 seeds/m. Estimates of seed prices were obtained from three seed suppliers in each year. Average prices of seed were $1.79/kg in 2019 and $1.91/kg in 2020. Since Florunner is no longer available for commercial seed, the same price per kg was used as for Georgia-16HO. Estimates of seed costs were $192.92/ha for Georgia -16HO and $181.95/ha for Florunner.
Insecticide costs
Estimates of the prices of the imidacloprid and phorate insecticides were obtained from a confidential survey of several input suppliers in the peanut growing region of Georgia. An average price calculated from the aggregated prices provided was used for calculation of treatment costs in this study. For the rates used in this study, costs were $34.59/ha for imidacloprid and $40.77/ha for phorate. Since each was applied in-furrow at planting, no additional cost of application was included.
Crop value calculations
For crop value comparisons, price ($ U.S./metric ton) was estimated for each plot using the following formula derived from the 2019 pod price schedule (USDA-FSA 2020).
$U.S.⁄metric ton= (%TSMK×$5.35)+ (%OK×$1.54) – (%FM – 4) × $1.10 – (%DD)
where TSMK is total sound mature kernels,
OK is other kernels,
FM is foreign material, and
DD is damaged deduction.
There was no deduction for damaged kernels if the level was below the threshold of 2% damaged kernels. There was a deduction of $3.75 per metric ton for each 1% incremental increase in damaged kernels above 2%. Total revenue/ha was calculated by multiplying the price per metric ton by yield in metric tons/ha. Adjusted revenue was calculated for each plot by subtracting seed cost and insecticide cost estimates for each cultivar-insecticide combination and estimated drying costs from the total revenue/ha.
Statistical analysis
Data were analyzed using SAS v.9.3 software (SAS Institute, Cary, NC). A mixed model procedure was used with maximum likelihood estimation of variance components (PROC MIXED). The Satterthwaite method was used for computing the denominator degrees of freedom ("ddfm=satterth" in the model statement). Since epidemics differed greatly between years, analysis of all variables was made independently for each year. Replication was considered a random effect, and insecticide, cultivar, and insecticide by cultivar interactions were considered fixed effects. Effects were considered significant when P < 0.05. Fisher’s least significant difference (LSD) values were computed using standard errors and t-values of adjusted degrees of freedom.
RESULTS
In furrow insecticide effects
Marginal chlorosis and necrosis on leaves of young plants were observed on both cultivars treated with phorate in both years. Severity of the phytotoxic effect was not assessed for each plot, but there were no indications of variability in response between the cultivars evaluated in either experiment.
Effect on thrips feeding injury
In 2019, insecticide (P < 0.0001) effects on thrips feeding injury were significant, but neither cultivar (P > 0.23) nor cultivar × insecticide (P > 0.45) effects were (Table 1). Across cultivars, thrips feeding injury ratings were lowest in the imidacloprid treatment and highest in the nontreated control (Table 1). Across insecticide treatments, thrips injury ratings were similar for the two cultivars (Table 1). In 2020, insecticide (P < 0.0001) and cultivar (P < 0.0001) effects were significant, but insecticide × cultivar (P > 0.06) was not. Across cultivars, thrips injury ratings were lower for both insecticide treatments than the nontreated control, and ratings were lowest in the phorate treatment (Table 1). Across insecticide treatments, injury ratings were lower for Georgia-16HO than for Florunner (Table 1).
Effects on tomato spotted wilt epidemics
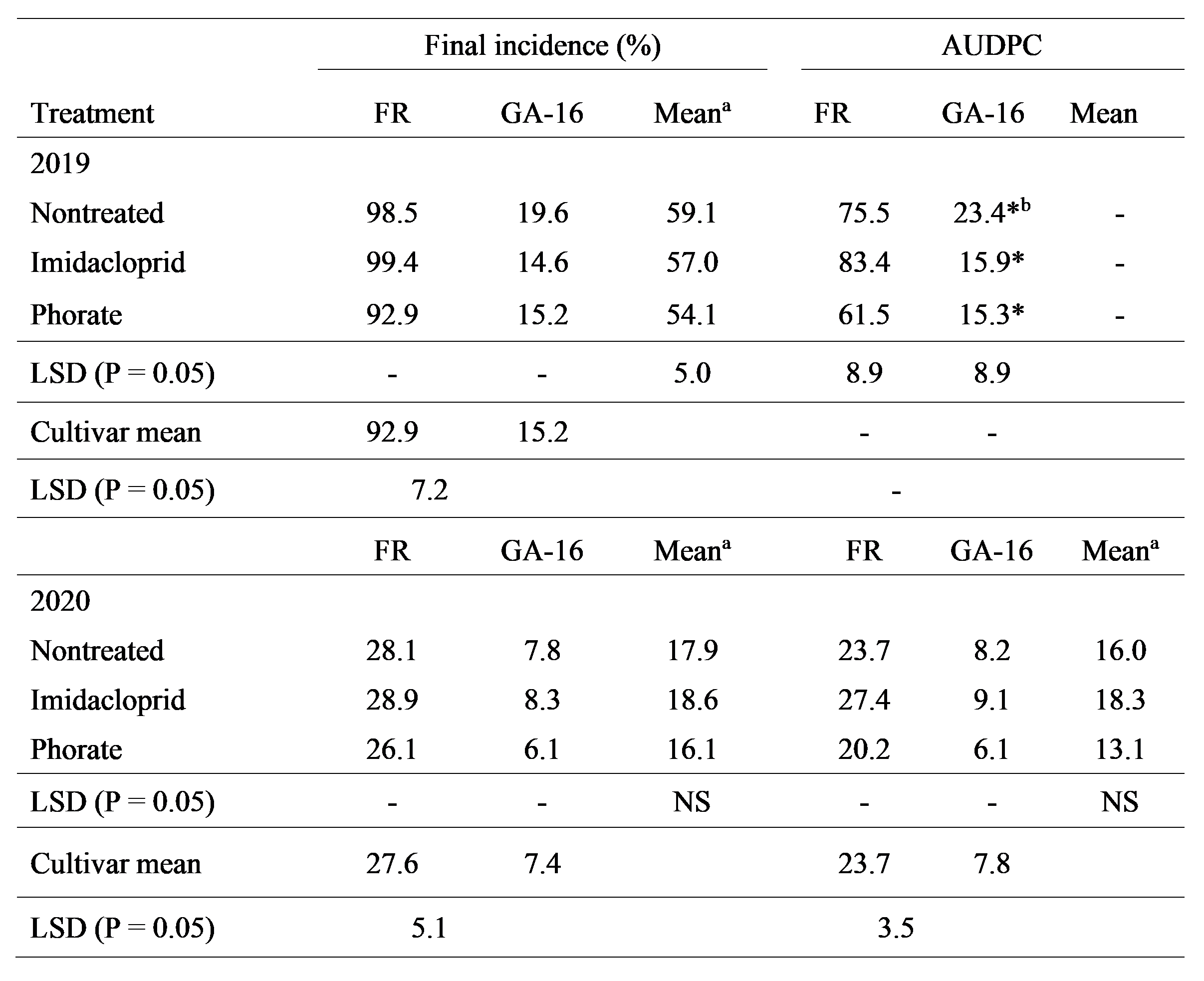
For final incidence of spotted wilt in 2019, cultivar main effects were significant (P < 0.0001), but insecticide (P > 0.15) and cultivar × insecticide (P > 0.33) were not (Table 2). Across insecticide treatments, final incidence in Florunner was higher than in Georgia-16HO (Table 2). For AUDPC, cultivar (P < 0.0001), insecticide (P = 0.001) and cultivar × insecticide (P = 0.006) effects were significant. On Florunner, AUDPC was lowest for the phorate treatment, and AUDPC did not differ for the other two treatments (Table 2). On Georgia-16HO, AUDPC did not differ among the three insecticide treatments (Table 2). AUDPC was lower for Georgia-16HO than for Florunner within each of the insecticide treatments (Table 2).
In 2020, cultivar main effects (P < 0.0001) were significant for both final spotted wilt incidence and AUDPC, but neither insecticide nor cultivar × insecticide were significant for either variable (P > 0.63). Across insecticide treatments, final incidence of spotted wilt and AUDPC were lower for Georgia-16HO than for Florunner (Table 2).
Effects on yield, grade, and crop value
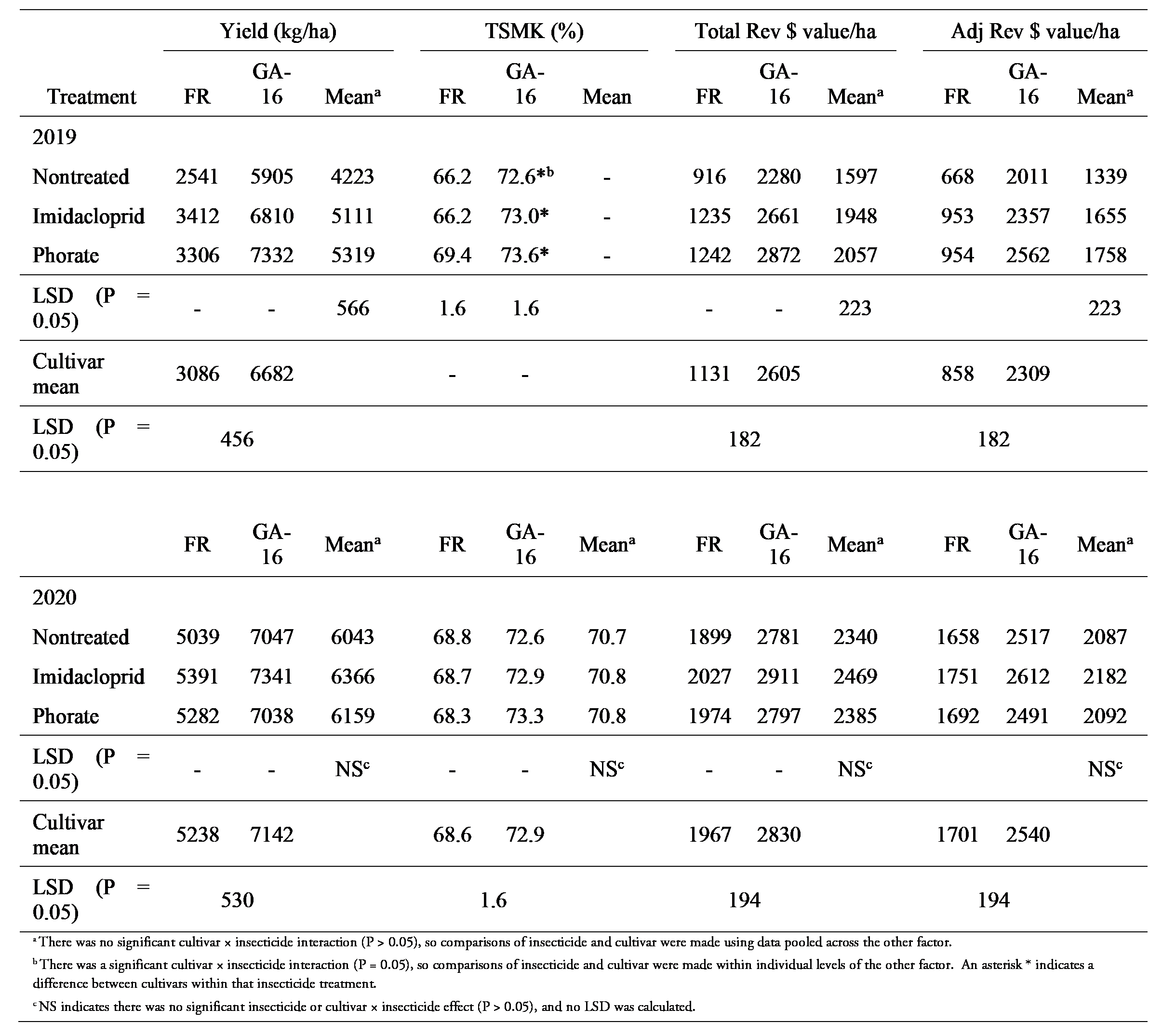
In 2019, cultivar (P < 0.0001) and insecticide (P = 0.0014) main effects were significant on yield, but cultivar × insecticide (P = 0.42) was not (Table 3). Across insecticide treatments, yield was greater for Georgia-16HO (Table 3). In 2020, only cultivar main effects were significant (P < 0.0001) on yield. Across insecticide treatments, yield was greater for Georgia-16HO than Florunner (Table 3).
Cultivar (P < 0.0001), insecticide (P = 0.0013), and cultivar × insecticide (P = 0.05) effects were significant for percent TSMK in 2019 (Table 3). Phorate treated plots of Florunner had higher percent TSMK than the other two treatments, whereas percent TSMK did not differ among insecticide treatments in Georgia-16HO (Table 3). TSMK percentages were higher for Georgia-16HO than for Florunner within each insecticide treatment (Table 3). In 2020, cultivar (P < 0.0001) effects were significant for percent TSMK, but neither insecticide (P = 0.99) nor cultivar × insecticide effects (P = 0.73) were significant. Across insecticide treatments, percent TSMK was higher for Georgia-16HO than for Florunner (Table 3). There were no differences among insecticide treatments (Table 3).
In 2019, cultivar (P < 0.0001) and insecticide (P < 0.002) main effects were significant for both total crop value and adjusted crop value, but cultivar × insecticide (P > 0.47) was not significant. Across insecticides, total crop value and adjusted crop value were higher for Georgia-16HO than for Florunner (Table 3). Both total crop value and adjusted crop value were similar for imidacloprid and phorate treatments, and were higher in each than in the nontreated control (Table 3). In 2020, cultivar (P < 0.0001) effects were significant for both total and adjusted crop value, but insecticide (P > 0.55) and cultivar × insecticide (P > 0.95) effects were not significant for either (Table 3). Across insecticide treatments both total crop value and adjusted crop value were greater for Georgia-16HO than Florunner (Table 3). There were no differences among insecticide treatments for either variable.
DISCUSSION
Results from this study illustrate a large advantage of the cultivar Georgia-16HO over the previous standard runner-type cultivar Florunner for suppression of TSW epidemics, and in yield and crop value when TSW is a factor. The advantage was evident in two years in which severity of spotted wilt epidemics varied greatly, and was most evident in 2019 in which spotted wilt epidemics were more severe. Although comparisons of these two cultivars have not been reported previously, these results corroborate the expectation that could be deduced from a series of previous reports. Branch (2017) reported lower incidence of TSW in Georgia-16HO than in Florida-07, one of its parents. Culbreath et al. (2008, 2016) reported incidence of spotted wilt in Florida-07 that was lower than in the cultivar Georgia Green. Culbreath et al. (1996) reported lower incidence of TSW in Georgia Green than in Florunner.
Similarly, differences between the cultivars in yield and dollar value per hectare were obvious in both years. It is not possible to ascertain exactly how much of the yield and crop value differences are due to field resistance to TSW. Relative yield potential of the two cultivars in the absence of TSW would be difficult to determine in this area. The potential yield of Florunner with fungal disease control comparable to what is available today was not realized in large-scale production. Tomato spotted wilt had emerged as a yield limiting factor before fungicides that provide improved levels of soilborne disease control were labeled for use on peanut in the U.S. (Culbreath and Srinivasan 2011, Culbreath, et al. 2018). Therefore, historic reports of average yield in Georgia when the peanut crop was predominantly Florunner compared to yields with current cultivars would include confounding factors from soilborne fungal diseases. However, Culbreath et al. (1992c) reported yields of Florunner of over 7,400 kg/ha in treatments that provided excellent control of foliar and soilborne diseases, so potential yield is substantially greater than was observed in this study. Although other factors besides field resistance to TSWV may also be involved with yield advantages in Georgia-16HO, the greater differences in yield and value of the yields were observed in 2019, when spotted wilt epidemics were much more severe than in 2020. Differences in yield of over 3500 kg/ha in 2019 and 1904 kg/ha in 2020 and resultant differences in adjusted value of $1451/ha and $839/ha in 2019 and 2020 respectively illustrate the importance of field resistance to TSWV and serve as an indication of the impact that cultivars with improved field resistance to TSWV can have.
Although both insecticide treatments decreased feeding injury by thrips in both years, the effects of insecticide on epidemics of spotted wilt and yield were not consistent across the two years. Final incidence of spotted wilt across cultivars was lower in the phorate treatment than the nontreated control in 2019, but did not differ in 2020. AUDPC was lower in the phorate treatment than for the nontreated control on Florunner in 2019 but not on Georgia-16HO in 2019, and the treatments did not differ in 2020. Across cultivars, yield and both revenue/ha values were higher for imidacloprid and phorate treatments in 2019, but there were no insecticide effects on yield or revenue/ha values in 2020. Although comparisons were not made to determine year effects on yield, it is interesting to note the similarity of yield of the phorate treatment on Georgia-16HO in 2019 to yields of all treatments in that cultivar in 2020.
These results demonstrate the strong advantage of the newer field resistant cultivar Georgia-16HO over the previous standard cultivar Florunner in two years that varied widely with regard to severity of TSW epidemics.
Acknowledgements
The essential efforts of Michael Heath, Ron Hooks, Trip McGhee, Sam Hudson, Nick Hughes, and Simmy McKeown are gratefully acknowledged.
Literature Cited
Black M.C., and Smith D.H.. 1987. Spotted wilt and rust reactions in south Texas among selected peanut genotypes. Proc. Amer. Peanut Res. Educ. Soc. 19:31. (abstr.).
Branch W.D. 1996. Registration of 'Georgia Green' peanut. Crop Sci. 36:806.
Branch W.D. 2017. Registration of 'Georgia-16HO' Peanut. J. Plant Regist. 11:200-203.
Culbreath A.K., Branch W.D., Beasley, Jr. J.P., and Tubbs R.S.. 2012. Peanut genotype and seeding rate effects on spotted wilt. Plant Health Prog. 13:doi:10.1094/PHP-2012-0227-03-RS.
Culbreath A.K., Brenneman T.B. , Shokes F.M. , Csinos A.S. , and Mclean H.S. . 1992c. Tank-mix applications of cyproconazole and chlorothalonil for control of foliar and soilborne diseases of peanut. Plant Dis. 76:1241-1245.
Culbreath A.K., Gevens A.J., and Stevenson K.L.. 2018. Relative effects of demethylation-inhibiting fungicides on late leaf spot of peanut. Plant Health Prog. 19:23-26.
Culbreath A.K., Selph A.C., Williams B.W., Kemerait, Jr. R.C., Srinivasan R., Abney M.R., Tillman B.L., Hollbrook C.C., and Branch W.D.. 2016. Effects of new field resistant cultivars and in-furrow applications of phorate insecticide on tomato spotted wilt of peanut. Crop Prot. 81:70-75.
Culbreath A.K., and Srinivasan R.. 2011. Epidemiology of spotted wilt disease of peanut caused by Tomato spotted wilt virus in the southeastern U.S. Virus Res. 159:101-109.
Culbreath A.K., Tillman B.L., Gorbet D.W., Holbrook C.C., and Nischwitz C.. 2008. Response of new field resistant peanut cultivars to twin row pattern or in-furrow applications of phorate insecticide for management of spotted wilt. Plant Dis. 92:1307-1312.
Culbreath A.K., Tillman B.L., Tubbs R.S., Beasley, Jr. J.P., Kemerait, Jr. R.C., and Brenneman T.B.. 2010. Interactive effects of planting date and cultivar on tomato spotted wilt of peanut. Plant Dis. 94:898-904.
Culbreath A.K., Todd J.W., and Brown S.L.. 2003. Epidemiology and management of tomato spotted wilt in peanut. Annu. Rev. Phytopathol. 41:53-75.
Culbreath A., Todd J.W., and Demski J.W.. 1992a. Productivity of Florunner peanut infected with Tomato spotted wilt virus. Peanut Sci. 19:11-14.
Culbreath A.K., Todd J.W., Demski J.W., and Chamberlin J.R.. 1992b. Disease progress of spotted wilt in peanut cultivars Florunner and Southern Runner. Phytopathology 82:766-771.
Culbreath A.K., Todd J.W., Gorbet D.W., Branch W.D., Sprenkel R.K., Shokes F.M., and Demski J.W.. 1996. Disease progress of tomato spotted wilt virus in selected peanut cultivars and advanced breeding lines. Plant Dis. 80:70-73.
Culbreath A.K., Todd J.W., Gorbet D.W., Shokes F.M., and Pappu H.R.. 1997. Field response of new peanut cultivar UF 91108 to tomato spotted wilt virus. Plant Dis. 81:1410-1415.
Culbreath A.K., Tubbs R.S., Tillman B.L., Beasley, Jr. J.P., Branch W.D., Holbrook C.C., Smith A.R., and Smith N.B.. 2013. Effects of seeding rate and cultivar on tomato spotted wilt of peanut. Crop Prot. 53:118-124.
Gorbet D.W. 1999. University of Florida peanut breeding program. Soil Crop Sci. Soc. Fla. Proc 58:2-7.
Gorbet D.W., Norden A.J., Shokes F.M., and Knauft D.A.. 1987. Registration of 'Southern Runner' peanut. Crop Sci. 27:817.
Gorbet D.W., and Tillman B.L.. 2009. Registration of 'Florida-07' peanut. J. Plant Regist. 3:14-18.
Hagan A.K., Weeks J.R., French J.C., Gudauskas R.T., and Mullen J.M.. 1990. Tomato spotted wilt virus in peanut in Alabama. Plant Dis. 74:615.
Herbert D.A., Malone S., Aref S., Brandenburg R.L., Jordan D.L., Royals B.M., and Johnson P.D.. 2007. Role of insecticides in reducing thrips injury to plants and incidence of Tomato spotted wilt virus in virginia market-type peanut. J. Econ. Entomol. 100:1241-1247.
Holbrook C.C., Timper P., Culbreath A.K., and Kvien C.K.. 2008. Registration of 'Tifguard' peanut. J. Plant Regist. 2:92-94.
Norden A.J., Lipscomb R.W., and Carver W.A.. 1969. Registration of Florunner peanuts. Crop Sci. 9:850.
Shaner G., and Finney R.E.. 1977. The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathology 67:1051-1056.
Sholar J.R., Mozingo R.W., and Beasley, Jr. J.P. 1995. Peanut cultural practices, pp.354-382, In H. E. Pattee and H. T. Stalker (eds.) Advances in Peanut Science. Amer. Peanut Res. and Educ. Soc., Stillwater, OK.
Todd J.W., and Culbreath A.K.. 1995. Thrips populations and spotted wilt disease progress on resistance/susceptible cultivars treated with various insecticides. Proc. Amer. Peanut Res. Educ. Soc. 27:35. (abstr.).
Todd J.W., Culbreath A.K., Chamberlin J.R., Beshear R.J., and Mullinix B.G.. 1995. Colonization and population dynamics of thrips in peanuts in the southern United States. In Parker B., Skinner M., and Lewis T. (eds.) Thrips Biology and Management. pp. 453-460. Plenum Press, New York.
Todd J.W., Culbreath A.K., Rogers D., and Demski J.W.. 1994. Contraindications of insecticide use relative to vector control and spotted wilt disease progress in peanut. Proc. Amer. Peanut Res. Educ. Soc. 26:42. (abstr.).
Todd J.W., Gorbet D.W., Culbreath A.K., and Brown S.L.. 2005. Comparison of final TSWV severity and yield of peanuts treated with acephate, aldicarb, or phorate insecticide at planting. Proc. Amer. Peanut Res. Educ. Soc. 37:79-80 (abstr.).
Tubbs R.S., Beasley, Jr. J.P., Culbreath A.K., Kemerait R.C., Smith N.B., and Smith A.R.. 2011. Row pattern and seeding rate effects on agronomic, disease, and economic factors in large-seeded runner peanut. Peanut Sci. 38:93-100.
USDA-FSA. 2019. Peanut buyers and handlers program guidelines for 2019 and subsequent crop years. USDA Farm Service Agency, Washington, D.C. https://www.fsa.usda.gov/Internet/FSA_File/1-ppg_r00_a04.pdf.
USDA-FSA. 2020. 2019 Peanut loan processing and required corrections: Notice PS-744. USDA Farm Service Agency, Washington, D.C. https://www.fsa.usda.gov/Internet/FSA_Notice/ps_744.pdf.
Notes
- Department of Agricultural and Applied Economics, The University of Georgia, Coastal Plain Experiment Station, Tifton, GA 31793-5766, USA [^]
- Department of Plant Pathology, The University of Georgia, Coastal Plain Experiment Station, Tifton, GA 31793-5766, USA [^]
- Department of Agricultural Economics and Rural Sociology, Auburn University, Auburn, AL 36849, USA [^]
- Department of Entomology, The University of Georgia, Griffin Campus, Griffin, GA 30223-1797, USA [^]
- Department of Entomology, The University of Georgia, Coastal Plain Experiment Station, Tifton, GA 31793-5766, USA [^]
- Department of Crop and Soil Sciences, The University of Georgia, Coastal Plain Experiment Station, 31793-5766 USA [^] Corresponding Author e-mail: spotwilt@uga.edu