Introduction
Early leaf spot caused by Passalora arachidicola (Hori) U. Braun syn.Cercospora arachidicola (Hori) and late leaf spot caused by Nothopassalora personata (Berk. & M.A. Curtis) U. Braun, C. Nakash., Videira & Crous syn. Cercosporidicum personatum (Berk. & Curt.) Deighton, are major endemic foliar diseases in southeastern U.S. peanut (Arachis hypogaea L.) production. Fungicides are routinely used to control these two leaf spot diseases each year. Such foliar fungicides are very effective but are also costly with estimates over $40 million for cost of control for leaf spot in Georgia alone in 2017 (Little, 2019).
Development of general leaf spot field resistant cultivars to reduce production costs and increase yield would be highly desirable. Planting dates coupled with cultivar resistance have been shown to affect leaf spot intensity and severity in peanut production (Jordan et al., 2019). These authors found that later plantings increased late leaf spot severity in two years (2015 and 2016), and pod yields were higher for ‘Georgia-12Y’ (Branch, 2013) compared to ‘Georgia-06G’ (Branch, 2007).
Earlier, Branch and Culbreath (2008, 2013) found high levels of leaf spot resistance and high yields in later-maturing cultivars: ‘Georganic’ (Holbrook and Culbreath, 2008), ‘Georgia-01R’ (Branch, 2002), ‘Georgia-05E’ (Branch, 2006), ‘Georgia-10T’ (Branch and Culbreath, 2011), and Georgia-12Y (GA 072531). The objective of this study was to evaluate peanut genotypes at three planting dates in each of three years to assess the effect of planting date on pod yield and leaf spot disease pressure.
Materials and Methods
During 2012, 2015, and 2018, a set of 18 peanut genotypes (some common and some different across years) were used to evaluate the effect of planting date on leaf spot severity and pod yield. Within each year, the same set of 18 peanut genotypes were included for each of the three planting dates at the Gibbs Farm near the University of Georgia, Coastal Plain Experiment Station, Tifton, GA.
Randomized complete block field design with five replications was used each year without fungicides or insecticides but with irrigation. These trials were conducted on a Tifton loamy sand soil type (fine-loamy, siliceous, thermic Plinthic Kandidult). Plots consisted of two-rows, 6.1m long x 1.8 m wide (0.8 m within row and 1.0 m between rows on adjacent plots).
The three planting dates were April 20, May 11, and June 1 in 2012; April 14, May 11, and June 1 in 2015; and April 12, May 11, and June 1 in 2018. Consistent production practices included conventional tillage, fertilization, and irrigation as recommended by the Georgia Cooperative Extension Service, but excluded all pesticides, except for seed treatment and herbicides as needed to maintain weed control throughout the growing season. These field tests were in a three-year rotation following corn (Zea mays L.) and cotton (Gossypium hirsutum L.).
Leaf spot ratings among all genotypes were recorded on an individual plot basis within a few days prior to actual digging during each growing season. Both early and late leaf spot were prevalent and evaluated together on a 0-9 visual canopy rating scale, where 0 = no visible leaf spot (immune) and 9 = dead and defoliated plants (very highly susceptible). The 0-9 leaf spot rating scale used in this study is most similar to the 1-9 scale of Pittman (1995), except for the addition of the 0 = immune rating.
Multiple harvest dates were used within each planting date test during each of the three years. In general, highly susceptible genotypes (8-9 rating) were harvested based on plant defoliation due to leaf spot disease severity; while the more resistant entries were dug near optimum maturity based on the hull-scrape determination from adjacent border plants (Williams and Drexler, 1981). After harvest, peanut pods from individual whole plots were dried with forced warm air to approximately 6% moisture. Entire plot pod samples were then hand-cleaned over a screen table before weighing for yield determinations.
Data from each test and year was statistically analyzed separately by analysis of variance (ANOVA). Waller-Duncan’s T-test (k-ratio=100) was used for mean separation at P≤0.05 in SAS (SAS, Cary, NC).
Results
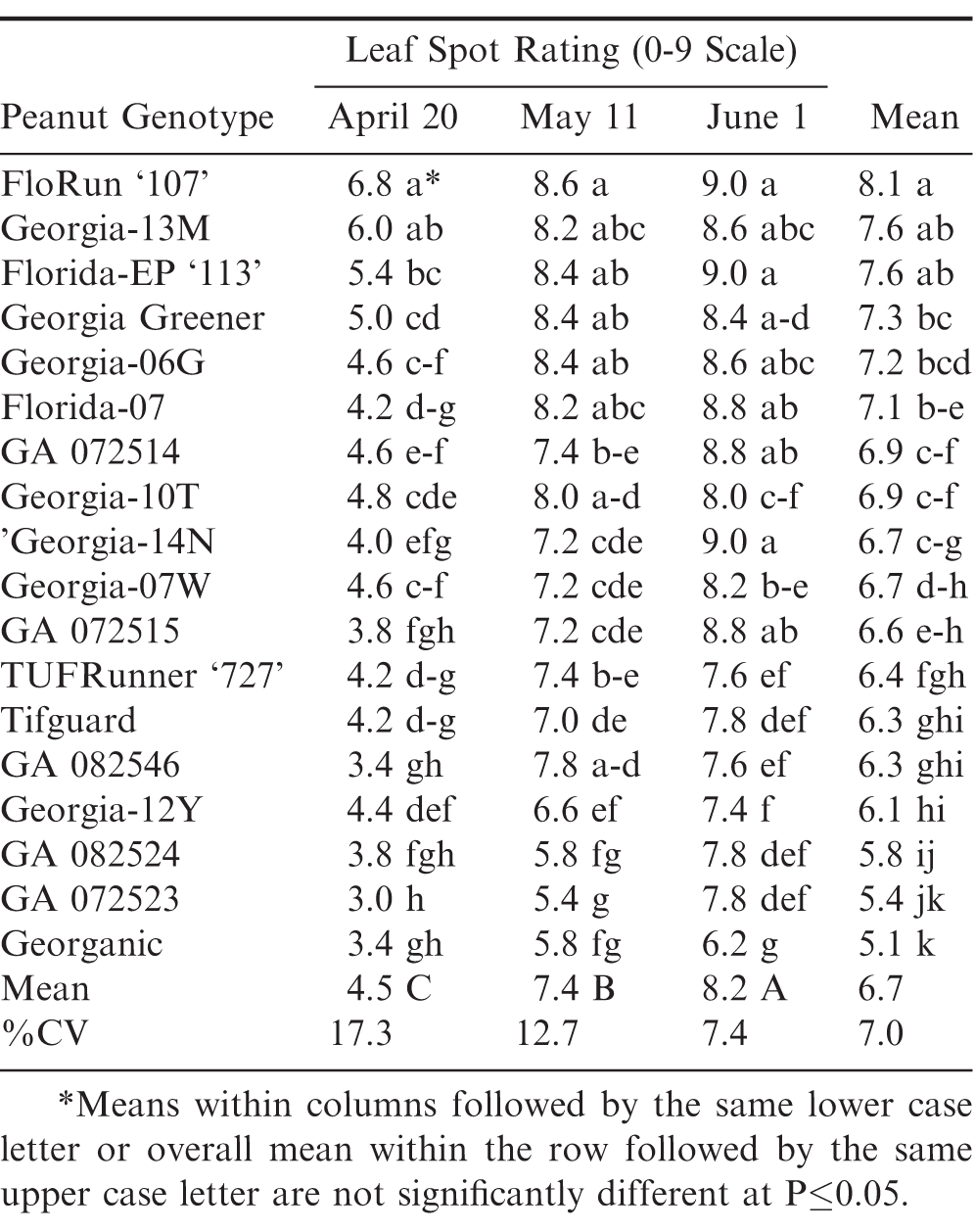
Significant differences among genotypes were found both within each planting date and between each planting date for leaf spot ratings during 2012 (Table 1). Georganic had the lowest leaf spot rating (highest resistance) averaged across the three planting dates; however, it was not significantly different from GA 072523, GA 082524, GA 082546, and GA 072515 in the 20 April 2012 planting date. Likewise, Georganic was not significantly different from GA 072523 and GA 082524 in the 11 May 2012 planting date. There was a consistent and significant increase in leaf spot pressure (higher ratings) from April to May to June ranging from 4.5 to 7.4 to 8.2, respectively.
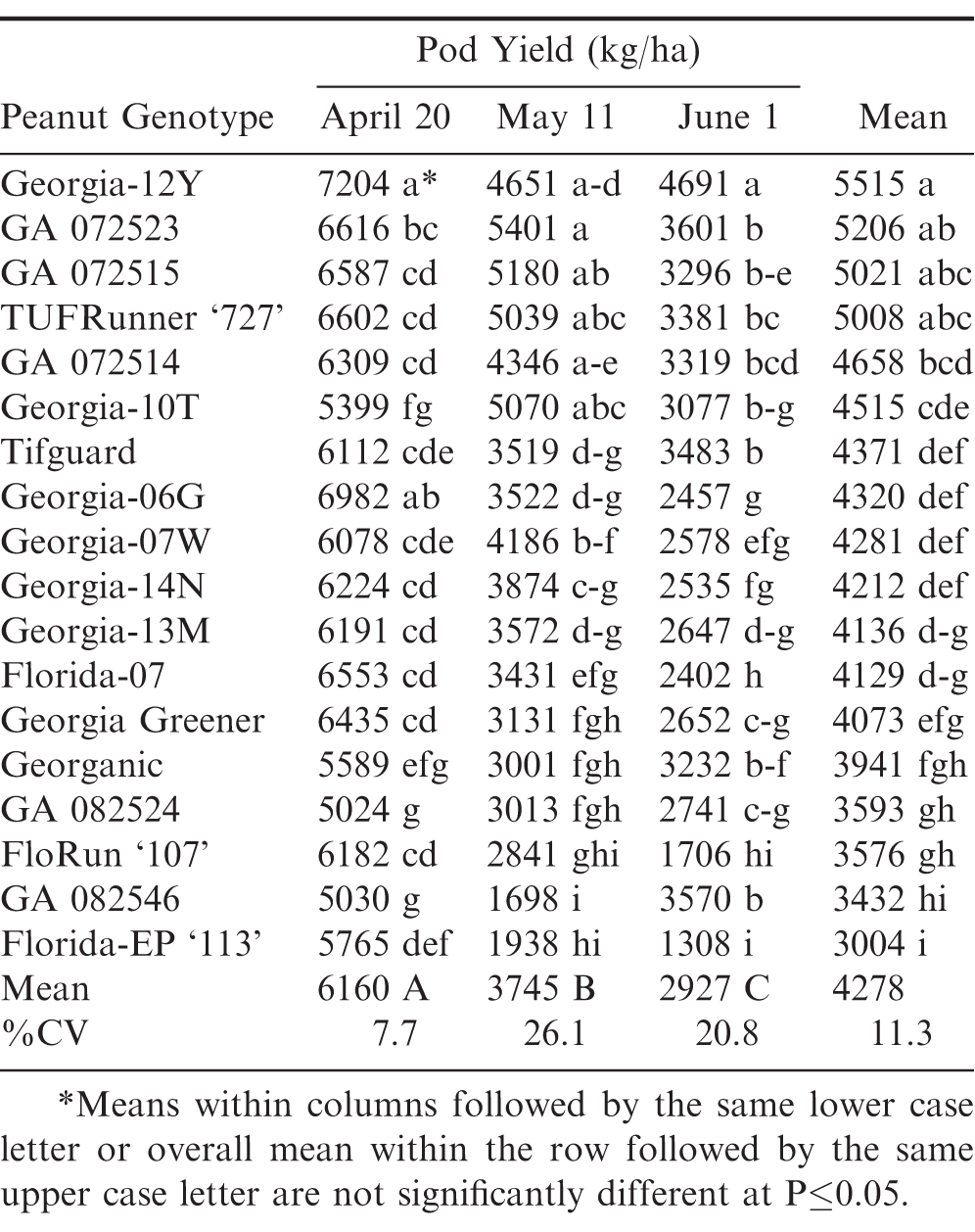
Conversely in 2012, pod yields decreased incrementally from April to May to June from 6160 to 3745 to 2927 kg/ha, respectively (Table 2). On average, the later June planting date resulted in the lowest pod yields and the highest leaf spot ratings. Georgia-12Y resulted in the highest pod yield across the three planting dates, but it was not significantly different from GA 072523, GA 072515, and TUFRunner ‘727’. However, Georgia-12Y did have a significantly higher yield than all other genotypes during April and June planting dates, except it was not significantly different than Georgia-06G in the April planting.
Significant genotypic differences were also found within and between planting dates for leaf spot ratings during 2015 (Table 3). Georganic again had the lowest leaf spot rating averaged across the three planting dates, but it was not different from GA 132705, GA 132706, and GA 132708. In 2015, similar to 2012, there was a significant and consistent increase in leaf spot from April to May to June with June having the highest leaf spot rating averaged over the same 18 peanut genotypes compared to May and April planting dates. In 2015, GA 072523 had significantly higher overall leaf spot rating than Georganic compared to 2012 (Table 1).
Also in 2015, pod yields were similar to 2012 in that the June planting date resulted in the lowest pod yields followed by May; whereas, April had the highest pod yields averaged over the same 18 different genotypes (Table 4). Geogia-12Y had the highest overall pod yield, but it was not significantly different from several other genotypes during April, May, and June planting dates, including Georgia-06G in the April planting. Even though Georganic exhibited better leaf spot resistance based upon ratings, its pod yield was among the lowest of the 18 genotypes across the three planting dates.
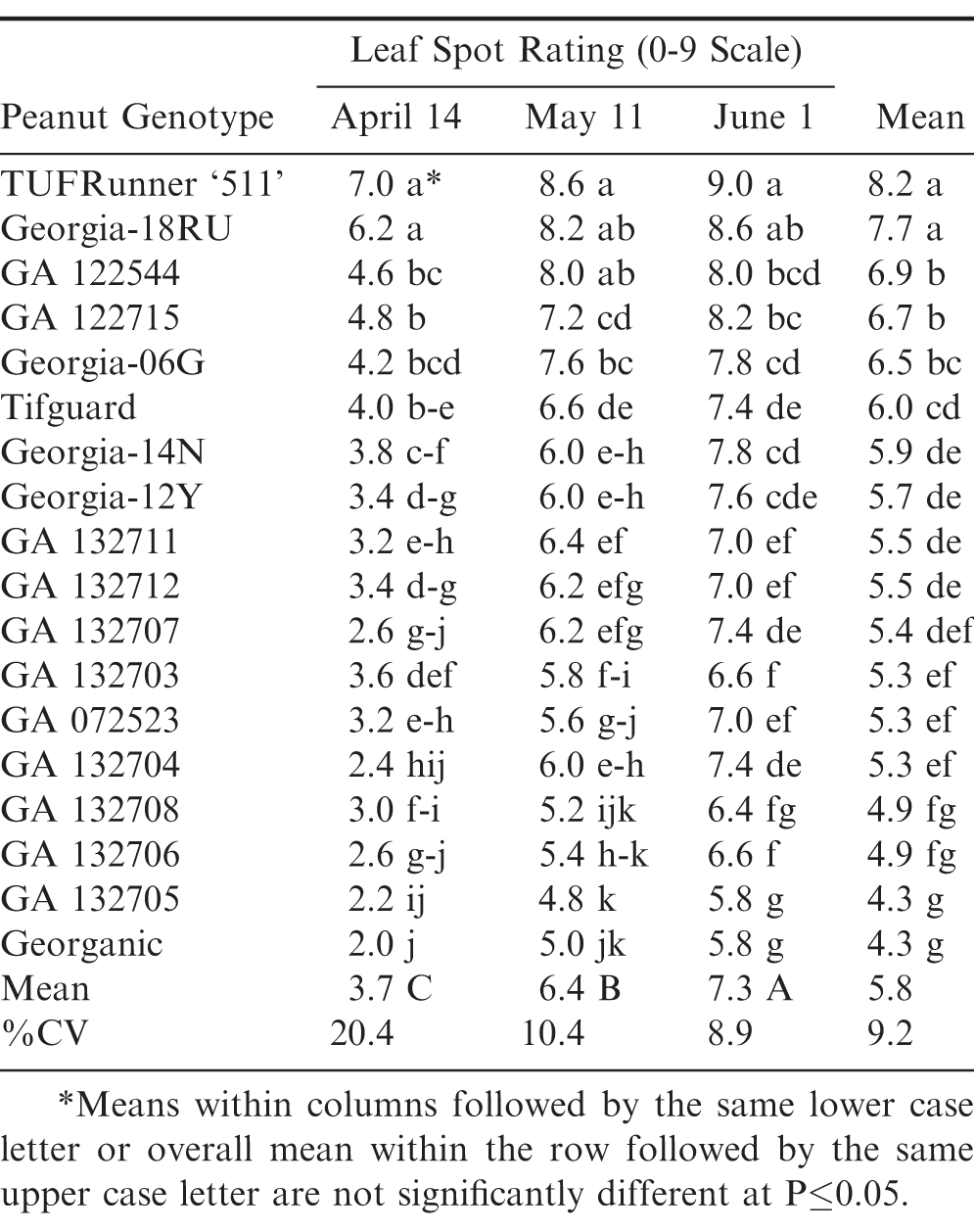
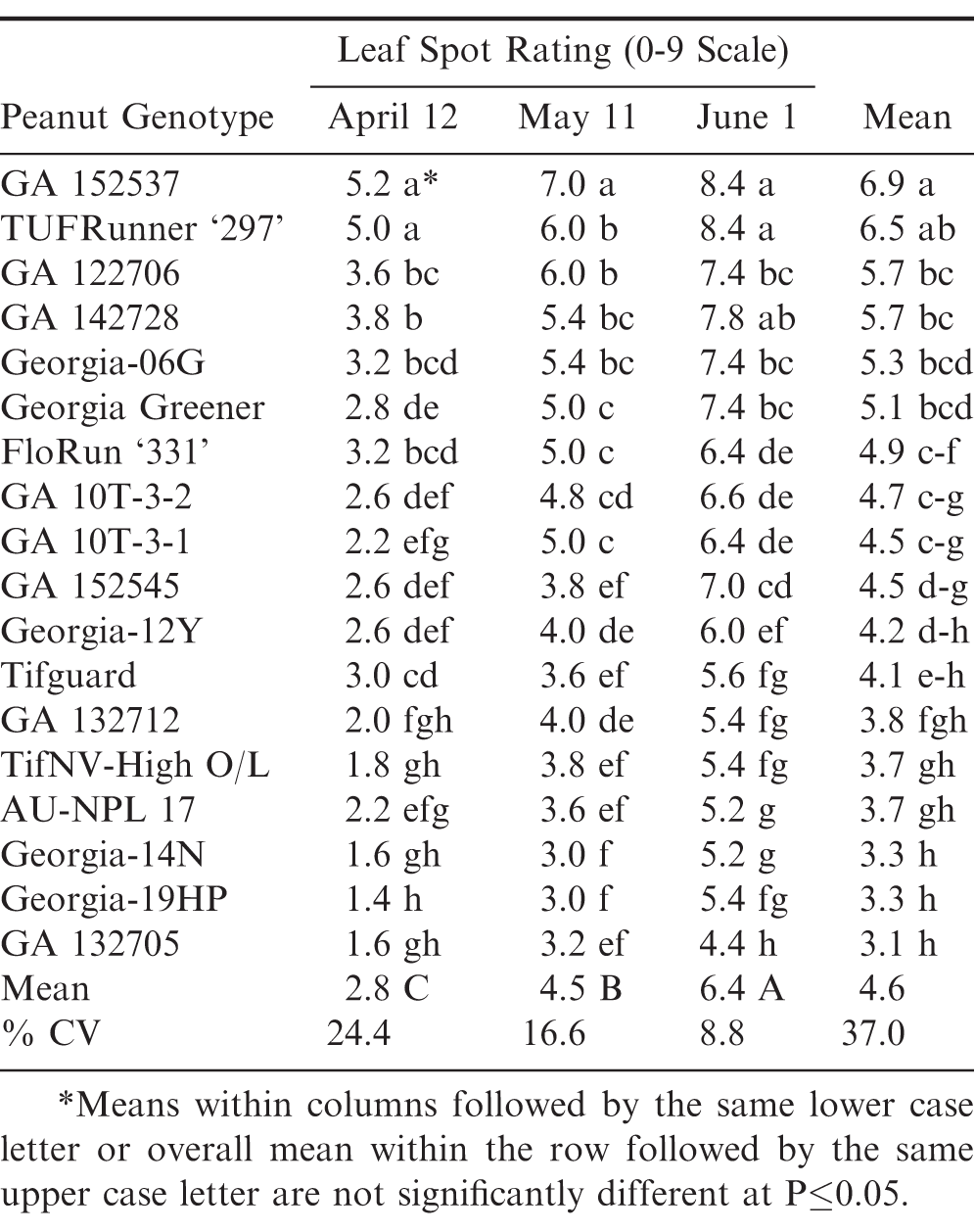
Similar to 2012 and 2015, significant genotypic differences were again found within and between planting dates for leaf spot ratings during 2018 (Table 5). GA 132705 had the lowest leaf spot rating averaged across planting dates, but it was not different from several other genotypes in April and May. However in the highest leaf spot pressure June planting, it did have a significantly lower leaf spot rating compared to all other genotypes.
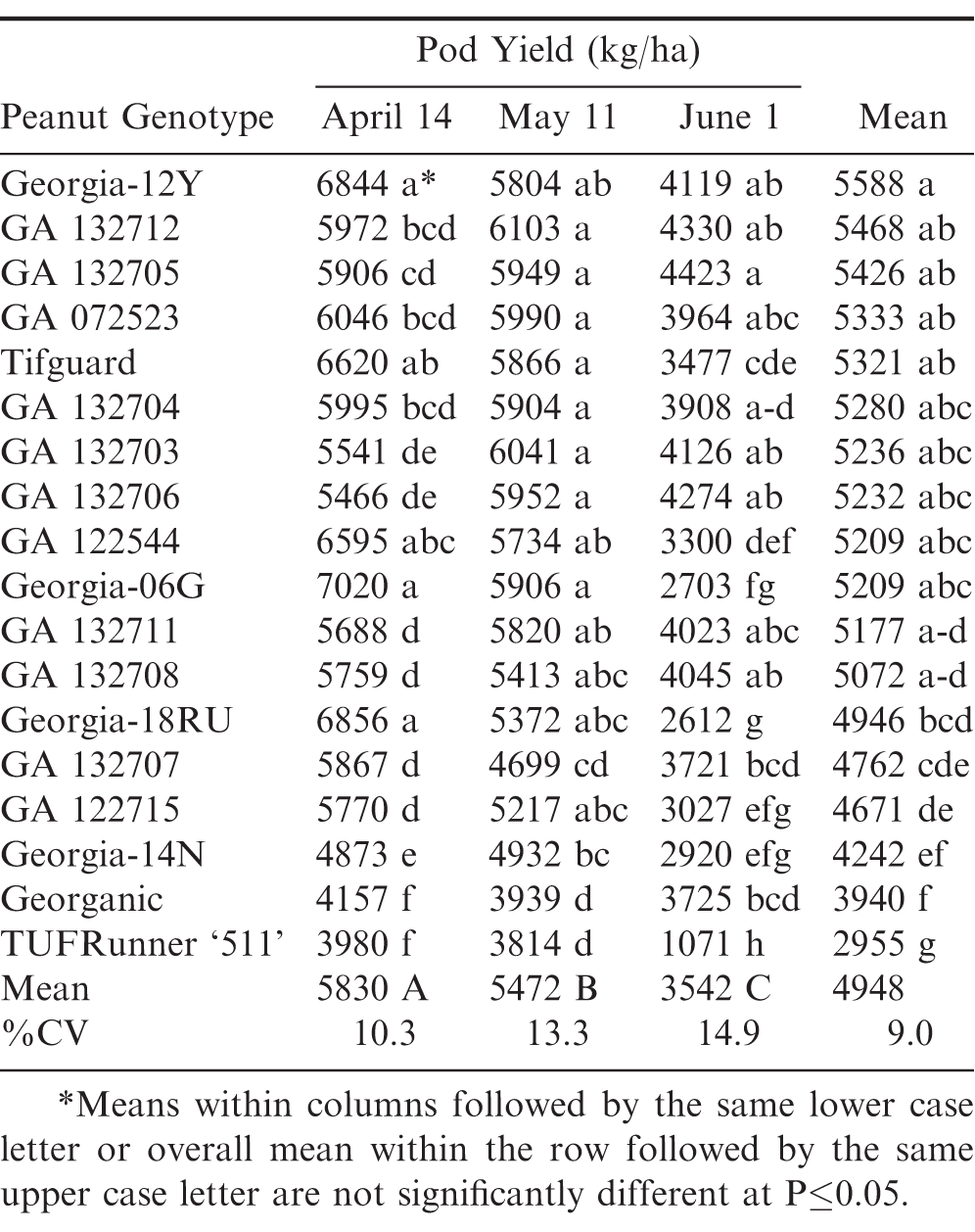
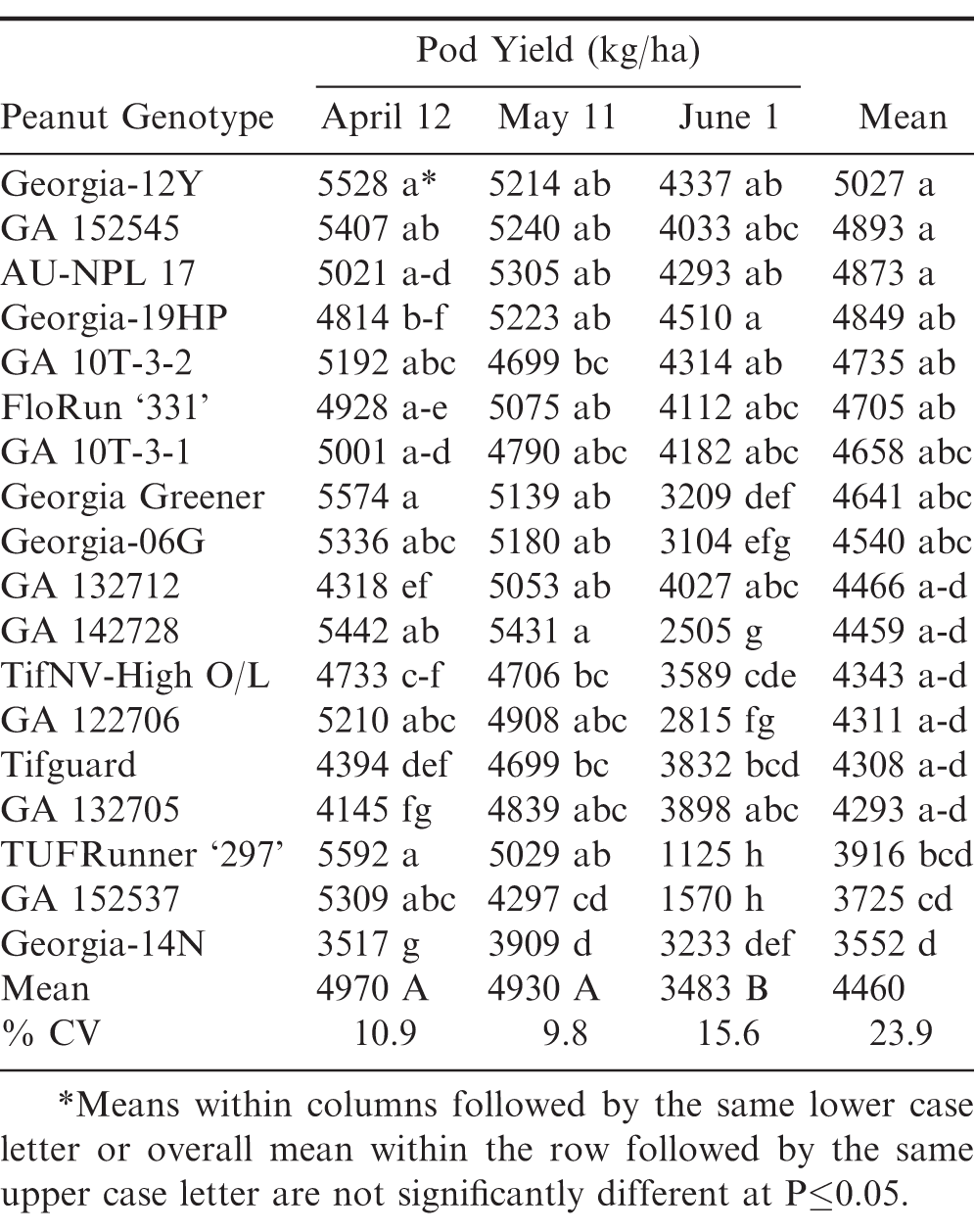
April pod yields in 2018 were lower than in 2012 and 2015. This resulted in the April and May planting dates not being significantly different. However, June planting date was again the significantly lowest compared to April and May in pod yield averaged over the same genotypes (Table 6). Georgia-12Y again had the highest pod yield, but it was not significantly different from several other genotypes during April, May, and June. ‘Georgia-19HP’ (Branch and Brenneman, 2020) had the highest pod yield in the June planting date, but it was not significantly different from several other genotypes including Georgia-12Y and GA 132705.
Discussion
Results from this three-year field study clearly shows the effect of planting date on leaf spot disease and pod yield across different peanut genotypes. April planting date consistently resulted in significantly less leaf spot pressure compared to May and June. However, it should be noted that these field tests were in a good crop rotation following corn and cotton during this planting date study. The trend was similar to that reported by Jordan et al., 2019) where shorter rotations, every other year cotton and peanut, were used.
Geogia-12Y did not have the lowest leaf spot ratings, but it did have the highest pod yield during each of the three years. The high yield performance and stability of Georgia-12Y could be attributed not only to its leaf spot resistance but also to its white mold resistance (Standish et al., 2019). These results further demonstrate that high leaf spot resistance may not solely be associated with high yield performance among peanut genotypes.
In summary, the overall pod yields decreased across the three planting dates with April planting dates having significantly highest pod yields, May intermediate, and June planting dates having the lowest average pod yields. These results also confirm and expand upon the findings of an earlier report (Jordan et al., 2019) in which April planting dates decrease severity of late leaf spot for two peanut cultivars, Georgia-06G and Georgia-12Y. However with early April planting dates, peanut genotypes in the southeast U.S. also need to have Tomato spotted wilt virus (TSWV) resistance, since early planting dates results in greater TSWV pressure (Brown et al., 2005). Georgia-12Y has a good combined general field resistance to all three of these major peanut diseases (leaf spot, white mold, and TSWV).
These results also have implications for field characterization of leaf spot resistance. Maximum disease potential with later planting dates would likely reduce the likelihood of escapes that could occur with early planting dates. Ideally, evaluation of genotypes with multiple planting dates should ensure the most accurate characterization of effect on leaf spot epidemics. However, various factors may limit the number of planting dates that can be used, especially for trials comparing larger numbers of genotypes for initial screenings or for mapping populations. If only one planting date is feasible, later plantings such as June plantings utilized in this study should increase the potential for leaf spot epidemic development and likely increase the ability to identify more resistant and/or tolerant genotypes.
Literature Cited
Branch, W. D. 2002. Registration of ‘Georgia-01R’ peanut. Crop Sci. 42: 1750– 1751.
Branch, W. D. 2006. Registration of ‘Georgia-05E’ peanut. Crop Sci. 46: 2305.
Branch, W. D. 2007. Registration of ‘Georgia-06G’ peanut. J. Plant Reg. 1: 120.
Branch, W. D. 2013. Registration of ‘Georgia-12Y’ peanut. J. Plant Reg. 7: 1– 3.
Branch, W. D. and Brenneman. T. B. 2020. Registration of ‘Georgia-19HP’ peanut. J. Plant Reg. 14: 306– 310.
Branch, W. D. and Culbreath. A. K. 2008. Disease and insect assessment of candidate cultivars for potential use in organic peanut production. Peanut Sci. 35: 61– 66.
Branch, W. D. and Culbreath. A. K. 2011. Registration of ‘Georgia-10T’ peanut. J. Plant Reg. 5: 279– 281.
Branch, W. D. and Culbreath. A. K. 2013. Yield performance and pest resistance among peanut genotypes when grown without fungicides or insecticides. Crop Prot. 52: 22– 25.
Brown, S. L., Culbreath, A. K. Todd, J. W. Gorbet, D. W. Baldwin, J. A. and J. P. Beasley, Jr. 2005. Development of a method of risk assessment to facilitate integrated management of spotted wilt of peanut. Plant Dis. 89: 348– 352.
Holbrook, C. C. and Culbreath. A. K. 2008. Registration of ‘Georganic’ peanut. J. Plant Reg. 2: 17.
Jordan, B. S., Culbreath, A. K. Brenneman, T. B. Kemerait, R. C.and Stevenson. K. L. 2019. Effect of planting date and peanut cultivar on epidemics of late leaf spot in Georgia. Plant Dis. 103: 990– 995.
Little, E.L., 2019. 2017 Georgia Plant Disease Loss Estimates, Univ. Georgia Coop. Ext. Annual Publication 102-10, Athens, GA, p. 21.
Pittman, R. N. (ed). 1995. United States Peanut Discriptors. USDA, ARS-132. U. S. Govt. Print. Ofc., Washington, DC.
Standish, J. R., Culbreath, A. K. Branch, W. D. and Brenneman. T. B. 2019. Disease and yield response of a stem-rot-resistant and -susceptible peanut cultivar under varying fungicide inputs. Plant Dis. 103: 2781– 2785.
Williams, E. J. and Drexler. J. S. 1981. A non-destructive method for determining peanut pod maturity. Peanut Sci. 8: 134– 141.