INTRODUCTION
Peanut (Arachis hypogaea L.) contributes to food security and serves as a cash crop in Ghana (Angelucci and Bazzucchi, 2013; Quiñones and Diao, 2011). Peanut is cultivated by approximately 74% of households in the Northern Savannah of Ghana and is an important source of protein in human diets (Quiñones and Diao, 2011). It is the most popular legume crop cultivated in Ghana. In 2016, farmers in Ghana produced approximately 425,825 MT of peanut on 336,450 ha of land. In 2017 and 2018, 433,772 MT and 521,032 MT were produced on about 338,000 ha and 394,231 ha, respectively (FAOSTAT, 2020). However, the current production of peanut has not been able to supply the increasing demand by consumers. Historically, peanut yield in Ghana was lower than the estimated attainable yield of 2.50 to 3.50 MT/ha (MOFA, 2021). For example, average yield in 2016 and 2020 was 1.3 Mt/ha and 1.67 Mt/ha in the country, respectively (MOFA, 2016, 2017, 2021).
Numerous factors can affect yield, quality, and contamination by aflatoxin for peanut (caused by Aspergillus flavus and A. parasiticus) (Jordan et al., 2018; Nigam et al., 2018). Limited access to improved cultivars and quality seed are major contributors to low yields and quality (Akpo et al., 2021; DFID, 2014). The seed sector in Ghana is characterized by two sources that include informal and formal seed systems. The informal seed system is the primary source of seed for planting and consists of seed saved by farmers (Anonymous, 2016; Puozaa et al., 2021). The formal seed system includes certified seed. Certified peanut seed accounts for only 1% of plantings by farmers in Ghana (Anonymous, 2016). Farmers also rely on seed purchased in local markets as well as exchanges with other farmers. Owusu-Adjei et al. (2017) reported that 59% of peanut producers source seeds from their previous harvest, 28% purchase seeds from local markets, and 13% obtain seeds as gifts from non-governmental organizations, friends, and relatives. However, the quality of seeds obtained from these sources is a concern and documentation of quality is limited. According to FAO (2018), the level of seed quality can vary within the informal seed system depending on the source of seed. Less than ideal stand establishment is associated with seed quality (Matthews et al., 2012). Greater seed quality is associated with a 15-20% increase in yield (Abebe et al., 2017; Chauhan et al., 2015).
Information relative to quality across all sources of peanut seed in Ghana is limited. Therefore, the objective of this research was to compare seed quality attributes that exist in peanut seed obtained from farmers, local seed markets, and research institutions.
MATERIALS AND METHODS
Seeds were collected in Ghana from 46 farmers, 45 open markets, and 9 research institutes across the Northern, Upper West, Upper East, Ashanti, and Bono East Regions in November 2019. These regions constitute 98% of peanut production in Ghana (MOFA, 2017). The research institutes where samples were collected included the Council for Scientific and Industrial Research-Crops Research Institute (CSIR-CRI) and the Council for Scientific and Industrial Research-Savanna Agricultural Research Institute (CSIR-SARI). Seed was stored in poly sacks at ambient temperature for approximately three months prior to the seed quality evaluations. This approach to storage is similar to that of farmers in Ghana.
Seed moisture was determined using a portable seed moisture tester (Moisture Chek-Plus-SW08120, John Deere, AgraTronix, Streetsboro, OH) for each sample using three replicated seed lots. Physical purity of seed was determined using 400 g of seed removed from samples. Seed in each sample was separated into three components including 1) seed only, 2) other crop seed, and 3) foreign matter. Each component was weighed and percent of total sample calculated.
Germination was determined using the pure seed fraction collected from each sample. Two-hundred seeds were divided into four replicates of fifty seed and planted on sand substrate in 34 cm × 11.5 cm size plastic dishes. Two-thirds of the plastic dishes were filled with sterilized loamy-sand soil. Germination dishes were maintained in a greenhouse at 25 ± 2℃. The number of germinated seed was recorded 5 d after water imbibition daily through 10 d and calculated as a percentage of seed placed in each dish. At the end of day 10, normal seedlings were counted and expressed as percentage of total seed. Seed vigor was measured using Mean Germination Time (MGT) and Mean Germination Rate (MGR) procedure (Ranal et al., 2009).
A sub-set of collected samples was randomly selected to document the presence of pathogens associated with seed and included 11 samples from farmer-saved seed, 30 samples of seed from open markets, and 9 samples of seed from research institutions. Potato Dextrose Agar media (PDA) was prepared from a commercial product (Sigma-Aldrich, Steinhein, Germany). Samples were individually sterilized in 50 ml of a 10% sodium hypochlorite solution for 30 s followed by a rinse in 50 ml of distilled water. The sterilized seeds were then inoculated on 9.0 cm petri dishes containing PDA media. Five seed in ten replicates were arranged equidistantly on the media. Samples were incubated at 25 ± 2℃ for 7 d under 12 h alternating light and dark conditions. On the eighth d, seed was examined for fungal infection. Fungal infection was examined through visual assessment of the fungal colonies. Fungi were identified based on morphological features of colonies including color and texture (Diba et al., 2007; Klich, 2002). The number of seeds with visible infection was determined and expressed as percentage of total number of seeds.
Phenotype uniformity of plants produced from seed from 95 samples was assessed after planting in the field. Fifty seed from each sample were planted in rows spaced 75-cm apart with seed spaced 30-cm apart with a plot length of 4 m. Seed samples were replicated three times. Individual plants were examined throughout the growing season to determine the number of plants with the same phenotype characteristics deviating from the majority of plants. The total number of plants and those deviating were recorded at maturity and expressed as percentage relative to the majority of morphological features of plants.
The experimental design in all experiments was a completely randomized design with three replications. Data for average moisture content, germination percentage, emergence percentage, germination time, germination rate, and plant phenotype uniformity for the 45 collections from farmers, 46 collections from open markets, 9 collections from research institutes were subjected to analysis of variance. Data for infection by pathogens for 11 collections from farmers, 30 collections from open markets, and 9 collections from research institutes were also subjected to analysis of variance. Means were separated using the Fisher’s Protected LSD test at p < 0.05.
RESULTS AND DISCUSSION
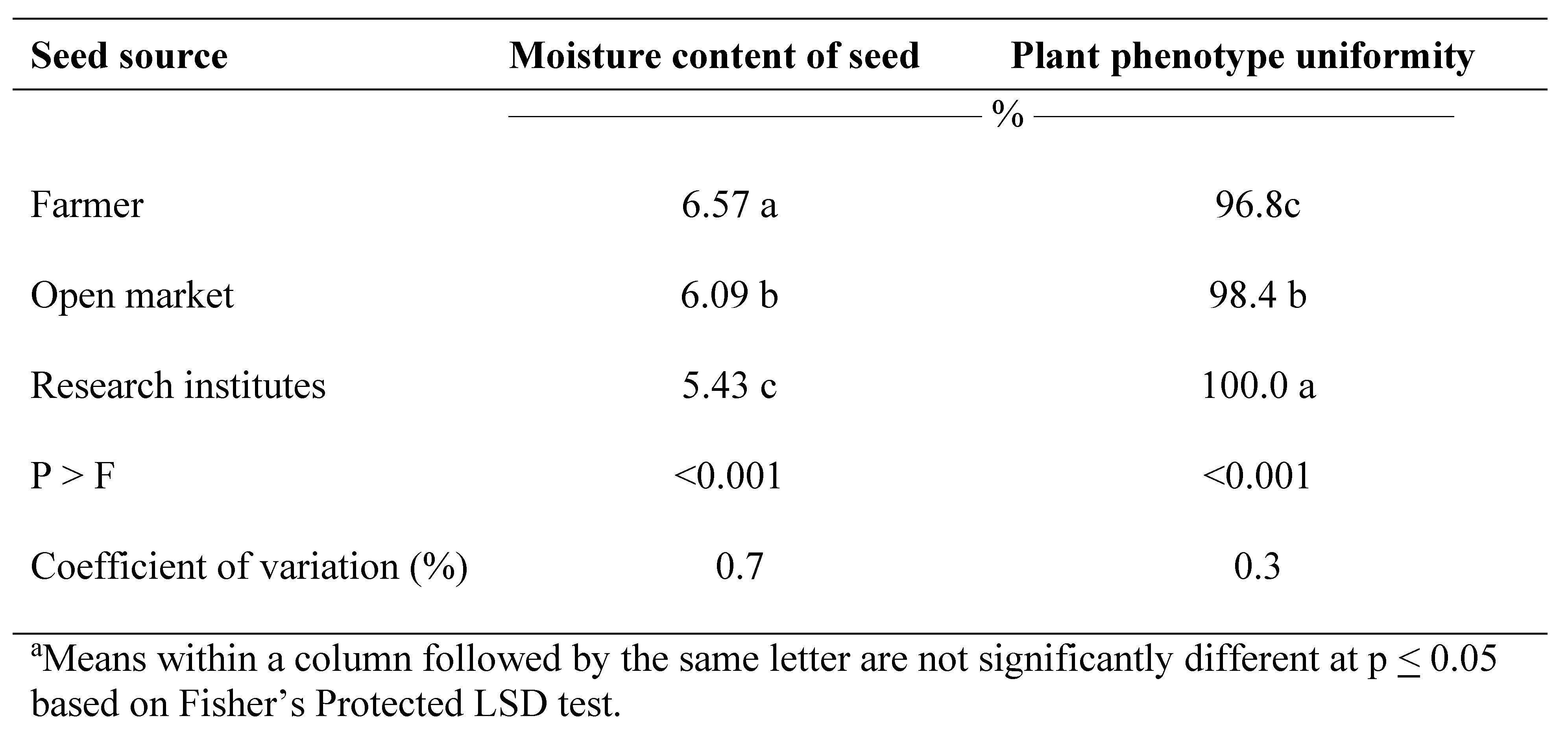
Moisture content of seeds from the research institutes was lower than seed obtained from the market or saved by farmers (Table 1). Farmer-saved seed recorded the highest average moisture content (6.57%) while seed obtained from the research institutes recorded the lowest moisture content (5.42%). Dhedhi et al. (2017) reported mean moisture content of 5.07% for peanut in India. Moisture content of 7.5% is widely reported as the most appropriate storage for peanut (Beuchat and Koehler, 1979; McDonald and Copeland, 2012; Nautiyal, 2002; Smith et al., 1995). However, lower amounts of moisture content can be achieved with oil crops (Sastry et al., 2007). The low moisture content recorded in the present study may be attributed to the dry climatic conditions of seed collection, suggesting that seeds were dried adequately before storage. Gebeheyu et al. (2019) reported similar results in rice (Oryza sativa L.) seeds in Tanzania.
No difference in purity among seed sources was observed and exceeded 98% (data not shown). This is most likely because individuals in all three segments of the seed industry shell farmer stock by hand and separate fractions by hand for the market (e. g., splits, damaged kernels, and foreign material).
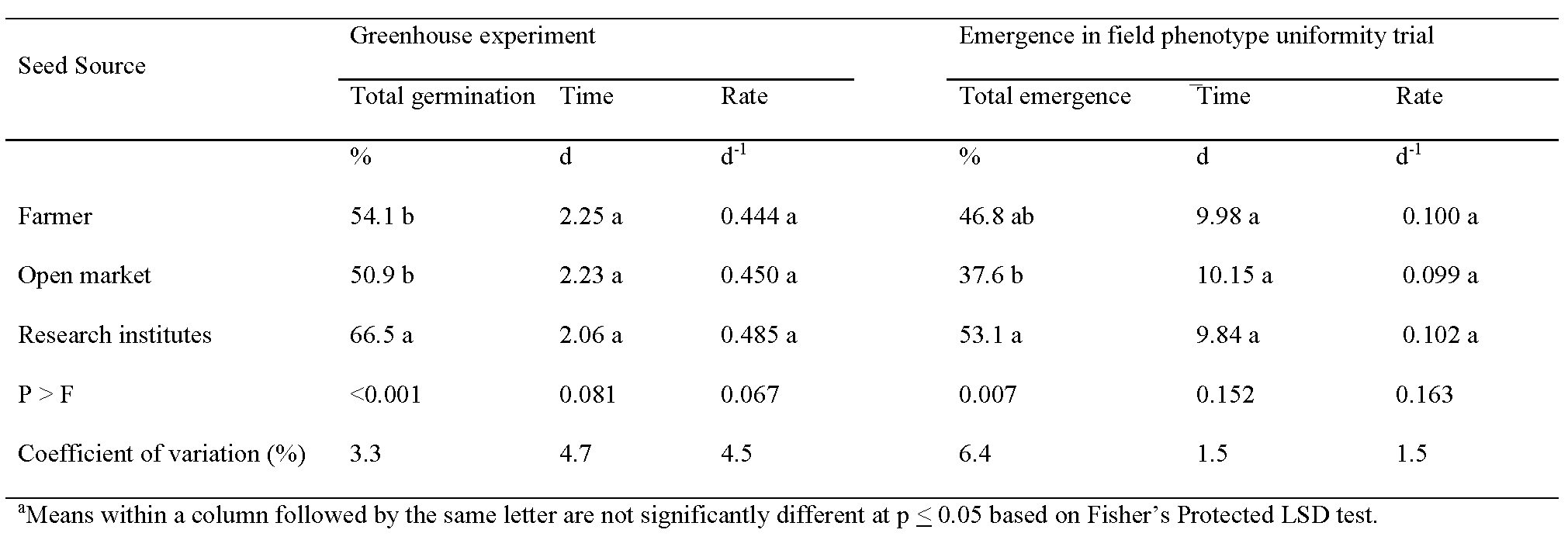
Germination in the greenhouse and emergence in the field evaluating phenotype uniformity were affected by source (Table 2). Germination in the greenhouse was 54.1%, 50.9%, and 66.5% when seed was collected from farmers, local markets, and research institutes, respectively. Emergence in the field from seed derived from these respective sources was 46.8%, 37.6%, and 53.1%. Germination was greater for seed collected at research institutes than seed from farmers and local markets: germination was similar for famer-saved and market-purchased seed. When comparing emergence in the field, a higher percentage was noted for seed from research institutes than local markets while emergence of famer-saved seed was intermediate. Although differences were noted in final germination and emergence, no difference in germination was observed with respect to MGT or MGR (Table 2).
Low germination and plant emergence in the field from three seed sources is likely an indication of environmental and edaphic conditions during the previous growing cycle, nutrition in seed, timing and handling of peanut pods at harvest, and storage conditions after harvest but before collection for these experiments. Germination of seed and peanut emergence from deteriorated seed can result in establishment of uneven populations and seedlings with low vigor (Biabani et al., 2011; Cho and Scott, 2000; Hamman et al., 2002; Sharanappa et al., 2018).
Differences in plant phenotype uniformity were observed based on seed source (Table 1). Seeds from research institutes recorded the highest phenotypic uniformity levels (100%) while uniformity of plants derived from farmer-saved seeds was 97%. Uniformity of plants from local markets was 96%.
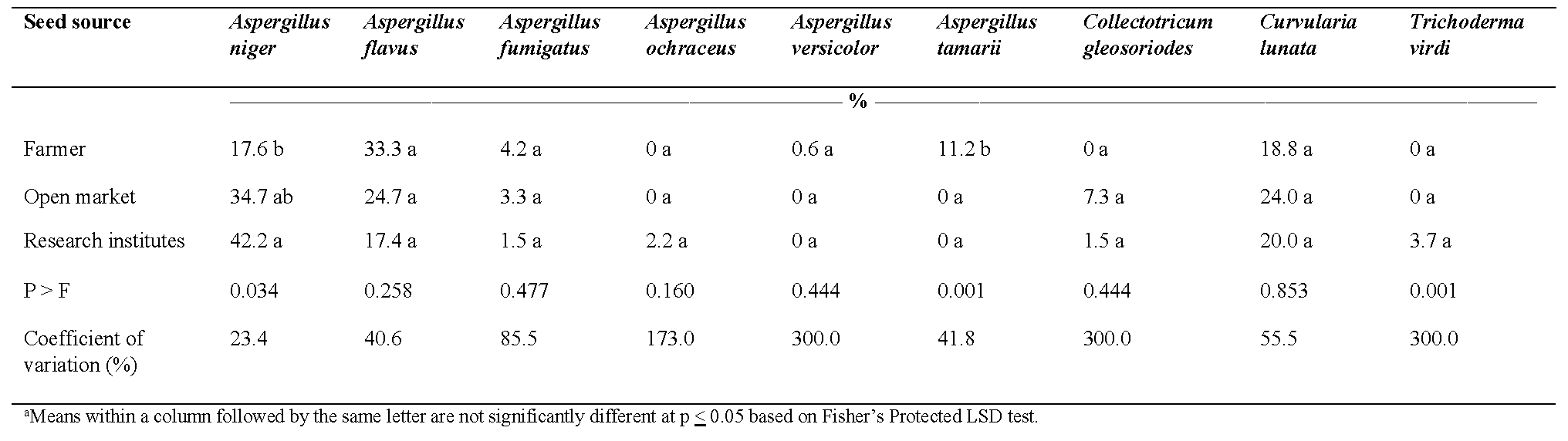
Nine fungal species were isolated from seed and included: Aspergillus niger Tiegh., Aspergillus flavus Link, Aspergillus fumigatus Fresenius, Aspergillus ochraceus Wilhelm, Aspergillus versicolor (Vuillemin) Tiraboschi, Aspergillus tamarii Kita, Collectotricum gleosporiodes Penz., Curvularia lunata (Wakker) Boedijn, and Trichoderma viride Persoon. Out of these nine fungal species, there was no difference in infection for seven fungal species when comparing seed sources (Table 3). Infection by Aspergillus tamarii was higher in samples collected from local markets and research institutes than seed saved by farmers. With respect to Aspergillus niger, infection was greater for seed collected from research institutes compared with farmer-saved seed: infection of seed from local markets was intermediate. Although infection by Aspergillus flavus and Curvularia lunata was relatively high, there were no significant differences in infection among seed sources.
Even though seed samples from all three sources were of low moisture content, high incidence of fungi were identified and associated with the seed. Genetic factors such as cultivar type can influence seed micro-flora as seed infection, micro-flora and pathogens are cultivar-dependent and usually a result of cultivar resistance (Cochran, 2015). Additionally, the limited availability of inputs for crop production and storage facilities may impede the ability of these three sources to produce and maintain quality seeds, especially in Ghana characterized by warm climate where disease incidence can be high. Similar results were reported by Adithya et al. (2017) where A. niger was predominant followed by A. flavus in peanut seed samples collected from farmers and markets in India. Additionally, Rathod et al., (2015) reported high incidence of A. niger (40%) followed by A. flavus (15%) in seed samples obtained from the research institute. Seed samples from the markets recorded the highest fungal incidence of pathogens.
In conclusion, in some instances quality of farmer-saved peanut seed was similar to quality of seed obtained from the research institutes. Seed from the research institutes was of higher quality than seeds from local markets and farmer-saved seed in relation to physical purity and plant phenotype uniformity. Seed from all three sources were poor in physiological quality and seed health. The poor performance of farmer-saved seeds and that of seed from the local markets with respect to physiological quality and seed health is most likely a result of inadequate quality control at production, selection, storage and marketing stages of the seed value chain by actors in these sectors. The formal seed system in Ghana has limited capacity to deliver high quality seed to smallholder farmers. Strengthening farmer-saved seed production and community-based seed systems should be recognized as possibilities until a national seed system for peanut can be developed and maintained.
Acknowledgements
This research was supported by the United States Agency for International Development, as part of the Feed the Future initiative, under the CGIAR Fund, award number BFS-G-11-00002, the predecessor fund the Food Security and Crisis Mitigation II grant, award number EEM-G-00-04-00013, and the Office of Agriculture, Research and Policy, Bureau of Food Security, U.S. Agency for International Development, under the terms of Award No. AID-ECG-A-00-07-0001 to The University of Georgia as management entity for U.S. Feed the Future Innovation Lab for Peanut. The opinions expressed herein are those of the authors and do not necessarily reflect the views of the U.S. Agency for International Development.
Literature Cited
Abebe G. and Alemu A.. 2017. Role of improved seeds towards improving livelihood and food security at Ethiopia. International Journal of Research – Granthaalayah. 5:338-356.
Adithya G., Rajeshwari B., Keshavulu K., Sudini H., & Swathi Y. ( 2017). Mycoflora associated with groundnut seeds collected from selected groundnut growing districts of Telangana State, India. International Journal of Current Microbiology and Applied Sciences. 6(7):4335-4342.
Akpo E., Ojiewo C.O., Kapran I., Omoigui L.O., Diama A. and Varshney R.K., 2021. Enhancing smallholder farmers' access to seed of improved legume varieties through multi-stakeholder platforms: Learning from the TLIII project experiences in sub-Saharan Africa and South Asia. Page 205). Springer Nature.
Angelucci F., and Bazzucchi A.. 2013. Analysis of incentives and disincentives for groundnuts in Ghana. Technical Notes Series, MAFAP, FAO, Rome.
Anonymous. 2016. Ghana early generation seed study. AGRA-SSTP. https://www.agrilinks.org/sites/default/files/resource/files/ghana_early_generation_seed_report.pdf.
Beuchat L. R., and Koehler P. E.. 1979. Effect of moist heat treatment on sensory qualities of peanut kernels. Peanut Sci. 6(2):93-95.
Biabani A., Boggs L. C., Katozi M., and Sabouri H.. 2011. Effects of seed deterioration and inoculation with Mesorhizobium cicerion yield and plant performance of chickpea. Australian Journal of Crop Science. 5(1):66-70.
Chauhan J. S., Prasad S. R., and Satinder P.. 2015. Quality seed: A mega factor in enhancing crop productivity. Pages 357-366 in A. L. Singh, ed. In: Recent Advances in Crop Physiology. Vol. 2. Daya Publishing House, New Delhi.
Cho Y., and Scott R. A.. 2000. Combining ability of seed vigor and seed yield in soybean. Euphytica. 112(2):145-150.
Cochran K. A. 2015. Soybean seed quality and vigor: influencing factors, measurement, and pathogen characterization. Graduate Dissertations. Available at: https://scholarworks.uark.edu/etd/1261.
Dhedhi K. K., Dhobi C. B., Chaudhari N. N., Sorathiya J. S., and Khanpara M. D.. 2017. Assessment of farmers saved groundnut seed quality of Devbhoomi Dwarka district of Gujarat, India. Agricultural Science Digest-A Research Journal. 37(1):16-21.
Diba K., Kordbacheh P., Mirhendi S. H., Rezaie S., and Mahmoudi M.. 2007. Identification of Aspergillus species using morphological characteristics. Pakistan Journal of Medical Sciences. 23(6):867.
FAO [Food and Agricultural Organization] 2018. Farmer seed systems and sustaining peace. Rome. 52 pp. License: CC BY-NC-SA 3.0 IGO. Available at: https://www.fao.org/resilience/resources/resources-detail/ar/c/1160196/.
FAOSTAT [Food and Agriculture Organization Corporate Statistical Database] 2020. Statistical data on crops, groundnut, area, production quantity of Ghana, Africa and World. Available at: https://faostat.fao.org.
Gebeyehu S., Kangile J., and Mwakatobe E.. 2019. Assessment of seed quality along the rice seed value chain in Tanzania. Development in Practice. 29(7):854-866.
Hamman B., Egli D. B., and Koning G.. 2002. Seed vigor, soilborne pathogens, preemergent growth, and soybean seedling emergence. Crop Science. 42(2):451-457.
Jordan D., Brandenburg R., Payne G., Hoisington D., Magnan N., Rhoads J., Abudulai M., Adhikari K., Chen J., Akromah R., Appaw W., Ellis W., Balota M., Mallikarjunan K., Boote K., MacDonald G., Bowen K., Bravo-Ureta B., Jelliffe J., Budu A., Chalwe H., Mweetwa A., Ngulube M., Dankyi A., Mochia B., Hoffman V., Muitia A., Mwangwela A., Njoroge S., Okello D., and Opoka N.. 2018. Preventing mycotoxin contamination in groundnut cultivation. Pages 181-214 in S. Sivasankar, D. Berguinson, P. Gaur, S. Kumar, S. Beebe, and M. Tamó, eds., Achieving Sustainable Cultivation of Grain Legumes; Improving Cultivation of Particular Grain Legumes. Volume 2. Burleigh Dodds Series in Agricultural Science. Burleigh Dodds Science Publishing, Cambridge, UK.
Klich M. A. 2002. Identification of common Aspergillus species. Utrecht, Netherlands: Centraalbureau voor Schimmelcultures.
Matthews S., Noli E., Demir I., Khajeh-Hosseini M., and Wagner H. M.. 2012. Evaluation of seed quality: from physiology to international standardization. Seed Science Research. 22:S69-S73.
McDonald M. F. and Copeland L. O.. 2012Seed production: principles and practices. Springer Science and Business Media, Dordrecht, Germany.
MOFA [Ministry of Food and Agriculture]. 2016. Agricultural sector progress report 2016. Ministry of Food and Agriculture, Monitoring and Evaluation Directorate. Available at: file:///C:/Users/dljorda2/Downloads/Agricultural%20Sector%20Progress%20Report%202016.pdf.
MOFA [Ministry of Food and Agriculture] 2017. Agriculture in Ghana: facts and figures (2016). Ministry of Food and Agriculture Statistics, Research and Information Directorate (SRID). Available at: https://mofa.gov.gh/site/images/pdf/AGRICULTURE%20IN%20GHANA%20F&F%202017.pdf.
MOFA [Ministry of Food and Agriculture] 2021. Agriculture in Ghana: Facts and Figures (2020). Ministry of Food and Agriculture Statistics, Research and Information Directorate (SRID). Available at: https://srid.mofa.gov.gh/sites/default/files/Agriculture%20In%20Ghana%20Facts%20%26%20Figures_%202020%20FINAL.pdf.
Nautiyal P. 2002. Groundnut: Post-harvest operations, New Delhi, India: ICAR-National Research Centre for Groundnut. Available at: http://www.icar.org.in/.
Nigam S. N., Jordan D. L., and Janila P.. 2018. Improving cultivation of groundnuts. Pages 155-180 in S. Sivasankar, D. Berguinson, P. Gaur, S. Kumar, S. Beebe, and M. Tamó, eds., Achieving Sustainable Cultivation of Grain Legumes; Improving Cultivation of Particular Grain Legumes. Volume 2. Burleigh Dodds Series in Agricultural Science, Burleigh Dodds Science Publishing, Cambridge, UK.
Owusu-Adjei E., Baah-Mintah R., and Salifu B.. 2017. Analysis of the groundnut value chain in Ghana. World J. Agriculture. 5(3):177-188.
Puozaa D. K., Jinbaani A. N., Adogoba D. S., Busagri D., Rasheed M. A., Issah A. R., and Oteng-Frimpong R.. 2021. Enhancing access to quality seed of improved groundnut varieties through multi-stakeholder platforms in Northern Ghana. Pages 65-79 In E. Akpo, C. O. Ojiewo, I. Kapran, L. O. Omoigui, A. Diama, and R. K. Varshney, eds., Enhancing Smallholder Farmers' Access to Seed of Improved Legume Varieties Through Multi-stakeholder Platforms. Springer, Singapore. Available at: http://oar.icrisat.org/11690/1/Akpoetal2021_Book_EnhancingSmallholderFarmersAcc.pdf.
Quiñones E. J., and Diao X.. 2011. Assessing crop production and input use patterns in Ghana –What can we learn from the Ghana Living Standards Survey (GLSS5)? Ghana Strategy Support Program (GSSP) GSSP Working Paper No. 0024. Available at: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.227.442&rep=rep1&type=pdf.
Ranal M.A., Santana D. G. D., Ferreira W. R., and Mendes-Rodrigues C.. 2009. Calculating germination measurements and organizing spreadsheets. Brazilian Journal of Botany. 32:849-855.
Rathod L. R., Naikade S. M., and Mote M. R.. 2015. Biodiversity of Aspergillusspp. on groundnut seeds. International Journal of Life Sciences. Special Issue A 4: 47-50.
Sastry D. V. S. S. R., Upadhyaya H. D., and Gowda C. L. L.. 2007. Survival of groundnut seeds under different storage conditions. Journal of SAT Agricultural Research. 5:3.
Sharanappa S. B., Shakuntala N. M., Vasudevan S. N., and Kuchanur P. H.. 2018. Influence of packaging materials on storability of groundnut (Arachis hypogaea L.). Journal of Pharmacognosy and Phytochemistry. 7(3):3013-3016.
Smith J. S., Blakenship P. D., and Mcintosh F. P.. 1995. Advances in peanut handling, shelling and storage from stock to processing. Pages 500-527 in H. E. Pattee and H. T. Stalker, eds. Advances in Peanut Science. American Peanut Research and Education Society, Inc. Stillwater, OK.
Notes
-
Author Affiliations
- Department of Horticulture, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana; [^]
- Department of Crop and Soil Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana; [^]
- Department of Agricultural Economics, Agribusiness and Extension, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana; [^]
- Department of Biochemistry and Biotechnology, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana; [^]
- Department of Crop and Soil Sciences, North Carolina State University, Raleigh, NC 27695. [^] Corresponding author’s e-mail: david_jordan@ncsu.edu.