Introduction
Cultivated peanut (Arachis hypogaea L.) is one of the most economically important legumes in the world. Peanut is susceptible to many pathogens, with most damage being caused by fungi (Melouk and Backman,1995). Soil-borne fungi cause diseases that adversely affect peanut health and productivity, often requiring management by fungicide treatment throughout the growing season which is expensive to the producer and harmful to the environment. Sclerotinia blight [causal agent Sclerotinia minor (Jagger)] is of major concern to peanut producers in many parts of the world, including Argentina. Depending upon the severity of field infestation, yield losses due to Sclerotinia blight may be as high as 50% (Melouk and Backman,1995).
Host plant resistance provides the most effective solution to managing Sclerotinia blight, but limited progress has been made in the development and release of cultivars with enhanced tolerance to the disease (Smith et al.,1991, 1998; Baring et al., 2006; Baring et al., 2013; Melouk et al., 2013; Chamberlin et al., 2017, 2018). Factors that influence the development of cultivars resistant to Sclerotinia blight include a complex inheritance pattern, plant morphology, the narrow genetic base of cultivated peanut, and available sources of resistance. The inheritance mechanism of host resistance to Sclerotinia blight is not well understood but is known to be quantitative with possible cytoplasmic effects (Wildman et al.,1992; Coffelt and Porter, 1982). Plant morphology can play an important role in resistance to fungal disease because of the environment required for development and progression (Chappell et al., 1995; Coffelt and Porter, 1982; Coyne et al., 1974; Schwartz et al., 1978). Cultivated peanut has an extremely narrow genetic base which has been explained to have resulted from a single domestication event (Simpson et al., 2001) and subsequent inbreeding among a few select parental lines in commercial breeding programs. In order to develop new cultivars with enhanced Sclerotinia blight resistance, breeders must search for new sources of resistance outside the cultivated peanut background.
Fortunately, there are peanut germplasm collections globally that contain a wealth of genetic diversity from which breeders can incorporate traits of interest. The largest collections are held by International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), the United States Department of Agriculture (USDA), the Oil Crops Research Institute, Chinese Academy of Agricultural Sciences (OCRI-CAAS) and the Empresa Brasileira de Pesquisa Agropecuaria (EMBRAPA) in Brazil. Other peanut producing countries also maintain smaller collections. These collections are vast and contain thousands of accessions collected from around the world but are difficult to screen in their entirety due to the financial and personnel limitations of most peanut breeding programs. In most cases, a sub-set of these large collections called 'core collections', have been developed and are representative of the genetic diversity available in the complete collection. These core collections, along with the development and use of molecular markers associated with key traits, greatly increase the efficiency of mining these vast collections for sources of breeding material to enhance cultivated peanut. A molecular marker associated with Sclerotinia blight resistance in peanut has been identified (Chenault et al., 2009), and has been used to screen multiple core collections (Chamberlin et al., 2010; Chamberlin, 2014; Chamberlin and Puppala, 2018).
Argentina ranks 7th world-wide in peanut production (USDA Foreign Agricultural Service, 2020), producing more than 350,000 ha and harvesting approximately 1.1 million tons, annually. The Instituto Nacional de Tecnología Agropecuaria (INTA) Manfredi, Argentina peanut collection consists of 3443 active entries, from 40 countries (although most come from South America). The entries include mostly landraces, but also encompass cultivars and experimental lines. A core collection of 154 entries representing the total genetic variability of the entire collection has been developed (Baldessari et al., 2017), but has not yet been screened for sources of Sclerotinia blight resistance. The objective of this study was to characterize the Argentinean INTA core collection using the molecular marker associated with Sclerotinia blight resistance.
Materials and Methods
Genetic Materials
Genomic DNA from 154 accessions from the INTA peanut core collection and 33 supplemental genotypes, many of which had been extensively phenotyped for Sclerotinia blight resistance in Argentina (Table 1), was provided by E. Mamani and V. Moreno of Instituto Nacional de Tecnología Agropecuaria (INTA), Manfredi, Argentina. The DNA extraction was performed from 20 mg of dried leaves in silica gel. The samples were ground for two 5-s cycles in bead mill at 20,000 rpm (Super FastPrep-2 Bead Beating System, MP Biomedicals LLC, Irvine, CA, USA). A modified CTAB method with a sorbitol cleaning wash before the lysis step was used (Inglis et al., 2018). DNA was re-suspended in 100 μL of Tris-EDTA buffer and stored at -20 C until further use.
Phenotyping of Control Genotypes
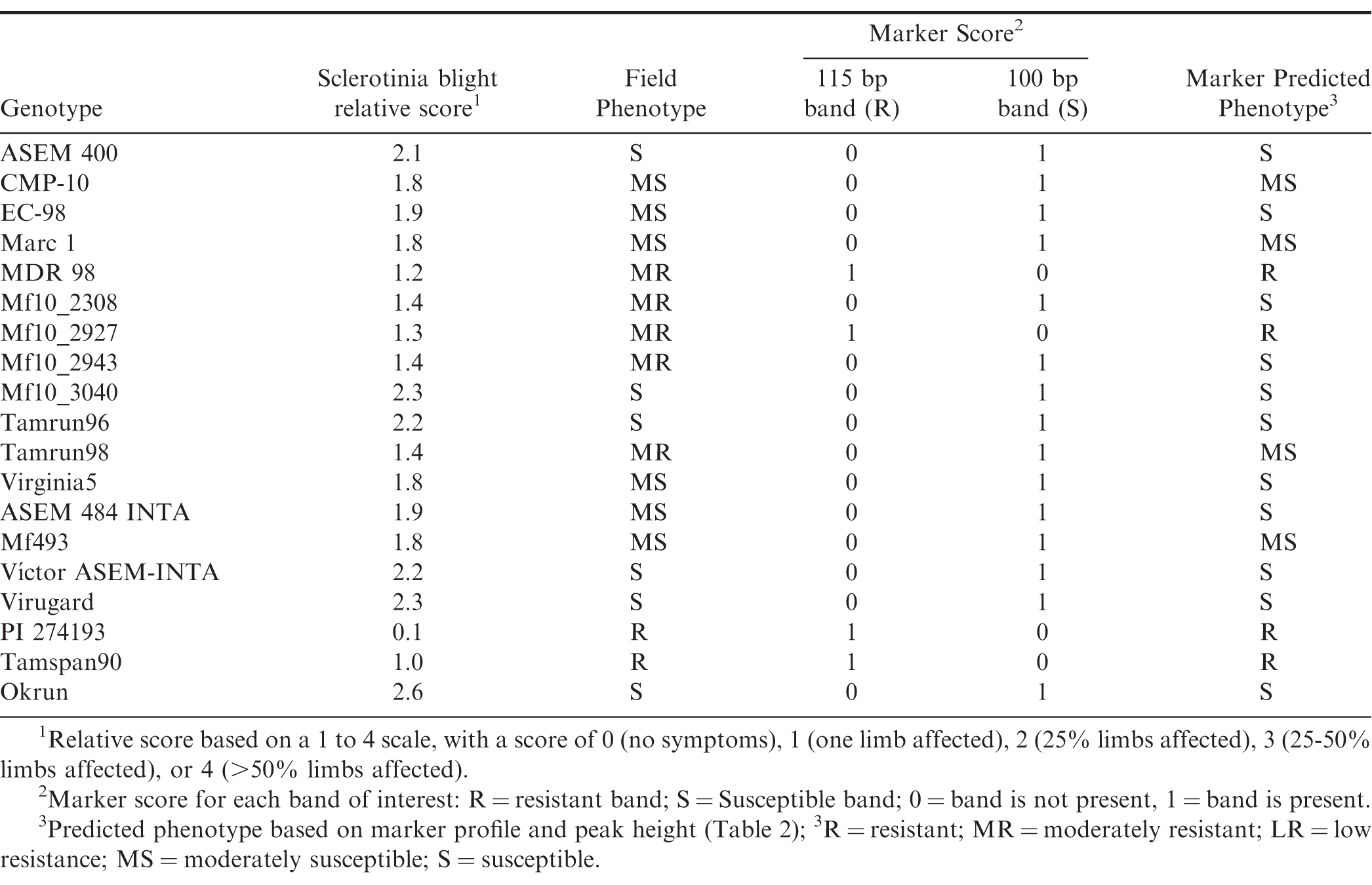
Phenotyping trials were arranged in a randomized complete-block design with three replications and planted at INTA's Manfredi Experimental Station near Manfredi, (Córdoba Province), Argentina. Individual plots consisted of single rows of 4 m length with a spacing of 1.4 m and planted at a seeding rate of 10 seeds/m. Entries included in these phenotyping trials include those listed in Table 1, with the exception of Okrun (Banks et al., 1989) and PI 274193 which were similarly phenotyped in the U.S. Plots were inoculated with 70 cc wheat grain containing active S. minor mycelium at approximately 120 d after planting (DAP). Individual plants within a plot were rated in the field after digging according to the following scale: 0 (no symptoms), 1 (one limb affected), 2 (25% limbs affected), 3 (25-50% limbs affected), and 4 (>50% limbs affected). Trials were repeated for 3 yr. Sclerotinia blight relative score was calculated by averaging individual plant scores within each plot.
Marker Analysis
Prior to amplification, DNA was quantified using a NanoDrop nd-3300 spectrofluorometer using the Pico Green dsDNA Assay Kit (ThermoFisher Scientific, Waltham, MA), and concentrations were adjusted to 25 ng/uL prior to PCR amplification. Amplification was performed in triplicate using a SSR marker derived from the SSR primer pair pPGPseq2E6, which has been reported to be associated with Sclerotinia blight resistance in peanut (Chenault et al., 2009). Amplification was carried out in an Applied Biosystems (Foster City, CA) MiniAmpPlus thermal cycler under conditions previously optimized. Primers were labelled with 5-FAM fluorophor. Fragment analysis of PCR products was done using an Applied Biosystems (Foster City, CA) 3730 DNA Analyzer and sized using a LIZ120 labelled size standard. Amplification with this primer set generally produces two bands of interest, one at 100 bp (predominant in susceptible genotypes) and one at 115 bp (predominant in resistant genotypes). Peaks of amplified products were analysed using PeakScanner 1.0 software (ThermoFisher Scientific). Peak heights were recorded for each genotype. DNA from known susceptible cultivar control Okrun and known resistant control PI 274193 (USDA Peanut Germplasm Collection) were included in each assay. Genotypes possessing the 115 bp band associated with Sclerotinia blight resistance were given a score of one (1), while those carrying only the 100 bp band were given a zero (0) rating. Genotypes possessing amplified products but neither band of interest were not rated (NR). Genotypes that did not possess any amplified products were not included in the analysis. Correlation analysis of phenotypic and genotypic data was conducted using SAS ver. 9.3 (Cary, NC).
Results and Discussion
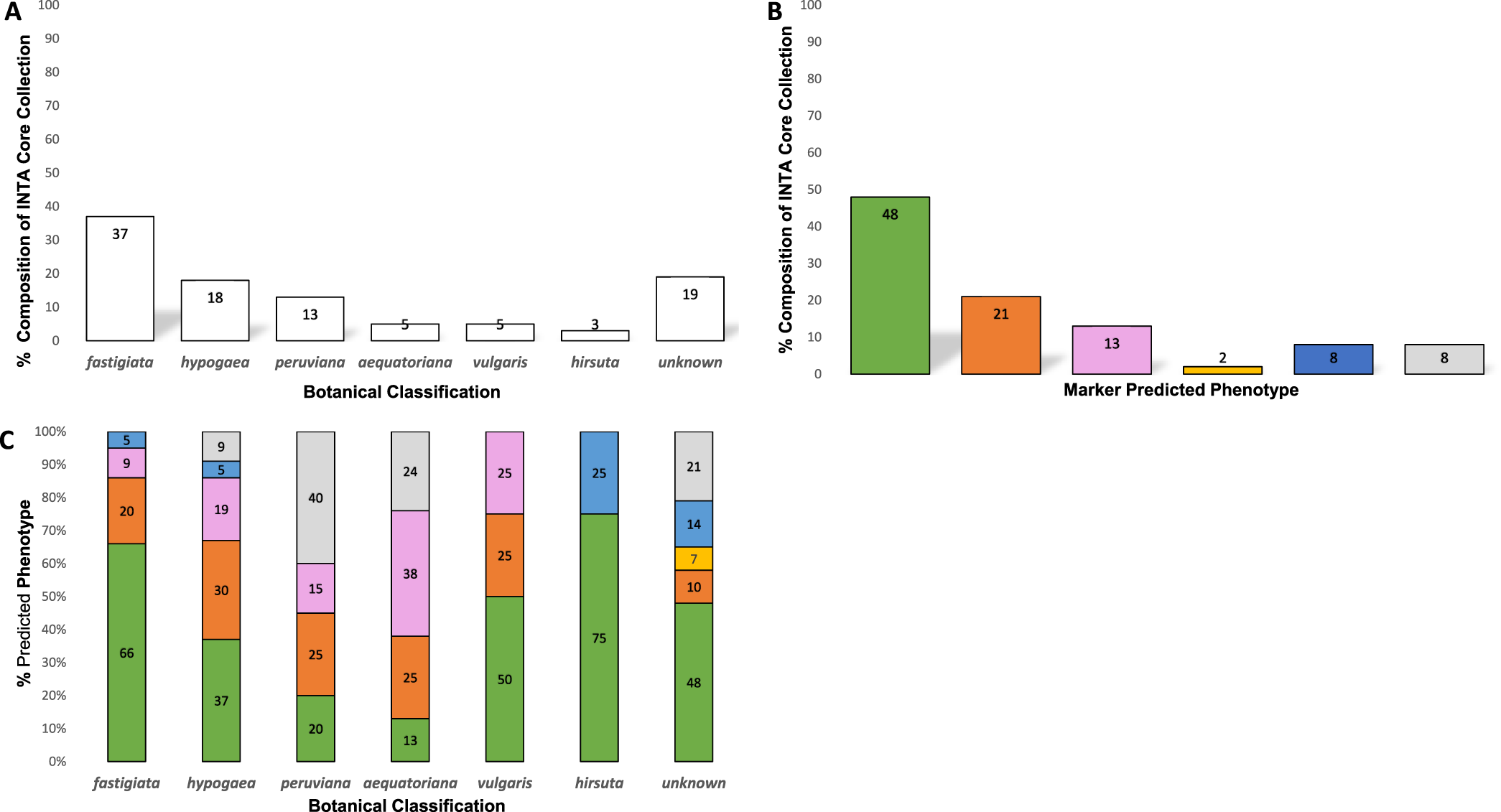
In addition to the 154 members of the INTA peanut core collection genotyped in this study, we included a set of 33 other peanut genotypes, many of which had also been phenotyped for Sclerotinia blight resistance in Argentina for 3 yr. Also included in this study were two genotypes (Okrun and PI 274193) that have been similarly genotyped and phenotyped in the U.S., and have been reported previously (Chenault et al., 2009; Chamberlin et al., 2010; Chamberlin, 2014) to serve as resistant and susceptible controls for this marker analysis. The INTA peanut core collection accessions have been classified by botanical variety and consists of 37% fastigiata, 18% hypogaea, 13% peruviana, 5% vulgaris, 5% aequatoriana, and 3% hirsuta (Figure 1A). Nineteen percent of the accessions have not been botanically classified.
Classification of the Instituto Nacional de Tecnología Agropecuaria (INTA) core collection, (A) Botanical variety categorization of INTA core collection accessions; (B) Percent composition of INTA core collection by marker predicted phenotype. Green = resistant (R), orange = moderately resistant (MR), pink = low resistance (LR), yellow = moderately susceptible (MS), blue = susceptible (S), grey = not rated (NR); (C) Marker predicted phenotypes of INTA core collection accessions by botanical variety. Green = resistant (R), orange = moderately resistant (MR), pink = low resistance (LR), yellow = moderately susceptible (MS), blue = susceptible (S), grey = not rated (NR).
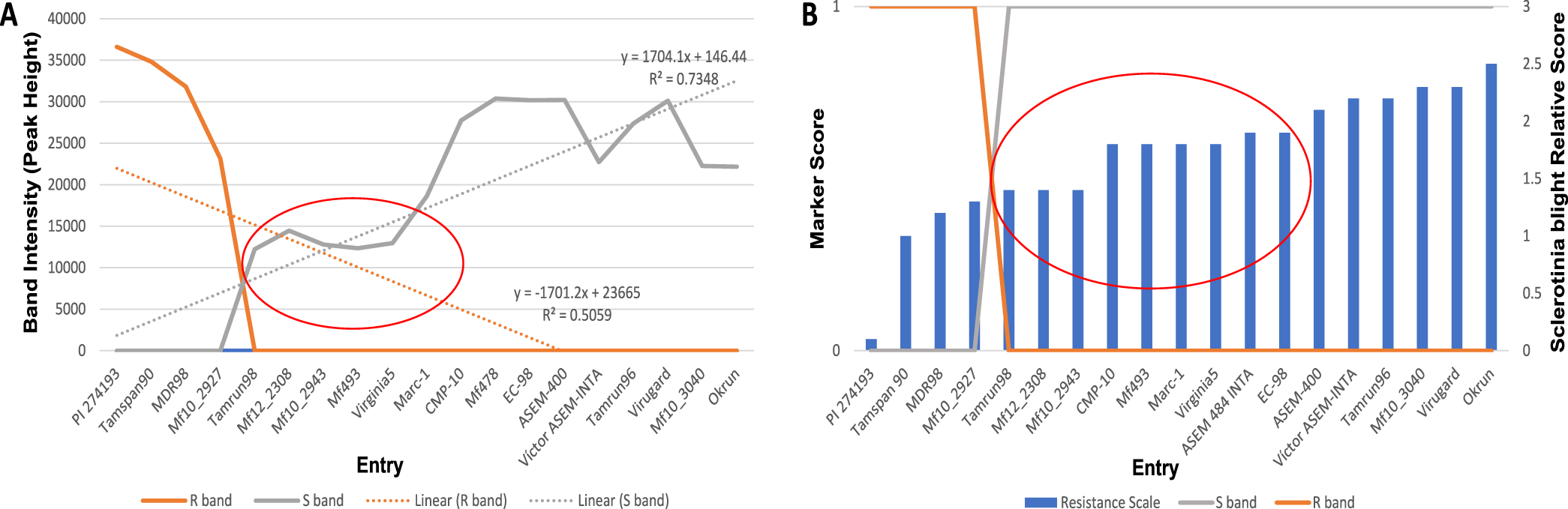
Using the SSR marker previously associated with Sclerotinia blight resistance (Chenault et al., 2009), successful amplification was achieved for all but 14 INTA core collection accessions. Lack of amplification for those accessions appears to be due to the absence of primer binding site(s) since amplification of the same templates using other primer sets and control primers were successful (data not shown). When comparing genotypic and phenotypic data for the genotypes already phenotyped in the field, the marker predicted phenotype agreed with the phenotyped observed in field trials with few exceptions (Table 1). Linear regression analysis of known phenotypes and genotypic peak height (Figure 2A) indicated there is a correlation between presence of a the 100 bp band with susceptibility to Sclerotinia blight (R2= 0.7348) and presence of the 115 bp band with resistance (R2= 0.5059). Genotypic score and resistance level of control phenotyped entries are shown in Figure 2B. Stronger correlations exist for the extremes of susceptibility or resistance (Figure 2A and 2B), whereas the marker is less likely to accurately predict phenotypic reactions in the moderate range (denoted by red ovals, Figure 2). These results are similar to those reported previously by others using this marker to screen other germplasm collections (Chamberlin et al, 2010; Chamberlin, 2014; Bennett et al., 2018; Chamberlin and Puppala, 2018). Resistance to S. minor has been shown to be complex and quantitative (Wildman et al., 1992). Resistance genes to Sclerotinia blight are not the only factor influencing disease in peanut. Plant architecture also influences resistance to S. minor and other pathogens, with drier open canopies found in fastigiata and vulgaris botanical-types (Valencia and Spanish market-types, respectively) being less favorable to disease than the more humid and dense canopies of the hypogaea botanical-types (runner and Virginia market-types, respectively) (Coffelt and Porter, 1982; Chappell et al., 1995; Goldman et al., 1995). The interaction between growth habit and Sclerotinia blight resistance on disease incidence may account for the exceptions in the marker analysis.
Correlation of marker profile with resistance of control genotypes. (A) Linear regression analysis of peak height with resistance levels of entries with control phenotypes. Red oval denotes region of uncertainty for marker predicted phenotypes; (B) Genotypic score and resistance levels of entries with control phenotypes. Red oval denotes region of uncertainty for marker predicted phenotypes.
Given that the template DNA has been quantified and normalized across all reactions, band intensity or peak height of the identified resistance and susceptible bands has previously been correlated with level of resistance seen among genotypes tested with this marker system (Chamberlin, 2014; Chamberlin and Puppala, 2018), allowing the prediction of field reaction to S. minor infection for genotypes not yet tested in phenotyping trials. Table 2 lists the botanical variety and genotypic profiles (peak heights) for each entry along with the respective predicted field reaction to S. minor. Of the 154 core accessions tested, the marker predicted phenotype (Figure 1B) was 48% resistant (R), 21% moderately resistant (MR), 13% low resistant (LR), 2% moderately susceptible (MS), and 8% susceptible (S). Eight percent of the accessions did not produce a marker genotype and thus were not rated (NR) for predicted phenotype. The predicted phenotypes by botanical variety group (Figure1C) were as follows: fastigiata 66% R, 20% MR, 9% LR and 5% S; hypogaea 37% R, 30% MR, 15% LR, 5% S and 9% NR; peruviana 20% R, 25% MR, 15% LR, 40% NR; aequatoriana 13%R, 25% MR, 38% LR, 24% NR; hirsuta 75% R, 25% S; vulgaris 50% R, 25% MR, 25% LR.
Previously, the marker used in this study was shown to be significantly associated with resistance to Sclerotinia blight in peanut cultivars and PIs that had been thoroughly evaluated in field trials (Chenault et al., 2009). The marker has been used previously to screen numerous germplasm collections (Chamberlin et al, 2010; Chamberlin, 2014; Chamberlin and Puppala, 2018) and the results obtained have been shown to closely agree with phenotypic reaction to S. minor in field trials (Chenault et al, 2009; Chamberlin et al., 2010; Damicone et al., 2010; Bennett et al., 2018). The results of the current study are similar to those reported when the marker was used to screen other collections, with the marker for resistance being prominent in the fastigiata, vulgaris, and hirsuta botanical types.
Markers closely associated with phenotypic traits can be used to increase screening efficiency when working with large germplasm collections. Such markers can also be used to track inheritance of traits while developing new cultivars. Although primitive compared with some other crops, markers have been identified in peanut for resistance to bacterial wilt (Ren et al., 2008), rust (Hou et al., 2007; Shoba et al., 2010), late leaf spot (Xia et al., 2007; Shoba et al., 2010), root-knot nematode (Chu et al., 2007), and Aspergillus flavus (Lei et al., 2005). The marker used in this study was used to identify PI 274193 of the USDA peanut germplasm collection as a source of excellent resistance to Sclerotinia blight (Damicone et al., 2010; Chamberlin et al., 2010) which was then incorporated into a cultivated background, leading to the development of the cultivar Lariat (Chamberlin et al., 2018), which requires no fungicide treatment for Sclerotinia blight control.
Keeping in mind that molecular markers, including the one used in this study, cannot accurately predict the level of disease resistance that will be demonstrated in field trials, use of molecular markers should be used as a tool only to screen vast collections and identify germplasm with the potential of field resistance. The results obtained in this study have identified accessions from the INTA core collection worthy of evaluation in the field for S. minor resistance and identified those with low probability of being a source of resistance, reducing the amount of field work required to select from the collection by 52%. Accessions within each botanical variety represented in the Argentinean INTA core collection worthy of field testing have been identified which may assist in defining breeder choices when designing cultivar development. Although screening for traits with molecular markers cannot necessarily predict the level of high disease resistance that will be demonstrated in the field and will not replace the breeder's eye in cultivar development, the reduction in resources required to screen large collections of germplasm for a trait of interest will ultimately increase the breeder's efficiency and reduce the time required for improved cultivar release.
Acknowledgements
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Literature Cited
D.J., Banks, J.S Kirby, and J.R Sholar (1989). Registration of 'Okrun' peanut. Crop Sci 29: 1574.
Baldessari, J., E.M.C Mamani, M.B Conde, M.V Moreno, R.M Gallardo, N.G Grandon, F Funes, M.M Manifesto, and V.J Etchart 2017 Development of an Argentinean peanut core collection and establishment of an association mapping population Proceedings of the 2017 Advances in Arachis through Genomics and Biotechnology Conference, Cordoba, Argentina.
Baring, M.R., C.E Simpson, M.D Burow, M.C Black, J.M Cason, and J Ayers 2006 Registration of 'Tamnut OL07' peanut Crop Sci 46: 2721- 2722 doi:10.2135/cropsci2006.06.0413
M.R., Baring, C.E Simpson, M.D Burow, J.M Cason, and J.L Ayers (2013). Registration of 'Tamrun OL11' peanut. J. Plant Reg 7: 154- 158.
R.B., Bennett, K.D Chamberlin, and J.P Damicone (2018). Sclerotinia blight resistance in the US Peanut mini-core collection. Crop Sci 58: 1306- 1317.
K.D Chamberlin, (2014). Characterization of ICRISAT peanut mini-core accessions with regards to a molecular marker associated with resistance to Sclerotinia blight. Peanut Sci 41: 42- 49.
Chamberlin, K.D., R.S Bennett, and J.P Damicone 2017 Registration of 'VENUS' peanut J. Plant Reg 11: 33- 37.
K.D., Chamberlin, R.S Bennett, and J.P Damicone (2018). Registration of 'Lariat' peanut. J. Plant Reg 12: 36- 42.
K.D.C., Chamberlin, H.A Melouk, and M.E Payton (2010). Evaluation of the U.S. peanut mini core collection using a molecular marker for resistance to Sclerotinia minor Jagger. Euphytica 172: 109- 115.
K.D Chamberlin, and N Puppala (2018). Genotyping of the Valencia peanut core collection with a molecular marker associated with Sclerotinia blight resistance. Peanut Sci 45: 12- 18.
G.F., Chappell, B.B Shew, J.M Ferguson, and M.K Beute (1995). Mechanisms of resistance to Sclerotinia minor in selected peanut genotypes. Crop Sci 35: 692- 696.
K.D., Chenault, A.L Maas, J.P Damicone, M.E Payton, and H.A Melouk (2009). Discovery and characterization of a molecular marker for Sclerotinia minor (Jagger) resistance in peanut. Euphytica 166: 357- 365.
Y., Chu, C.C Holbrook, P Timper, and P Ozais-Akins (2007). Development of a PCR-based molecular marker to select for nematode resistance in peanut. Crop Sci 47: 841- 847.
T.A., Coffelt, and D.M Porter (1982). Screening peanuts for resistance to Sclerotinia blight. Plant Dis 71: 811- 815.
D.P., Coyne, J.R Steadman, and F.N Anderson (1974). Effect of modified plant architecture of great northern dry bean varieties (Phaseolus vulgaris) on white old severity, and components of yield. Plant Dis Rep 58: 379- 382.
J.P., Damicone, C.C Holbrook, D.L Smith, H.A Melouk, and K.D Chamberlin (2010). Reaction of the core collection of peanut germplasm to Sclerotinia blight and pepper spot. Peanut Sci 37 ((1)): 1- 11.
J.J., Goldman, O.D Smith, C.E Simpson, and H.A Melouk (1995). Progress in breeding Sclerotinia blight-resistant runner-type peanut. Peanut Sci 22: 109- 113.
H.M., Hou, B.S Liao, Y Lei, X.P Ren, S.Y Wang, D Li, H.F Jiang, J.W Huang, and B.Y Chen (2007). Identification of AFLP markers for resistance to peanut rust. Chin. J. Oil Crop Sci 29: 89- 92.
Inglis, P.W., Md.C.R. Pappas, L.V Resende, and D Grattapaglia 2018 Fast and inexpensive protocols for consistent extraction of high-quality DNA and RNA from challenging plant and fungal samples for high-throughput SNP genotyping and sequencing applications PLoS ONE .13: e0206085. https://doi.org/10.1371/journal.pone.0206085
Y., Lei, B.S Liao, S.Y Wang, Y.B Zhang, D Li, and H.F Jiang (2005). Identification of AFLP markers for resistance to seed invasion by Aspergillus flavus in peanut (Arachis hypogaea L.). Acta Agronomica Sinica 31: 1349- 1353.
Melouk, H.A and P.A Backman 1995 In "Peanut Health Management" H.A Meloukand F.M Shokes, Eds., p.75-85 APS Press, St. Paul, Minnesota.
H.A., Melouk, K.D Chamberlin, C.B Godsey, J.P Damicone, M.D Burow, M.R Baring, C.E Simpson, K.E Dashiell, and M Payton (2013). Registration of 'Red River Runner' peanut. J. Plant Reg 7: 22- 25.
Ren, X.P., H.F Jiang, and B.S Liao 2008 Identification of molecular markers for resistance to bacterial wilt in peanut (Arachis hypogaea L.) J. Plant Genet 9: 163- 167, SAS Institute. 2011. SAS 9.3. SAS Inst., Cary, NC.
H.F., Schwartz, J.R Steadman, and D.P Coyne (1978). Influence of Phaseolus vulgaris blossoming characteristics and canopy structure upon reaction to Sclerotinia sclerotiorum. Phytopathology 68: 465- 470.
Shoba, D 2010 Identification of molecular markers for resistance to rust (Puccinia arachidis) and late leaf spot (Phaeoisariopsis personata) in groundnut (Arachis hypogaea L.). Ph.D. thesis. Submitted to Tamil Nadu Agricultural University, Coimbatore.
C.E., Simpson, A Krapovickas, and J.M Valls (). History of Arachis including evidence of A. hypogaea progenitors. Peanut Sci 28: 79- 81.
O.D., Smith, C.E Simpson, W.J Grichar, and H.A Melouk (1991). Registration of 'Tamspan 90' peanut. Crop Sci 31: 1711.
O.D., Smith, C.E Simpson, M.C Black, and B.A Besler (1998). Registration of 'Tamrun 96' peanut. Crop Sci 38: 1403.
USDA Foreign Agricultural Service 2020 World Agriculture Production, Circular Series, WAP 1-20.
L.G., Wildman, O.D Smith, C.E Simpson, and R.A Taber (1992). Inheritance of resistance to Sclerotinia minor in selected spanish peanut crosses. Peanut Sci 19: 31- 34.
H.Q., Xia, B.S Liao, J.N Li, Y Lei, H.F Jiang, F.H Cui, and X.P Zeng (2007). Identification of AFLP markers linked to resistance to late leaf spot in peanut (Arachis hypogaea L.). Chin. J. Oil Crop Sci 29: 318- 321.