Introduction
Thrips are an important early season insect pest of peanut (Arachis hypogaea L.) that can reduce pod yield and delay harvest maturity (Brecke et al. 1996). Multiple species of thrips (Thysanoptera: Thripidae) infest peanut throughout the growing season in the southern U.S., with tobacco thrips, Frankliniella fusca (Hinds), being the predominant species during the seedling stage (Todd et al. 1995). Tobacco thrips injure seedling peanut plants by feeding on developing leaves and leaf buds with rasping sucking mouthparts. This feeding can cause leaf discoloration, leaf distortion, and plant stunting in peanuts and other crops (Todd et al. 1995, Marasigan et al. 2016). The severity and persistence of thrips feeding on peanut seedlings often varies from year to year as weather is a strong driver influencing the number of thrips in a population. Specific weather parameters linked to thrips populations cycles throughout peanut seedling development have been linked to minimum daily temperatures, number of rain days, and evapotranspiration in the spring months preceding peanut planting (Olatinwo et al. 2009). Whereas, high precipitation intensity events at the time of planting and seedling development generally reduces thrips prevalence. Tobacco thrips injury and its associated yield loss can also be exacerbated in situations where other stress factors impact peanut growth and development, or from thrips transmitted Tomato spotted wilt virus (Ullman et al. 1992, Herbert et al. 2007). Multiple studies have shown that thrips can cause yield losses in peanuts where injury from a pre- or early post-emergence herbicide has occurred (Funderburk et al. 1998, Drake et al. 2009, Moor et al. 2021). Additionally, thrips can reduce peanut yields when excessive drought slows the growth of seedlings (Funderburk et al. 1998) or when saturated soils affect peanut growth (Moor et al. 2021). Given the complex interaction between environmental conditions, thrips populations, and seedling development, integrated pest management strategies to reduce thrips injury may vary across peanut producing geographic regions.
Peanut production in the midsouthern region of the U.S., including Mississippi, Louisiana, Arkansas, and Missouri, has been increasing in recent years. This region has unique challenges for peanut production that are different than other peanut production regions in the U.S. In particular, the cultivars planted in this region include runners, such as ‘Georgia 06G’ (Branch 2007), developed as medium maturing varieties within the lower coastal plain production region. Although these varieties are adapted and perform well in the midsouthern U.S., the relatively shorter growing season experienced in this region makes timely plant establishment critical to maximize potential cumulative thermal units (Kukal and Irmak, 2018). However, late April to mid- May planting windows are often interrupted with cool, wet weather that increases the risk of reduced stand and slow seedling development. Studies have documented increased foliar injury from thrips as peanut plant populations decrease (Mahoney et al. 2019). Additionally, if cool weather occurs prior to crop emergence, the benefits of planting earlier to maximize thermal unit accumulation are often negated as emergence time is increased. A possible strategy to address these management challenges is to evaluate earlier maturing peanut varieties whose crop development is better synchronized to the midsouthern U.S climate. Varieties of shorter maturation with faster stand establishment and seedling development may also aid in reducing yield reductions associated with reduced stand and thrips injury.
Although shorter maturing peanut varieties with high seedling vigor have potential to provide benefits to midsouthern peanut production systems, no information exists regarding the normal plant date for these varieties within the midsouthern peanut production environment. In-furrow insecticide control methods for thrips may also vary for varieties with different seedling vigor if planted either early or late within the planting window. For example, varieties with less seedling vigor planted in suboptimal environmental conditions may benefit from in-furrow insecticides that provide greater efficacy against thrips. Therefore, the objective of this research was to evaluate in-furrow insecticides when applied to peanut varieties of different maturity at early and late plant date timings. The effectiveness of in-furrow insecticide, planting date, and variety of different maturities was determined by quantifying their impact on seedling emergence, immature and adult thrips numbers, and pod yield.
Materials and Methods
A study was conducted in 2018 and 2019 at the Delta Research and Extension Center in Stoneville, MS to determine the impact of planting date, peanut variety, and at-planting insecticide on thrips management and yield. The study was arranged as a split-split-plot in a randomized complete block design with four replications. The main-plot factor was planting date and included a normal (based on grower practices) planting date and a late planting date. The first planting dates (normal) were May 7 in 2018 and 2019. The second planting dates (late) were May 30 in 2018 and June 10 in 2019. Each main-plot was 24 rows (1.02 m centers) wide by 12.2 m long. Both varieties were planted at 21 seed m-1 of row with a John Deere 1700 MaxEmerge vacuum planter. A Bradyrhizobium sp. inoculant (Primo Power CL, Verdesian Life Sciences, Cary, NC) was sprayed into the open seed furrow immediately in front of the closing wheels at a rate of 0.95 L ha-1 ensuring good seed coverage. Weeds and diseases were managed across the entire test area with labeled pre- and post-emergence herbicides and fungicides at their recommended rates. Insect pests other than thrips never exceeded current action thresholds throughout the duration of the experiment, so no insecticides were used other than the experimental treatments.
The sub-plot factor was peanut variety and included Georgia 06G (Branch 2007) and Algrano IPG QR14 (Algrano, Bledsoe, TX). Each sub-plot was 12 rows wide by 12.2 m long. Georgia 06G was developed in south Georgia and is currently the most common peanut cultivar planted in the southern U.S. Algrano IPG QR14 was developed in west Texas as an earlier maturing variety with an average harvest maturity of 125-130 days after planting. The sub-sub-plot factor was insecticide treatment at three levels. Each sub-sub-plot was 4 rows wide by 12.2 m long. The insecticide treatments included phorate (Thimet 20G, Amvac Corporation, Los Angeles, CA) at a rate of 1.12 kg ai ha-1, imidacloprid (Admire Pro, Bayer CropScience, Raleigh, NC) at a rate of 0.36 kg ai ha-1, and an untreated control. Phorate was applied into the open seed furrow through granular hopper boxes immediately behind the seed tube and in front of the closing wheels. Imidacloprid was sprayed into the open seed furrow with 8002 flat fan nozzles behind the seed tubes and immediately in front of the closing wheels. The spray pattern was directed into the open seed furrow with a flat fan nozzle oriented parallel to the seed furrow ensuring good coverage of seed and the seed furrow. The application volume for the in-furrow spray was 46.7 L ha-1 total volume at 138 kPa. The untreated control did not receive any insecticide treatment.
Plant population was assessed at 10 and 20 days after the first planting date and 7 and 14 days after the second planting date. Plant population was counted on the center two rows of each sub-sub-plot and averaged to obtain the number of plants in 12.2 m of row for each sub-sub-plot. Adult and immature thrips densities were determined at the 1-2 leaf stage and 3-4 leaf stage. These corresponded to 21 and 28 days after planting for the first planting date, and 14 and 21 days after planting for the second planting date. Assessments of plant populations and thrips densities were taken earlier for the second planting date because peanut emergence and growth was faster than for the first planting date. Thrips were sampled by cutting five random plants from the center two rows of each plot. The plants were transported to the laboratory in 0.95 L self-sealing plastic bags (Ziploc®, S. C. Johnson &Son, Inc., Racine, WI). In the laboratory, a whole plant washing method developed for cotton (Burris et al. 1990) was used to dislodge adult and immature thrips from plants. Plants were soaked in a solution of bleach and soapy water in the plastic bags. The contents of each bag were poured into a 2.0-mm sieve on top of a funnel to filter out plants and larger material. The plants were washed with a stream of water to ensure all thrips were dislodged from plants and washed into the funnel. A 45.0-µm sieve was placed under the funnel to collect thrips. The contents of the smaller sieve were washed into a Buchner funnel with 70% ethanol onto 9.0-cm diam ruled filter paper. A vacuum pump was used to remove excess ethanol and water from the filter paper. The number of adult and immature thrips were counted with the use of a dissecting microscope at 40X magnification.
The timing of peanut digging each year was determined using the days until digging prediction from the peanut profile board (Williams and Drexler 1981). Plants were inverted with a two-row KMC digger-shaker-inverter (Kelley Manufacturing, Tifton, GA). After 5 and 7 days, peanuts were harvested in 2018 and 2019, respectively, with a two-row KMC peanut picker modified with a bagging attachment. Peanuts in the first and second planting dates in 2018 were harvested 150 and 141 days after planting, respectively. In 2019, peanuts were harvested 143 and 140 days after planting in the first and second planting dates, respectively. Immediately after harvest, bags were weighed on a hanging digital scale (Model CS200, Intercomp, Medina, MN), and weights were converted to kg ha-1.
Daily meteorological data was recorded using an automated weather station located within 1500 m of the experiment. Daily maximum and minimum soil temperatures were recorded at a depth of 5 cm and used to determine the average soil temperature during the first 20 DAP for each planting date in 2018 and 2019. The total precipitation amount during this time period was also computed for each planting date. Daily thermal units were computed by averaging the minimum and maximum atmospheric temperatures and subtracting the base temperature of 13.3°C (Emery, 1969). Total thermal unit accumulation or growing degree days (GDD) was computed by summing the daily thermal unit accumulation from planting to digging for both planting dates in 2018 and 2019.
All data were analyzed with a general linear mixed model analysis of variance with a Gaussian distribution (PROC GLIMMIX, SAS 9.4, SAS Institute Inc., Cary, NC). Planting date, variety, thrips management and their interactions were considered fixed effects in the model. Replication, replication nested in year, replication by planting date nested in year, and replication by planting date by variety nested in year were considered random effects. The Kenward-Roger method was used to estimate denominator degrees of freedom (Kenward and Roger 1997). Means and standard errors reported in the tables and figures were calculated with PROC MEANS. Mean separation was determined based on the LSMEANS and adjusted according to Tukey’s HSD at α = 0.05 (Tukey 1953).
Results and Discussion
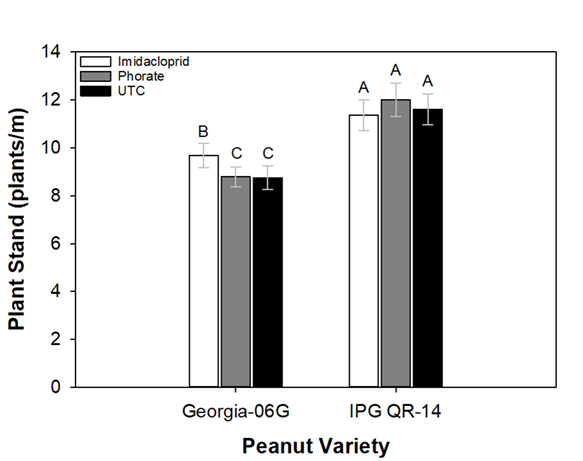
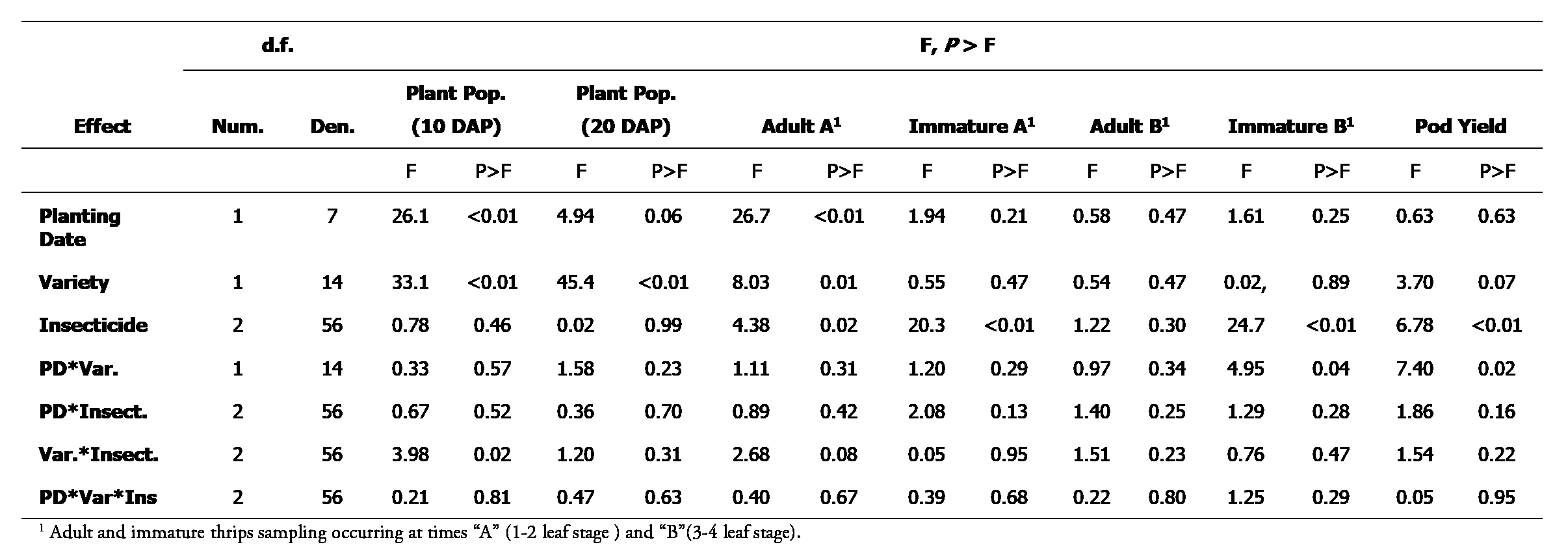
Treatments had an effect on plant population, thrips numbers, and yield in this experiment. At 7-10 days after planting, there was an effect of planting date and a variety by insecticide interaction for plant population (Table 1). The first planting date (normal) (11.7 ± 0.34 plants/m ) had a greater plant population than the second planting date (late) (9.0 ± 0.30 plants/m ). This was likely caused by warm and dry weather causing suboptimal soil moisture conditions reducing plant emergence (Table 2). Plant populations for Georgia 06G were also lower than IPG QR-14 at 7-10 days after planting. Plant population was similar among all insecticide treatments for the IPG QR-14 variety (Fig. 1). In contrast, plant population where imidacloprid was used in furrow was greater than where phorate was used and the untreated control for Georgia 06G peanuts. At 14-20 days after planting, variety was the only experimental factor that had an effect on plant population (Table 1). The IPG QR-14 variety (13.4 ± 0.32 plants/m) had a greater plant population than Georgia 06G (11.6 ± 0.29 plants/m). The effect of variety on plant population is not clear, but could be related to varietal maturity, in general early maturing varieties of most crops tend to have greater seedling vigor than later maturing varieties (Nautiyal et al. 2010, Zheng and Arima 2013). Other factors for increased plant population may be attributed to IPG QR-14 having a smaller seed size or greater cold tolerance during emergence/seedling stage (Prasad et al. 2006; Grey et al. 2016). Also, germination percentage was not recorded for either variety, but may have played a role in the different plant populations observed since both varieties were planted at the same seeding rate. Imidacloprid has been shown to influence several biochemical and physiological processes of cotton that enhance plant growth (Kaur et al. 2011). This may partially explain why Georgia 06G peanut plants treated with imidacloprid in-furrow had a greater initial plant population than other treatments with Georgia 06G. However, this result is unclear based on the data collected as insecticide treatment had no influence on the initial plant population of QR-14. Nonetheless, this effect was marginal as the final stand of Georgia-06G was similar among all insecticide treatments.
Effect of the interaction between peanut cultivar and insecticide treatment on plant populations in Stoneville, MS during 2018 and 2019. Data presented are results for the first sampling date occurring at 10 days after planting. Means followed by similar letter are not statistically different using Tukey’s Honestly Significant Difference at P < 0.05.
Planting date and variety each had an effect on adult thrips numbers at the 1-2 leaf stage (Table 1). More adult thrips were observed on peanuts at the second planting date (late) (5.8 ± 0.7 no./five plants) than the first planting date (normal) (1.9 ± 0.3 no./five plants). Additionally, more adult thrips were observed on Georgia 06G peanuts (4.8 ± 0.7 no./five plants) than IPG QR-14 peanuts (3.0 ± 0.4 no./five plants). This may be a result of the relatively lower plant population of Georgia-06G. Other research studies have reported similar results of increased visual thrips injury as plant populations decrease (Mahoney et al. 2019). Insecticide treatment had an effect on adult and immature thrips at the 1-2 leaf stage and on immature thrips at the 3-4 leaf stage (Table 1). At the 1-2 leaf stage, peanuts treated with phorate at-planting had fewer adult and immature thrips than imidacloprid treated and untreated peanuts (Table 2). Additionally, peanuts treated with imidacloprid had fewer immature thrips than the untreated peanuts. However, the number of adult thrips at the 1-2 leaf stage was similar on imidacloprid treated and untreated peanut. At the 3-4 leaf stage, no differences were observed among treatments for adult thrips. Insecticide affected immature thrips populations at the 3-4 leaf stage (Table 1). Similar to the 1-2 leaf stage, peanuts treated with phorate had fewer immature thrips than peanuts treated with imidacloprid and untreated peanuts (Table 2). Peanuts treated with imidacloprid had fewer immature thrips than untreated peanuts. These results indicate greater efficacy of phorate on both adult and immature thrips when compared to imidacloprid. Additionally, the number of immature thrips at both sampling times was significantly lower when comparing phorate to imidacloprid. At the 3-4 leaf stage, planting date and variety interacted to influence numbers of immature thrips (Table 1). Fewer immature thrips were observed on Georgia 06G at the second planting date (late) compared to Georgia 06G peanuts planted at the first planting date (normal), but no differences in numbers of immature thrips were observed for IPG QR-14 peanuts at either planting date (Table 3).
Insecticide treatment had a main effect on peanut pod yield regardless of variety or plant date (Table 4). Pod yield was greater in phorate and imidacloprid treated peanut than the non-treated control. Pod yield loss in the untreated control was likely a result of early season thrips injury reducing reproductive development since insecticide treatment had no influence on final plant population. The phorate and imidacloprid treatments had similar pod yield despite the phorate treatments having substantially greater thrips control. This suggests imidacloprid provided adequate thrips control to prevent pod yield loss in the two site years of this study. Although not reported, it is important to note that the level of Tomato spotted wilt virus (TSWV) incidence was low during both years of this study which is typical for midsouthern peanut production systems. The application of these research results could change if TSWV becomes more prevalent in midsouthern peanut production systems since phorate has been well documented to reduce TSWV incidence.
A variety by plant date interaction did occur for pod yield. Georgia-06G had reduced pod yield when plated later, whereas the pod yield for IPG QR-14 was similar for both plant dates (Table 4). This occurred despite no planting date by variety interaction for adult and immature thrips at the 1-2 leaf stage. Additionally, the later planting of Georgia 06G had lower immature thrips when compared to the later planting of QR-14. This suggest the relatively later maturing cultivar Georgia-06G may have never reached its full yield potential due to a lack of thermal unit accumulation when planted in late May and early June. Total thermal unit accumulation was reduced by 187 and 206 °C days at the second (late) planting date in 2018 and 2019, respectively. Overall, these results indicate that the greater pod yield of QR-14 when planted later was independent of thrips control and that early maturing cultivars may be better suited for later plant dates due to maturation limitations on pod yield. Future research evaluating variety maturation and plant dates within the midsouthern production system is needed.
Literature Cited
Branch W. D. 2007. Registration of ‘Georgia 06G’ peanut. J. Plant Regist. 1: 120-120.
Brecke B.J., Funderburk J.E., Teare I.D., and Gorbet D.W.. 1996. Interaction of early-season herbicide injury, tobacco thrips injury, and cultivar on peanut. Agron. J. 88: 14-18.
Drake W. L., Jordan D. L., Lassiter B. R., Johnson P. D., Brandenberg R. L., and Royals B. M.. 2009. Peanut cultivar response to damage from tobacco thrips and paraquat. Agron. J. 101: 1388-1393.
Emery D.A., Wynne J.C., and Hexem R.O.. 1969. A heat unit index for Virginia type peanuts. I. Germination to flowering. Oleagineux 23: 99-104.
Funderburk J. E., Gorbet D. W., Teare I. D., and Stavisky J.. 1998. Thrips injury can reduce peanut yield and quality under conditions of multiple stress. Agron. J. 90: 563.-566.
Grey T.L., Branch W.D , Tubbs R. S., Snider J.L., Webster T.M , Arnold J., and Li X.. 2016. The impact of genotype x environment effects on runner-type peanut seed vigor response to temperature. Agron. J. 108: 1424-1433.
Herbert Jr. D. A., Malone S., Aref S., Brandenburg R. L., Jordan D. L., Royals B. M., and Johnson P. D.. 2007. Role of insecticides in reducing thrips injury to plants and incidence of tomato spotted wilt virus in Virginia market-type peanut. J. Econ. Entomol. 100: 1241-1247.
Kaur N., Sohal B. S., and Singh K.. 2011. Biochemical and physiological changes on Bacillus thuringiensis cotton after imidacloprid foliar spray. Pest. Biochem. Physiol. 99: 280-284.
Kenward M. G., and Roger J. H.. 1997. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53: 983-997.
Kukal M.S., and Irmak S.. 2018. U.S agro-climate in the 20th century: growing degree days, first and last frost, growing season length, and impacts on crop yields. Sci. Rep. 8: 1-14.
Mahoney D.J., Jordan D.L., Brandenburg R.L., Shrew B.B., Royals B.R., Inman M.D., and Hare A.T . 2019. Influence of planting date, fungicide seed treatment, and phorate on peanut in North Carolina. Peanut Sci 46: 14-21.
Moor J. C., Gore J., Catchot A., Cook D., Crow W. D., Dodds D. M., Sarver J. M., Towles T. B., and Zurweller B.. 2021. Effect of imidacloprid and acephate for tobacco thrips (Thysonaptera: Thripidae) management on flumixazin injured peanut. Peanut Sci. 48: 6-14.
Nautiyal P. C., Misra J. B., and Zala P. V.. 2010. Influence of seed maturity stages on germinability and seedling vigor in groundnut. SAT eJournal 8: http://ejournal.icrisat.org/volume8/Groundnut/Influence_of_.pdf (accessed 9/22/ 2021).
Olatinwo R.O., Paz J.O , Brown S.L., Kemerait R.C., Culbreath A.K., and Hoogenboom G.. 2009. Impact of early spring weather factors on the risk of tomato spotted wilt in peanut. Plant Dis. 93:783-788.
Prasad P.V.V., Boote K.J , Thomas J.M.G., Allen Jr. L.H. and Gorbet D.W.. 2006. Influence of soil temperature on seedling emergence and early growth of peanut cultivars in field conditions. J. Agron. and Crop Sci. 192: 168-177.
Todd J. W., Culbreath A. K., Chamberlin J. R., Beshear R. J., and Mullinix B. G.. 1995. Colonization and population dynamics of thrips in peanuts in the southern United States, pp. 453-460, In B. Parker, M. Skinner, and T. Lewis (eds.), Thrips Biology and Management, Plenum Press, New York.
Tukey J. W. 1953. The problem of multiple comparisons. Department of Statistics, Princeton University, NJ.
Ullman D. E., Cho J. J., Mau R. F. L., Hunter W. B., Westcot D. M., and Custer D. M.. 1992. Thrips-tomato spotted wilt virus interactions: morphological, behavioral and cellular components influencing thrips transmission. Adv. Dis. Vect. Res. 9: 195-240.
Williams E. J., and Drexler J. S.. 1981. A non-destructive method for determining peanut pod maturity. Peanut Sci. 8: 134-141.
Notes
- First author: Assistant Professor, Department of Plant and Soil Sciences, Mississippi State University, Mississippi State, MS 39762 [^]
- Second, fourth, and sixth authors: Professor, Associate Professor, and Assistant Professor, Department of Biochemistry, Molecular Biology, Entomology, and Plant Pathology, Mississippi State University, Stoneville, MS 38776 [^]
- Third author: Assistant Professor, Entomology and Plant Pathology, Auburn University, Auburn University, AL 36849 [^]
- Fifth author: Assistant Professor, Entomology Department, Louisiana State University, Winnsboro, LA 71295 [^] Corresponding author’s E-mail: brendan.zurweller@msstate.edu