Introduction
Peanut ( Arachis hypogaea) is the only economically important geocarpic plant species, producing flowering structures above ground but forming fruits below ground. After pollination, a gynophore (peg) is produced which contains the fertilized ovules at the tip ( Smith, 1954). The peg is positively geotropic, and between 4 and 7 days after pollination it enters the soil to a depth of 2 to 8 cm below the surface, otherwise known as the pegging zone. Here the embryo within the peg begins to swell ( Bledsoe, Comar, & Harris, 1949; Moss, Rao, Pattee, & Stalker, 1995; Rashid et al., 2016 ; Zamski & Ziv, 1976; Ziv, 1981), and then forms the peanut pod and the seeds ( Smith, 1954; Xi, 1991). As the pod swells, it actively uptakes nutrients (calcium and other inorganic salts) and moisture from the surrounding soil, while photosynthates are translocated from mesophyll cells to developing pods ( Bledsoe et al., 1949 ; Inanaga & Yoshihara, 1997; Pattee & Mohapatra, 1987; Singh, 2004).
Although peanut pods and roots are both developed in soil, they have distinct interactions with soil microbiome and different needs for nutrition. Comparison of rhizosphere, geocarposphere and bulk soil microbiota revealed differentiated bacterial and fungal populations associated with each organ ( Kloepper & Bowen, 1991; Subrahmanyam & Rao, 1977). The preferential association of some fungi and bacteria with early developmental stages of pods indicates that these microorganisms may be adapted for colonizing the geocarposphere ( Kloepper & Bowen, 1991; Rashid et al., 2016 ; Subrahmanyam & Rao, 1977). Such pod-associated microbiota could influence the level of infection by fungal pathogen Aspergillus flavus and the production of aflatoxin ( Chourasia & Sah, 2017; Li et al., 2020 ). Selected geocarposphere bacteria were also proposed as a biological approach for controlling Aspergillus infection and aflatoxin biosynthesis in peanut ( Dong et al., 2020 ; Lyu, Yang, Wu, Zhang, & Li, 2020; Mickler, Bowen, & Kloepper, 1995; Yan et al., 2010 ).
As an essential nutrient for pod development, calcium is directly absorbed by developing pods from geocarposphere rather than being transported from roots ( Bledsoe et al., 1949 ; Brady, 1948; Skelton & Shear, 1971; Wiersum, 1951; S. Yang et al., 2020 ). Soluble calcium is critical for proper pod and embryo development. Deficiency of soluble calcium (<400 ppm) in the soil surrounding the developing pods leads to abortion of seed development ( Dey, Pal, Bhatt, & Chauhan, 2004; Kissel & Sonon, 2008; R. Yang, 2014). If the seed is not aborted, its quality is severely affected, diminishing its commercial value. A calcium-deficient soil promotes stress within the peanut plant and increases the severity of diseases caused by soil borne pathogens. For example, pod rot is caused by a group of fungal pathogens, and is more severe in peanuts grown in calcium-deficient soil ( Damicone, 2014). To mediate a lack of soluble calcium in the field, supplemental sources are used such as gypsum (CaSO 4) or lime (CaCO 3). Gypsum is highly soluble and therefore applied at pegging time, whereas lime is incorporated into the soil prior to planting to allow time for the calcium to become available; and is used primarily to alter pH. A few studies investigating the global transcriptional response to calcium deficiency revealed that auxin and gibberellin (GA) pathway genes were differentially expressed in pods grown in calcium-deficient or calcium-sufficient soil, as well as genes involved in Ca2+ signal transduction, nutrition absorption and microRNA function ( Chen et al., 2019 ; S. Yang et al., 2017 ; S. Yang et al., 2020 ). However, in these studies, both roots and pods were exposed to calcium deficiency, so it is unclear which responses observed in pods are secondary results of calcium deficiency sensed via root ( Chen et al., 2019 ; S. Yang et al., 2017 ; S. Yang et al., 2020 ).
The object of this study is to develop an experimental system that allows researchers to study pod development and microbial conditions in a controlled environment.
Materials and Methods
Plant source and growth conditions
Peanut varieties used in this study were Numex High Oleic 01 (Reg. No. CV-123, PI 670460) and Georgia-06G (CV- GA 011557). Seeds were germinated in soil and grown in a growth chamber (Model CMP3244 Winnipeg, Canada) at 26 C day/ 23 C night, with 12 hr day/ 12 hr night and with a 62% humidity. Plants were grown to full maturity within 1.3-gallon tulip pan circular pots containing Professional Growers Mix potting soil (Sungro Horticulture, Agawam MA, USA 01001). Plants were watered to soil saturation twice a week. Plants were fertilized with an all-purpose 24-8-6 water soluble fertilizer (Miracle-Gro, Marysville OH, USA 43040) every other week.
The In-Tube Growth (ITG) System
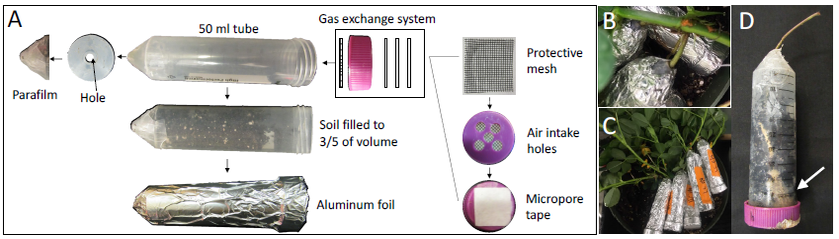
The ITG system was developed to support individual pod growth in a controlled environment ( Fig. 1). A 50 ml Falcon conical tube (Thermo Fisher Scientific Waltham MA, USA 02451) was modified to hold soil and the developing pod. The lid with five 6-mm holes served as a gas exchange system. The perforated lid was then covered in three alternating layers of 1-inch 3M Micropore tape (3M, Saint Paul MN, USA 5122). On the interior side of the cap was a 0.5 mm gridded mesh. The tube was sealed around the cap-tube junction with parafilm. The gas exchange system allowed proper moisture and humidity control, while simultaneously providing the exchange of gasses to prevent an anaerobic environment. The standard 50 mL tube had one 0.5mm hole drilled at its bottom, covered with Parafilm (Bemis Company, Neenah WI, USA 54956), and can be filled with various growth media depending on the purpose of a study ( Fig. 1A). The ITG system was encased in a layer of aluminum foil. After filling the tube with soil to a volume of 35 ml, 50 ml of sterile DI water was added to ran through the soil. The humidity of soil was maintained around 20.37% for the duration of the experiment. No additional water was added to the tubes throughout the experiment.
Assembly of the ITG system
A) Components of the ITG system. The lid was with five 6 mm holes, three layers of 3M micropore tape and a mesh. Soil was filled up to 35ml in a 50 ml tube. The bottom of the tube has a single hole for insertion of the peanut peg. The whole system is wrapped in aluminum foil. B) Close-up view of a peg inserted in a tube. C) A representative image showing multiple tubes hosting individual pods attached on a peanut plant. D) A pod growing in a tube. Arrow points to a developed pod.
Application of the ITG system
When a peg elongated to about 2.5 cm, it was inserted into a tube with soil ( Fig. 1B). Only aerial pegs were used for the system; pegs that contacted the soil surface were no longer used for the ITG system, preventing the initiation of nutrient uptake by a peg. Multiple ITG tubes could be assembled on a single plant ( Fig. 1C). A peanut pod was then grown for an average of 90 days in a tube ( Fig. 1C). The pegs used as open soil control were labeled with a tag at the same time when ITG tubes were applied onto experimental pegs.
Comparison of ITG system and open soil
Nine Georgia-06G plants were grown in potting mix soil to the flowering stage for tube attaching. Each tube contained 35g of potting soil; and adjusted to a 20.37% humidity. On each individual plant, 5-10 pegs as open soil control were labeled using a plant tag. At the same time, 4-6 tubes were assembled on the same plants. To reduce the noise coming from physiological variation of individual plant, pods grown in open soil or tubes from about 10 plants were pooled. Tubes were attached randomly based on peg availability. Three replicas were conducted. The same experiment was conducted for the Valencia type peanut Numex high oleic 01.
Low and high calcium growth medium
Low calcium growth medium used in ITG system contained 3 parts of sand and 1 part of perlite (Miracle Gro, Marysville, OH, 4304). Murashige & Skoog Medium Basal Medium without calcium, sucrose and agar (PT018, Himedia, Mumbai, India) was added to supplement nutrition.
High calcium growth medium was comprised of the same artificial soil and amended with 5 grams of gypsum (Pennington, Madison, GA, USA 30650) per 300 grams of sand.
Low and high calcium pod response
Nine pots of Georgia-06G plants were grown to flowering stage for the attaching tubes. Plants were grown in potting mix. 50 tubes filled with 35 g of either low or high calcium artificial soil were assembled on these 9 plants. On average, each plant carried about 5 low calcium and 5 high calcium tubes.
Data Collection and Analysis
Tubes were harvested at the end of the growing season which was at 90-120 days after applying them. All tubes were removed by cutting the pegs from a plant. The peanuts were then removed from the tubes and rinsed under DI water to remove attached soil. Extra water was removed using paper towel. Pod fresh weight and size were measured immediately after harvest. Pods were then shelled, and the fresh weight of seeds was also determined. Student t-test was used to test a null hypothesis.
Results and Discussion
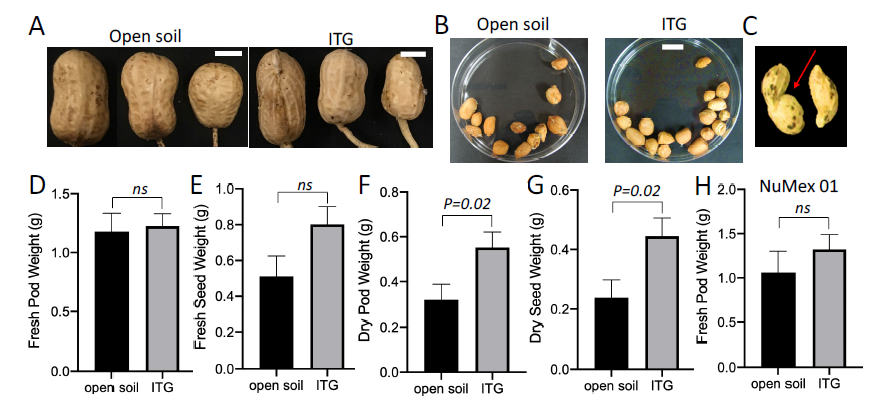
The assembly of ITG system was illustrated in Figure 1 ( Fig. 1A). Tubes were applied on Georgia-06G plants to examine the growth of pods in ITG system ( Figs. 1B-1D). The development of Georgia-06G pods and seeds in the ITG system was comparable to those from the same plants grown in open soil ( Figs. 2A and 2B). In some cases, deformed pods were found in tubes, probably due to the restriction in space ( Fig. 2C). The deformed pods were included in the analysis of pod weight and seed weight. A decrease in the number of deformed pods were observed when perlite was added into the soil mix, probably due to a reduction in the compaction. Pods grown in open soil had an abortion rate of 19%, while the abortion rate was slightly lower in tubes, at 13%. The abortion rate was calculated by dividing the number of aborted pods by the total number of pegs assembled in tubes. When compared to pods that were tagged at the same time and grown in open soil, pods development in tubes were slightly delayed. No significant differences in fresh pod and seed weight were observed ( Figs. 2D and 2E). The average dry weight of pods and seeds developed in tubes surpassed those developed in open soil ( Figs. 2F and 2G), probably due to the limited exposure to pathogens in the enclosed environment. The increased peanut pod performance in tubes could also be due to differences in environmental and nutritional conditions in open soil and a tube. The growth of a Valencia type variety, Numex High Oleic 01, was also tested in the ITG system. The development of pod and seeds were comparable to those in open soil. The average pod weight was 1.06g and 1.27g in open soil and in ITG, respectively ( Fig. 2H). Taken together, the data demonstrated that the ITG system could support normal pod growth for multiple varieties. The problem of deformed pods could be severe when assaying varieties with large pods, such as Georgia-11J. Adding perlite into the ITG system or using a large tube may alleviate the issue. Results showed that within a 120-day growth period, the ITG system supported comparable pod development as those in open soil. Supplementation of nutrition and water may be required for varieties with late maturity.
Comparisons of pod development in ITG and open soil
A) Representative images of Georgia-06G pods grown in the ITG system and open soil. Bar: 0.5 cm. B) Representative images of seeds developed in the ITG system and open soil. Bar: 1 cm. C) Representative image of deformed pods developed in the ITG system. D)-H): Measurement of fresh pod weight (D), seed weight (E), dry pod weight (F) and dry seed weight (G) from Ga-06G pods grown in ITG and open soil. n=42 for open soil samples, n=45 for ITG samples. H): Measurement of fresh pod weight of NuMex High oleic 01 in ITG and open soil. n=18 for open soil samples; n=40 for ITG samples. Error bars in C-G indicates SEM. ns: not significant based on a student two-tailed t-test. ITG tubes were opened at 90 days after attachment.
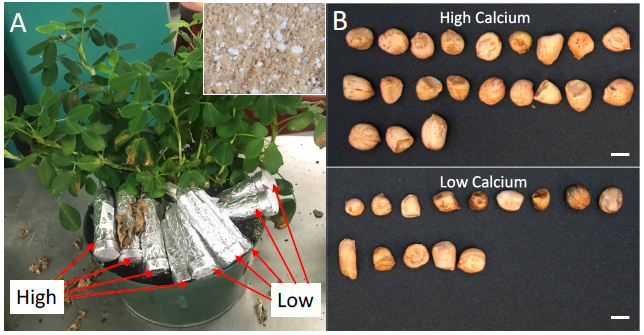
Developing pods directly absorb calcium from the geocarposphere, which is independent of the calcium level in the rhizosphere. The ITG system was used to test the pod-specific response to calcium deficiency. Plants were grown in a potting mix (calcium concentration at 12,000ppm). Artificial soil mixtures with either a low (42 ppm) or a high (1046 ppm) calcium concentration were added into tubes ( Fig. 3A). On average 5 tubes with low and 5 tubes with high calcium levels were assembled on one plant ( Fig. 3A). As expected, low calcium treatment caused reduced seed weight ( Fig. 3B and Table 1). Low calcium treatment led to a high rate of aborted pegs (50% in the low calcium condition vs 32% in the high calcium condition) and a low fresh pod weight ( Table 1). Pods grown in a low calcium condition produced less than half of seeds from high calcium-treated pods ( Table 1). These seeds from low calcium condition also showed reduced dry weight ( Table 1). Thus, the ITG system can be used to test a pod-specific response to nutritional conditions.
Experimental setting to compare pod growth in a low or high calcium condition.
A) An image showing tubes with low or high calcium artificial soil were assembled on a peanut plant. The insert at the up-right corner shows the artificial soil composed of sand and perlite. B) Seeds developed in tubes with a low or high calcium medium. Scale bar=5 mmA) An image showing tubes with low or high calcium artificial soil were assembled on a peanut plant. The insert at the up-right corner shows the artificial soil composed of sand and perlite. B) Seeds developed in tubes with a low or high calcium medium. Scale bar=5 mm
Summary and Conclusions
Research results showed that the ITG system could support pod growth in a controlled soil environment separated from the root. This system should be suitable to study pod-specific responses to a range of factors associated with the geocarposphere. It also has utility for investigation of soil nutritional conditions, specifically calcium levels. The ITG system may also be applied to studying the interactions between pods and microbes associated with the geocarposphere. For example, soilborne pathogens or plant growth-promoting rhizobacteria can be added into either open soil or tubes to test their specific impact on pod development.
Acknowledgements
The author thanks Carl Michael Deom for providing the seeds of Numex High Oleic 01. We thank Soraya Bertioli for helpful discussion of the manuscript. This research was partially supported by Georgia Peanut Commodity Commission grant UGA48-19/21.
Literature Cited
Bledsoe R. W., Comar C., and Harris H. . 1949. Absorption of Radioactive Calcium by the Peanut Fruit. Science 109( 2831): 329- 330.
Brady N. 1948. The Effect of Period of Calcium Supply and Mobility of Calcium in the Plant on Peanut Fruit Filling. Soil Sci. Soc. Am. J. 12( C): 336- 341.
Chen H., Yang Q., Chen K., Zhao S., Zhang C., Pan R., Cai T., Deng Y., Wang X., and Chen Y. . 2019. Integrated Microrna and Transcriptome Profiling Reveals a Mirna-Mediated Regulatory Network of Embryo Abortion under Calcium Deficiency in Peanut (Arachis Hypogaea L.). BMC Genomics 20( 1): 1- 17.
Chourasia H., and Sah P. K. . 2017. Control of Aflatoxin Biosynthesis in Peanut with Geocarposphere Bacteria: A Biotechnological Approach for Sustainable Development. pp. 65- 72. In Mukhopadhyay K., Sachan A. and Kumar M. (eds.) Applications of Biotechnology for Sustainable Development . Springer, New York, NY .
Damicone J. P. 2014. Soilborne Diseases of Peanut. pp. 1- 6. In Oklahoma Cooperative Extension Fact Sheet . Stillwater, OK .
Dey R., Pal K., Bhatt D., and Chauhan S. . 2004. Growth Promotion and Yield Enhancement of Peanut (Arachis Hypogaea L.) by Application of Plant Growth-Promoting Rhizobacteria. Microbiol. Res. 159( 4): 371- 394.
Dong X., Zhang Q., Zhang Z., Yue X., Zhang L., Chen X., Zhang W., Chen L., and Li P. . 2020. Inhibitory Effect of Enterobacter Cloacae 3j1ec on Aspergillus Flavus 3.4408 Growth and Aflatoxin Production. World Mycotoxin J. 13( 2): 259- 266.
Inanaga S., and Yoshihara R. . 1997. Translocation and Distribution of Assimilated Carbon in Peanut Plant. J. Soil Sci. Plant Nutr. 43( 2): 267- 274.
Kissel D. E., and Sonon L. S. . 2008. Soil Test Handbook for Georgia Athens, Georgia University of Georgia Cooperative Extension.
Kloepper J. W., and Bowen K. L. . 1991. Quantification of the Geocarposphere and Rhizosphere Effect of Peanut (Arachis Hypogaea L.). Plant Soil 136( 1): 103- 109.
Li P., Yao Y., Zhang Q., Mwakinyali S., Gao S., Ding X., Liang S., and Xia Z. . 2020. The Peanut Pods-Associated Microbiota and Their Effects on Aflatoxin Contamination. Res Sq. Retrieved from: https://www.researchsquare.com/article/rs-11688/v1?redirect=/article/rs-11688
Lyu A., Yang L., Wu M., Zhang J., and Li G. . 2020. High Efficacy of the Volatile Organic Compounds of Streptomyces Yanglinensis 3-10 in Suppression of Aspergillus Contamination on Peanut Kernels. Front. Microbiol. 11: 142.
Mickler C. J., Bowen K. L., and Kloepper J. W. . 1995. Evaluation of Selected Geocarposphere Bacteria for Biological Control of Aspergillus Flavus in Peanut. Plant Soil 175( 2): 291- 299.
Moss J. and Rao V. R. , 1995. The Peanut–Reproductive Development to Plant Maturity. pp. 1- 13. In Pattee H. and Stalker H. (eds.) Advances in Peanut Science. Am. Peanut Res. Educ. Soc . Stillwater, OK .
Pattee H. E., and Mohapatra S. C. . 1987. Anatomical Changes During Ontogeny of the Peanut (Arachis Hypogaea L.) Fruit: Mature Megagametophyte through Heart-Shaped Embryo. Bot. Gaz. 148( 2): 156- 164.
Rashid M. I., Mujawar L. H., Shahzad T., Almeelbi T., Ismail I. M., and Oves M. . 2016. Bacteria and Fungi Can Contribute to Nutrients Bioavailability and Aggregate Formation in Degraded Soils. Microbiol. Res. 183: 26- 41.
Singh A. 2004. Growth and Physiology of Groundnut. pp. 178- 212. In Groundnut Research in India. National Research Center for Groundnut (Icar) , Junagadh, India .
Skelton B. J., and Shear G. . 1971. Calcium Translocation in the Peanut (Arachis Hypogaea L.) 1. Agron. J. 63( 3): 409- 412.
Smith B. W. 1954. Arachis Hypogaea Reproductive Efficiency. Am. J. Bot. 41: 607- 616.
Subrahmanyam P., and Rao A. . 1977. Rhizosphere and Geocarposphere Mycoflora of Groundnut (Arachis Hypogaea Linn). Proc. Indian Acad. Sci. 85( 6): 420- 431.
Wiersum L. 1951. Water Transport in the Xylem as Related to Calcium Uptake by Groundnuts (Arachis Hypogaea L.). Plant Soil 3( 2): 160- 169.
Xi X.-Y. 1991. Development and Structure of Pollen and Embryo Sac in Peanut (Arachis Hypogaea L.). Bot. Gaz. 152( 2): 164- 172.
Yan P., Gao X., Wu H., Li Q., Ning L., and Guan S. . 2010. Isolation and Screening of Biocontrol Bacterial Strains against Aspergillus Parasiticus from Groundnut Geocarposphere. Journal of Earth Science 21: 309.
Yang R. 2014. Calcium Availability to Runner-Type Peanut (Arachis Hypogaea L.) in the Southeastern United States. Masters of Science thesis. Submitted to Auburn University, Alabama.
Yang S., Li L., Zhang J., Geng Y., Guo F., Wang J., Meng J., Sui N., Wan S., and Li X. . 2017. Transcriptome and Differential Expression Profiling Analysis of the Mechanism of Ca2+ Regulation in Peanut (Arachis Hypogaea) Pod Development. Front Plant Sci. 8: 1609.
Yang S., Wang J., Tang Z., Guo F., Zhang Y., Zhang J., Meng J., Zheng L., Wan S., and Li X. . 2020. Transcriptome of Peanut Kernel and Shell Reveals the Mechanism of Calcium on Peanut Pod Development. Sci. Rep. 10( 1): 1- 13.
Zamski E., and Ziv M. . 1976. Pod Formation and Its Geotropic Orientation in the Peanut, Arachis Hypogaea L., in Relation to Light and Mechanical Stimulus. Ann. Bot. 40( 3): 631- 636.
Ziv M. 1981. Photomorphogenesis of the Gynophore, Pod and Embryo in Peanut, Arachis Hypogaea L. Ann. Bot. 48( 3): 353- 359.
Notes
- Department of Plant Pathology, College of Agricultural and Environmental Sciences, University of Georgia, Athens, GA 30602. [^] Corresponding author’s Email: li.yang1@UGA.edu