Introduction
In the U.S., peanut (Arachis hypogaea L.) is planted from late April to June and plants begin to emerge seven to ten d later. Flowering normally occurs one month after planting depending on the genotype, with harvest at 120 to 160 d after planting (DAP). However, peanut is an indeterminate crop that continually flowers and sets pods throughout the growing season, so harvest includes mature and immature pods at various stages of development. The mature peanut hull or pericarp consists of a thin-walled parenchyma in two cell layers, an underlying mesocarp layer 5 to 15 cells thick, and an inner endocarp composed of two layers of sclerenchyma and parenchyma (Halliburton et al., 1975). Research indicated that catechol-type tannins accumulated in the mechanical and vascular conducting tissue, primarily in the mesocarp of the pod as it matured (Schenk, 1960), thus revealing various colors ranging from white, yellow, orange, brown, and black as the pod and associated seed developed (Williams and Drexler, 1981). For growers, maturity in peanut is determined by visual assessment of the color of the mesocarp region after the removal of the exocarp, with maximization of the percentage of brown and black pods harvested, since these represent the optimal level of crop maturity (Williams and Drexler, 1981).
Seed maturity has the potential to affect seed quality, albeit an imprecise term that often is assumed to include emergence and vigor but could also impact subsequent crop development and yield. Seed maturation is critically important to subsequent crop performance or vigor because it involves the processes after embryo growth inhibition that involve the accumulation of storage reserves, the acquisition of desiccation and other stress tolerance, DNA repair, the establishment of regulatory networks and signaling pathways, the establishment of dormancy in some species, and the achievement of metabolic quiescence allowing cellular processes to resume once imbibition takes place (Bewley, 1997; Qun et al., 2007; Ventura et al., 2012).
Peanut has relatively large seeds and cotyledons, and desiccation tolerance may be achieved relatively late in the maturation process, or even beyond mass maturity (Hay and Probert, 1995). Seed maturity can influence dormancy characteristics in many species (Robertson et al., 1978; Samarah et al., 2004). From an agronomic standpoint, seed vigor is important and contributes to uniform emergence and stand establishment under adverse environmental conditions (Ventura et al., 2012; Finch-Savage and Bassel, 2016). The direct effect of seed vigor on yield is manifested through poor stand establishment; a reduction in plant numbers per unit area, leading to decreased yields (Finch-Savage and Bassel, 2016).
While seed germination is defined as the first visual appearance of the radicle (Forcella et al., 2000), vigor is difficult to define because it encompasses a group of characteristics, including aspects of: 1) rate and uniformity of germination and eventual seedling growth; 2) early growth traits including emergence and subsequent field performance; and 3) storage performance and the ability to retain successful germination (Hampton, 2000; Finch-Savage and Bassel, 2016). Seed vigor is principally influenced by three factors: genetics, phenotypic development, and storage after harvest (Qun et al., 2007). Aside from differences in characterization and subsequent influences, germination and vigor diverge by the fact that they develop separately in the seed. Germination capacity commonly does not reflect seed vigor and develops prior to vigor (Finch-Savage and Bassel, 2016). In fact, the recognition that there can be wide variability in overall seed performance despite high germination rates among seed lots has been recognized for over a century, and led to the description of vigor in 1876 as some internal driving force (Hampton, 2000). This separation between germination capacity and vigor has led to the sometimes-frequent failure of standard germination tests to detect the level of variability in overall seed quality (referring to vigor) that can be the case even among high germinating seed lots (Hampton, 2000). Hence, there is a real need for describing and quantifying seed vigor in standard seed testing procedures.
As for other crops, the need to quantify seed vigor for peanut is essential, particularly with increasing issues with stand establishment noted by producers and other industry representatives over the past decade. Maturity may be a real key to determining seed vigor for peanut. With limited research reported about peanut seed maturity, Spears and Sullivan (1995) noted that germination increased for the cultivar NC7 as the crop matured, with concomitant decreases in membrane leakage. This research illustrated the link between maturity and germination for peanut, and seed vigor as measured by accelerated aging and electrical conductivity (Spears and Sullivan, 1995). Their work also indicated the link between seed weight and maturity is not always clearly defined (Spears and Sullivan, 1995). Others have shown a link between maturity and dormancy in peanut (Toole et al., 1964; Ketring and Morgan, 1970). Interestingly, this effect existed even between dormancy states of apical and basal peanut seed, likely because they develop at different times and therefore represent a range of seed maturity in a single pod (Schenck, 1961; Ketring and Morgan, 1970; Rucker et al., 1991). There are also biochemical changes that occur in peanut as it matures that likely impact vigor including increasing accumulation of storage reserves as evidenced by increasing sugar and lipid content with maturity (Pattee et al., 1974), leading to a lower density in fruits that are mature (Gilman and Smith, 1977).
To address the dearth of studies in peanut that link seed maturity to subsequent life history traits and overall crop performance, a field study was conducted over a two-year period quantifying the difference in physiological responses between seed obtained from immature and mature pods. The objective was to compare critical characteristics of crop performance between plants established using both mature and immature seed for two commercial peanut cultivars. Specifically, the plants evaluated were grown from seed in the immature yellow class and the combined mature brown/black classes. Crop characteristics measured include emergence, leaf area index (LAI), normalized difference vegetation index (NDVI), flower production, yield and the resulting maturity of the harvested seed.
Materials and Methods
Field management
The location of the field trial was the Agronomy Teaching Farm (+29.63, -82.36) at the University of Florida campus in Gainesville, Florida. Genotypes tested were TUFRunner™ '727' (University of Florida, BL Tillman- personal communication) and FloRun™ '107' (Tillman and Gorbet, 2015). Each experiment was a randomized complete block design with four replications for each genotype and maturity class, consisting of two-row plots for each treatment combination in 2014. In 2015, the design was identical except that the plot size was expanded to three-rows. Conditions at planting and harvest were comparable both years; planted 30 May, 29 April and harvested 3 Oct, 11 September: 2014 and 2015, respectively. Seed used for the trial each year was produced the year previous and treated similarly post-harvest for storage at the UF/IFAS North Florida Research and Education Center in Marianna. Seed were hand planted in rows that were 3.7 and 3.1 m in length (2014 and 2015, respectively) and 91.4 cm apart, with six seeds per 30.5 cm within the row.
All seed were treated with azoxystrobin (Syngenta Corp., North Carolina) fungicide at the registered rate before planting as a preventative for seed borne diseases prior to emergence. The foliar fungicide pyraclostrobin (BASF Corp., New Jersey) was applied at the registered rate in 2014 but delayed due to an expectation that the trial would be terminated after full emergence; however, when treatment differences persisted, fungicide application began at 63 d after planting (DAP) and was repeated every 21 d in order to maintain the plots through optimum maturity. However, the delayed fungicide application resulted in heavy leafspot pressure and the need to harvest prior to full maturity. Fungicide applications in 2015 followed typical recommendations and commenced at 40 DAP and were repeated every 21 d. Beside azoxystrobin and pyraclostrobin fungicides, no other pesticide treatments were applied and weeds were controlled manually in both years.
In 2014 and 2015 plots received 19.05 mm of irrigation at planting. In 2014 they received two irrigation applications of 19.05 mm the month after planting, whereas 2015 had adequate rainfall and did not need additional irrigation. Weather data for the trials in both years was collected from the Gainesville Regional Airport (approximately 15 km away from the field site) and a station established on the roof of the University of Florida Physics Department (approximately 2.7 km away from the field site).
Crop Development
Over the growing period, data were collected to analyze the differences pertaining to emergence, growth, and development. Data impacting canopy structure included emergence, leaf area index (LAI), normalized difference vegetation index (NDVI) and other reflectance parameters were collected. With exception of sampling for the maturity ratio, all measurements taken prior to harvest were nondestructive, so as not to remove or damage plant tissue. For this study, emergence was defined as the opening of the first set of true leaves which follow the cotyledons. Once emergence had begun, daily stand emergence was quantified each afternoon until the numbers reached maximum levels and no longer changed. Once the emergence rates had peaked, the daily counts ceased and a final count was taken one week later. As the crop canopy began to expand, canopy architectural changes were documented by quantifying LAI using a Licor LAI 2200 (Licor, Nebraska, USA). Two groups of five points were taken for each LAI measurement, ten points total per reading. Each group of five readings consisted of one above canopy reading, and then four below canopy readings approximately 23 cm apart (taken in that order); the first set of five points were taken parallel to the row, and the last five were taken perpendicular to the row. The LAI measurements were taken wk throughout the season in sections of row that contained no plants missing i.e. sections that had a contiguous stand.
Multiple reflectance vegetation indices were measured using the Crop Circle model ACS-470 (Holland Scientific, Nebraska, USA) on two rows per plot. Readings were averaged over the length of the plot. The Crop Circle was configured to measure spectral characteristics between 440 to 800 nm using 12.5 mm diameter filters, resulting in the calculation of five different vegetation indices including: the normalized difference red-edge (NDRE), NDVI, red-edge band reflectance, near infrared (NIR) waveband, and red band reflectance. The sensor measurements of red band reflectance (700-750 nm) is assumed to be representative of the chlorophyll status until the canopy LAI nears 2.0; while the orange, yellow, and green bands are assumed to be more appropriate for larger canopies until the LAI approaches 4.0 or more (Holland Scientific, 2005). The sensor readings of NIR radiation (760-900 nm) are assumed to be representative of the amount of biomass present, as NIR reflectance is indicative of living vegetation (Holland Scientific, 2005). In both years, the Crop Circle was used on a wk basis. As in LAI, only contiguous sections were measured.
Reproductive characteristics and harvest
The reproductive characteristics measured included average number of flowers per plant, maturity assessment at harvest, yield, and grade. The plants began flowering approximately 30 DAP in both years. After flowering had commenced, the number of new flowers (not wilted from previous d) per row were recorded daily for four consecutive days, followed by wk counts conducted at 10:00 a.m. until the number of flowers no longer increased. The number of flowers per plot was divided by the number of plants in the row as determined by a final stand count to determine the average number of flowers per plant.
In 2014 and 2015, a Peanut Field Agronomic Resource Manager (PeanutFARM) account was used to gauge adjusted growing degree days (aGDDs) for each DAP as well as an accumulated value through the season. Harvest was anticipated at 135-150 DAP for the cultivars used in the study, corresponding to approximately 2,400-2,500 aGDDs. Research has shown that optimum peanut maturity in the southeastern U.S. correlates to approximately 2,500 aGDDs, with a maturity ratio (mature pods compared to total pods) of a blasted sample at 70% or higher (Rowland et al., 2006). However, in 2014, rapid defoliation of the canopy due to leaf spot caused an early harvest at 126 DAP, with an accumulation of only 2,291 aGDDs. In 2015, harvest occurred at 135 DAP, with an accumulation of 2,446 aGDDs. Actual quantification of maturity at harvest was determined by the calculated maturity ratio (MR; Rowland et al., 2006) in both years:
The mesocarp color was determined for the MR using a pressure washer for removal of the exocarp (Williams, 2003) and then pods were visually categorized. Once the maturity ratio had been calculated, dry weight was recorded and added back into the final yield for that plot. The plots were then hand dug and plants with attached pods were dried using forced air (35 C) applied for three days prior to threshing until the moisture level was reduced to approximately 10%. After drying, the pods were removed from the plants using a Kincaid thresher (Haven, Kansas USA), cleaned by picking out stems and other debris by hand, and weighed. Weights were scaled to plot area to determine yield (kg/ha). To determine grade, samples of approximately 200 ± 0.1 g were taken from each plot. Adapted from (Anonymous, 2015), the grade process was modified from the AMS farmer stock grading instructions by calculating the summed totals for the sound mature kernels and sound splits, dividing that number by sample weight, and then multiplying by 100.
Statistical Analysis
All data were evaluated in JMP 10 (SAS, North Carolina USA) through univariate analysis using a mixed model ANOVA consisting of a factorial design. The model differed slightly for each data set, depending on whether the data was repeated (in time) or non repeated measures. The restricted maximum likelihood method was used with standard least squares and an emphasis on effect leverage. The following traits were analyzed using repeated measures: emergence, LAI, and NDVI. This model included the fixed factors of cultivar, maturity class, and date; the rep nested within cultivar and maturity class was treated as random. The traits of maturity ratio, yield, and grade were analyzed as non-repeated measures with fixed factors in the model of cultivar and maturity class and rep as a random factor. One standard error of the mean is displayed in all graphical representations of data from this experiment.
Results and Discussion
Emergence and Canopy Structure
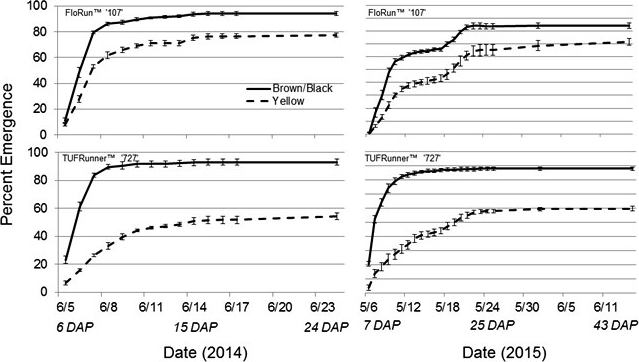
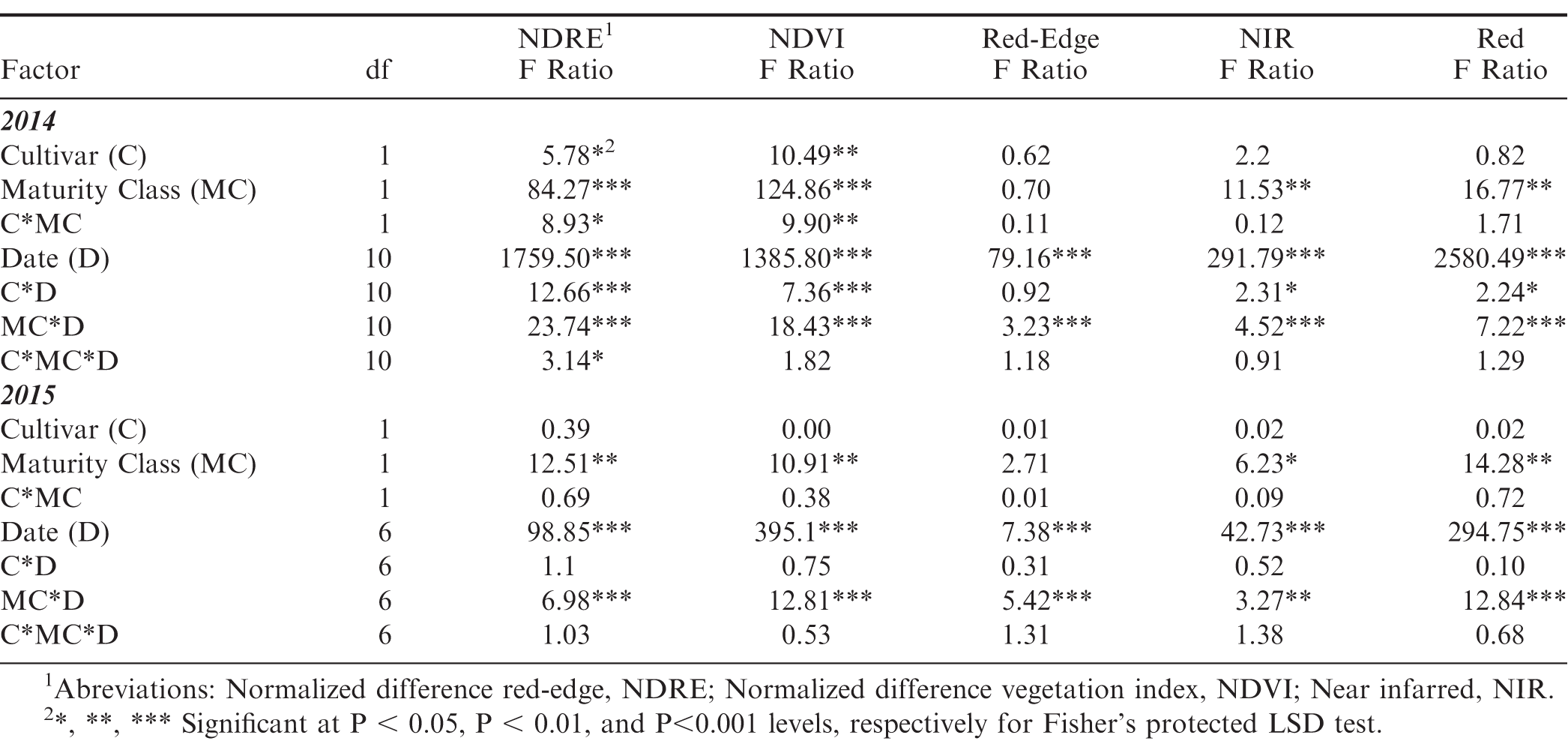
In 2014 and 2015, emergence was affected by cultivar, maturity class, and date with two and three-way interactions (Table 1). The three-way interaction in both years indicated differences between the emergence rates for both cultivars, between the yellow and black classes, and in the time it took to reach the maximum emergence, primarily for the yellow class (Figure 1). The maximum emergence for the black, mature pods for both cultivars approached 95% and occurred at approximately the same date in 2014: 8 June (9 DAP). The main source of the interaction among the cultivars, dates, and maturity classes occurred in the yellow maturity class. The maximum emergence for the yellow class of FloRun™ '107' occurred at approximately the same date as the black class (8 June 2014, 9 DAP) but only reached 76%. The maximum emergence for TUFRunner™ '727' yellow class was much lower than for FloRun™ '107' at only 52% and occurred three d later on 11 June 2014 (12 DAP). The source of the interaction among cultivar, date, and maturity class was more complex in 2015. The maximal emergence for the brown/black class of TUFRunner™ '727' took approximately the same period of time (15 May 2015, 16 DAP) and reached a similar maximum value (90%) as in 2014. This was not the case for the black maturity class of FloRun™ '107'; there were actually two plateaus in emergence, one at 16 May 2015 (17 DAP) when emergence reached 65% and another at 24 May (25 DAP) when emergence reached a maximum value of 84% (Figure 1). For the immature yellow class of TUFRunner™ '727', the time to reach maximum emergence was roughly double the time period for this cultivar's black class - 23 DAP (22 May) but reached only 60% emergence. The timing of emergence for the yellow class of FloRun™ '107' mirrored the emergence pattern of its black class; with two plateaus occurring at approximately 17 and 25 DAP. However, the yellow class of FloRun™ '107' reached a higher maximum value of 70% as compared to TUFRunner™ '727'.
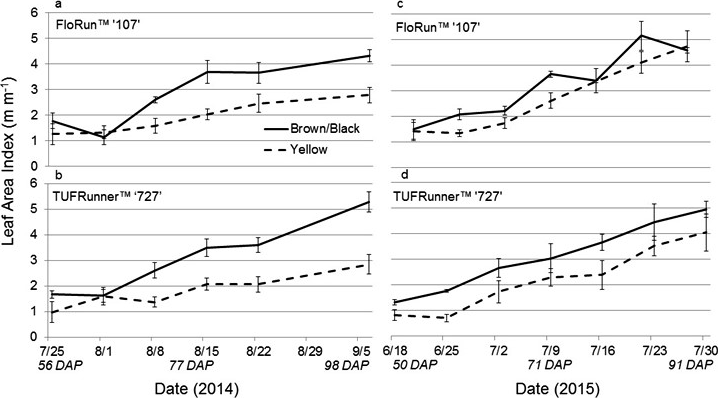
After emergence, LAI was measured through the season to determine if lasting differences in maturity class would be evident in later life history traits. The canopy development for the yellow classes of each variety were notably delayed when compared to the brown/black maturity classes (Figure 2). The yellow class of each genotype had a lower LAI at the beginning of the season and continued to have the lowest LAI at the end of the season with the exception of the yellow FloRun™ '107' which matched its black class (Figure 3). LAI was affected by maturity class and the interactions between maturity class and cultivar, and maturity class and date. In 2014, the significant interaction for LAI between maturity class and date was explained by a season-long increase in LAI in the black maturity class at a greater rate than for the yellow class. This difference between maturity classes was much larger for TUFRunner™ '727' than for FloRun™ '107' (Figure 3). This pattern repeated itself in 2015 for TUFRunner™ '727' but did not hold for FloRun™ '107' (Figure 3). The brown/black maturity class increased throughout the season, and while it was higher across the season, it did not rise at the increased rate of the yellow class. In 2015, no interactions occurred despite maturity class and date being significant.
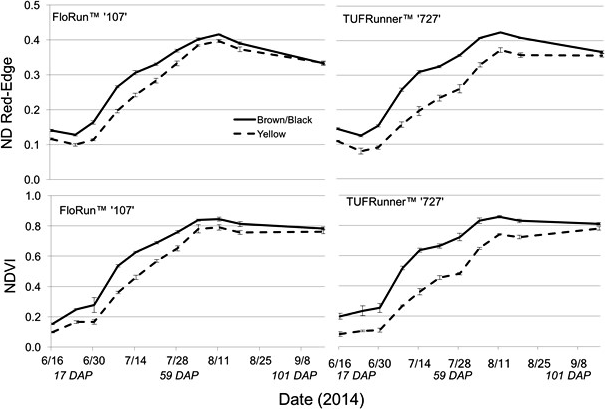
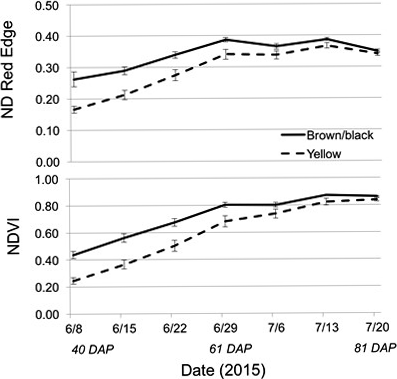
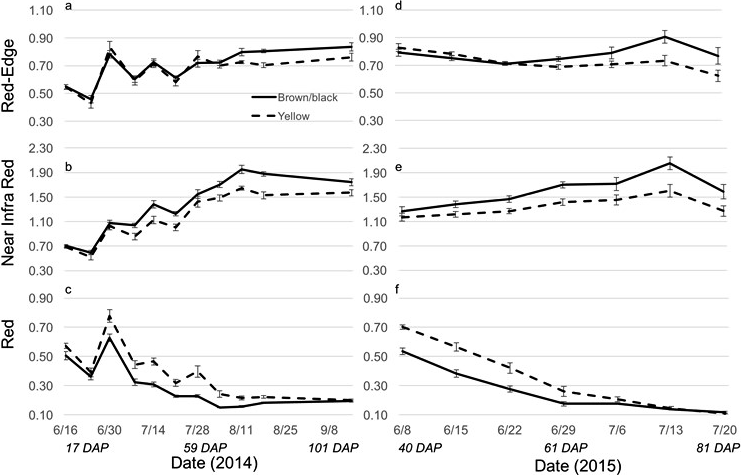
The Crop Circle also determined lasting influences of seed maturity class on the resulting canopy reflectance parameters across the season (Figure 4, 5 and 6). In 2014 and 2015, all reflectance parameters showed a significant interaction between maturity class and date (Table 2). Regarding NDRE and NDVI, the interaction was driven by a lower value for the yellow compared to the brown/black maturity class in both cultivars through most of the season until just prior to harvest, when these two parameters converged to similar values (Figure 4 and 5). The interaction between maturity class and date was driven by an opposite pattern for Red-Edge and NIR reflectance parameters; the values were similar for yellow and brown/black maturity classes in the early part of the season and began diverging by 50 DAP for NIR, and by 60 DAP in the case of Red-Edge reflectance (Figure 4). The patterns were very different for Red band reflectance than the other reflectance parameters, where the yellow maturity class had higher values than the brown/black class relatively early in the season at 17 and 40 DAP, before converging on similar numbers at 75 and 72 DAP in 2014 and 2015, respectively (Figure 6). The only reflectance parameter that showed a three-way interaction was NDRE, and this occurred between cultivar, maturity class and date (Table 2).
Reproductive characteristics
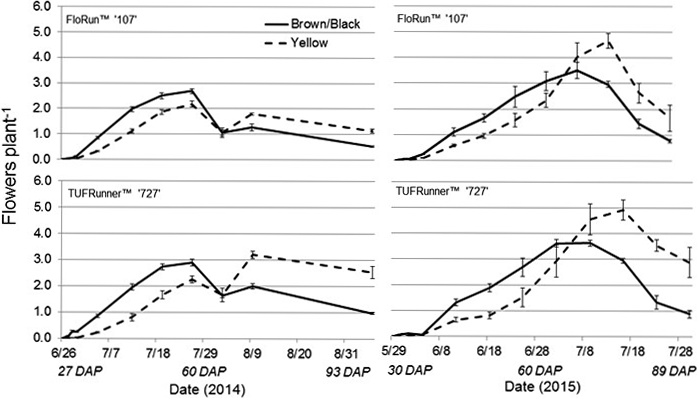
For 2014, flower production was affected by all the factors in the model with the exception of date, with two and three-way interactions. Interactions included cultivar by maturity class, cultivar by date, maturity class by date, and the three-way interaction among cultivar by maturity class by date (Table 3). These interactions demonstrate large differences between maturity class and cultivar, as well as the time needed to reach maximum flowering. The brown/black class of both cultivars displayed a higher flowering rate then the yellow class for the first half of the season; with the yellow class of each cultivar surpassing the more mature class after 2 August 2014 (64 DAP) (Figure 7). Flower production in July 2014 dropped due to an extended dry period, and later spiked following a rainfall event (Figure 7). Flower production in 2015 exhibited the same trend as the previous year, as the shift in flowering production occurred over the same time period between the maturity classes. The brown/black class of both cultivars had the higher flowering rate until their respective yellow classes exceeded the black classes after 6 July 2015 (69 DAP) (Figure 7). Date was significant, as well as the two-way interaction with maturity class by date (Table 3).
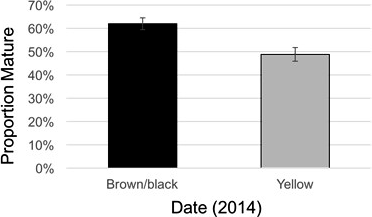
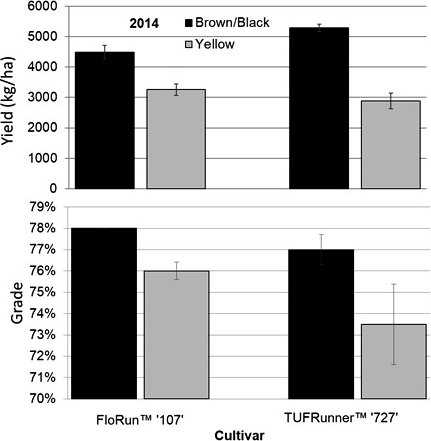
In 2014, the maturity ratio, yield, and grade were all different between maturity classes (Table 4). The maturity ratios in 2014 were 64 (brown/black) and 45 (yellow) for TUFRunner™ '727', and 60 (brown/black) and 52 (yellow) for FloRun™ '107' (Figure 8). In 2015, the maturity ratios were not different between cultivar, maturity class, or their interaction; proportions for each cultivar were 73 (brown/black) and 50 (yellow) for TUFRunner™ '727', and 54 (brown/black) and 56 (yellow) for FloRun™ '107'. In 2014, the brown/black class of each cultivar out-yielded the yellow classes in both years; further, a two-way interaction between cultivar and maturity class indicated that maturity class varied in its impact on yield depending on cultivar (Figure 9). In 2014, the brown/black class of TUFRunner™ '727' graded higher on average than its respective immature class, and the yellow class of FloRun™ '107' graded higher than the yellow class of TUFRunner™ '727' (Figure 9).
Peanut growers and researchers have assumed that seed maturity would likely affect crop emergence such that a delay in emergence would be exhibited for immature (yellow) as opposed to mature (brown/black) seed. However, most have assumed that this difference in emergence would be overcome quickly during the season, the assumption being that plants from yellow seed would 'catch up with those from brown/black seed and that plant performance would not vary through the majority of the season. This study has shown this assumption to be false and that it is critical to pay more attention to quantifying and optimizing seed maturity within the peanut industry. Therefore, the results of this study are important to disproving the catch-up assumption: seed maturity not only has an impact on emergence, but on subsequent life history and performance traits through the remainder of the season. This impact projects its influence even into the next crop, as the maturity and grade of the resulting seed is changed as well. However, given that the extremes measured in this study do not exist in practice, the impact of immature seed in commercial seed lots is likely less than observed in this research.
While seed vigor remains the central focus of the current study, defining this trait as derived from a single measure is complex and probably not appropriate. This research evaluated vigor in from measures of emergence, as well as subsequent evaluations of crop performance from LAI and reflectance. The first crop characteristic studied in this experiment was emergence. The cultivar and its maturity class clearly influenced emergence such that immature seed (yellow) had a delayed daily emergence and lower overall total emergence. This was expected, as the impact that maturity has on germination and emergence has been demonstrated in other crops such as soybean, common vetch, and canola (Miles et al., 1988; Samarah et al., 2003; Elias and Copeland, 2001). Emergence is a trait considered as indicative of vigor and necessarily includes the ability to germinate as well. However, oftentimes germination and vigor can diverge; seed may have the capacity to germinate but not be inherently vigorous. For example, Borowski et al. (1991) in one year found no influence of maturity on germination for two commercial maize (Zea mays L.) hybrid lines, while vigor tests did indicate variability among maturity levels with increased vigor in more mature seed. TeKrony et al. (1984) found that soybean (Glycine max L.) also showed mature seed having greater scores on standard germination tests as well as decreased levels of Phomopsis infection. At the far end of this divergence scale, Ajayi and Fakorede (2000) reported that immature maize seed had high germination rates, but relatively low emergence rates compared to mature seed. The current study results indicate maturity is similarly linked to vigor in peanut: in 2014 and 2015, the brown/black class of each genotype consistently outperformed the yellow class in regard to the overall traits measured.
The performance of each genotype and its maturity classes were also impacted by the climate conditions in each year and some of the variability between years may be linked to these varying weather patterns. In 2014, plots likely received an adequate amount of precipitation early in the season; while in 2015, there was little to no rain for the first three weeks after planting, leading to an extended period before maximum emergence. Planting date to final emergence encompassed 25 days in 2014, and nearly doubled in 2015 to 47 d. The total rainfall recorded for the 2014 growing season was 623 mm, whereas 666 mm was documented in 2015. Similar to a study by Mozingo et al., (1991), these results indicate that the difference associated with the timing and moisture levels between the two years lead to slowed vegetative growth and maturity in 2015 and the plants did not mature as fast as they would otherwise, as seen in 2014.
The LAI patterns overall showed a normal crop development with increasing LAI values up to 5 in 2014 and 2015. However, poor emergence for the yellow class of both cultivars resulted in gaps within the rows as well as delays in canopy development. To eliminate the influence of bare soil or plant gaps in measurements of subsequent crop performance and to concentrate on quantification of delayed development alone, measures of NDVI and LAI were conducted in spans within the rows that had intact stands. Reflective of the slower emergence in the yellow maturity classes for each genotype, this maturity class had reduced LAI development throughout the season in both years. Although the brown/black classes of each genotype were similar for emergence, TUFRunner™ '727' had the highest LAI at the end of the season for both yeas. This was most likely due to its different growth style and canopy structure when compared to FloRun™ '107'. It was observed that TUFRunner™ '727' grew outwards, while FloRun™ '107' grew more upright before the canopy proceeded to expand outwards. It could be expected that LAI would impact assimilation through its direct measure of assimilative area and link back to yield. Thus, the decreased yields in 2014 may be partially explained by the reduction in LAI for that year. However, the link between LAI and yield is not always the case; for example, in soybean, TeKrony et al., (1987) stated that differences in vegetative growth have little impact on yield. This is reflective of the study in 2015 which showed decreased LAI for the yellow class as in 2014, but no difference in yield between maturity classes.
In regard to flower production in both years, the brown/black class produced more flowers than the yellow class for each cultivar during the early to mid-season but then declined; while the yellow class for each cultivar continued to climb until surpassing the brown/black classes late in the season. This shift in production occurred for each cultivar 60 to 70 DAP in both years. These patterns would indicate that the yellow class had the potential to catch up to the black class, at least in flower production. However, with maximum flower production being reached only by 76 DAP on average for the yellow classes, this would result in needing nearly 150 to 160 DAP to reach an optimal harvest maturity level for the yellow class. This would be an unlikely scenario as temperatures would be decreasing and disease pressure increasing as the season progressed into the autumn. The differences in flower production from 2014 to 2015 can be attributed to a healthier canopy in 2015 with low disease pressure (leaf spot).
This study shows that crop performance may be differentially affected by maturity level throughout the season as emergence, LAI, and flower production differed between plants established from mature vs. immature pods. From a field management standpoint, it would be critical to determine maturity level on a whole field basis to predict yield and possibly grade variability influenced by seed maturity. The most logical choice would be utilizing remote sensing technology to help in decisions about harvest timing, vulnerability to stress effects leading possibly to aflatoxin or increased disease pressure, or even segregation of parts of the field with optimal maturity for seed peanut. In the current study, vegetative reflectance was capable of separating the maturity classes for both cultivars during the season. Four out of the five variables measured (NDRE, NDVI, Red-Edge, and NIR) increased throughout the season as the canopy grew and progressed (Figures 4, 5, 6), making these vegetation indices possible candidates for remote sensing of peanut maturity. Red band reflectance was the only variable to decrease from the beginning of the season to the end of the season, although it still showed a separation of maturity classes. There have been a few studies exploring the utility of reflectance indices for determining harvest timing. Robson et al. (2006) was able to utilize hyperspectral reflectance to accurately predict maturity level and harvest date from measurements of individual peanut canopies and then scaled these measurements up to multispectral satellite platforms to successfully predict variation in maturity across a field. In contrast, Danésha et al. (2008) found that NDVI was not a good predictor of maturity, and for one of the Virginia market-type cultivars tested, NC-V11, none of the reflectance patterns were indicative of maturity. While neither of these two studies were assessing differences in seasonal performance among seed from different maturity classes, their application of reflectance patterns was similar and indicates that additional studies need to be conducted examining particular wavelengths and indices that are predictive not only of harvest maturity, but of season long performance variability linked to maturity.
The maturity ratio quantified in this study reflects what was seen in the flower counts; that the maturity of the yellow class was delayed and never reached a comparable level with the black class. In general, the brown/black class typically had a higher percentage of mature pods in both years for TUFRunner™ '727' and in 2014 for FloRun™ '107'. There are critical biochemical and physiological processes that occur during the seed maturation process. For example, when peanut nears physiological maturity, the water content of seeds decreases from about 62 to 30-40%, and both the fat-carbohydrate and protein-carbohydrate ratios increase (Pickett, 1950). What is perhaps most critical about the characteristics of immature peanut seed is a probable lack of desiccation tolerance (Hay and Probert, 1995), a property that could be particularly critical for the storage capability of peanut. Hay and Probert (1995) found that processes occurring even after mass maturity were critical for foxglove because water potential was maintained at levels likely allowing metabolic activity in the seed to continue leading to improved seed longevity. The continued acquisition of characteristics leading to greater seed longevity later in the maturation process were also found in cultivars of barley (Hordeum vulgare L.) and wheat (Triticum aestivum L.) (Ellis and Filho, 1992). Completing the circle, improved desiccation tolerance and seed longevity are likely to contribute to an overall greater seed vigor. Elias and Copeland (2001) documented the increase in vigor for seeds of canola (Brassica napus L.) as they moved through the latter stages of maturity from physiological to harvest maturity. Similarly in maize, Ajayi and Fakorede (2000) found greater emergence rates for seed harvested at later maturity stages than more immature seed.
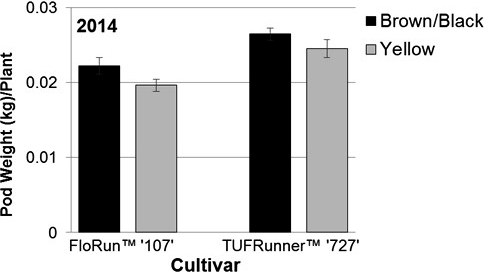
The reduced maturity level in the current study was certainly translated into yield in 2014 (Figure 9) with the brown/black class of each cultivar producing more than the yellow classes. However, this was not the case in 2015 despite the trends being the same with the yellow classes having numerically lower yields on average than the brown/black classes (data not shown). The lack of consistent yield impacts may imply that the differences in yield found in 2014 could have been related to plant density and not to seed maturity after all. However, by calculating the average pod weight per plant for the brown/black and yellow classes of both cultivars, it is clear that the brown/black class produced significantly more per plant than the yellow class (p= 0.0471 MC; p= 0.0012 C) (Figure 10). This difference in production potential per plant supports the finding that the yield differences between maturity classes found in 2014 resulted from inherent differences in plant performance related to maturity rather than differences in stand counts. In fact, there is evidence that immaturity does not always lead to a decrease in yield: TeKrony et al. (1987) reported that in soybean, yields were similar across a wide range of seed sizes and maturities. Maturity may also be reflected by grade as opposed to just the measure of yield. At the buying point, a grower's lot is evaluated for grade and yield when configuring the amount to be paid to the grower (Lamb and Blankenship, 2005). On average, the brown/black class of TUFRunner™ '727' was found to grade higher than its yellow class in both years, while the brown/black class of FloRun™ '107' only graded higher than its yellow class in 2014. This suggests that immature seed may negatively influence grade, while mature seed could be used to improve seed lot grades.
One of the most important findings of this study was the variability between the two cultivars in their level of tolerance to immaturity: for TUFRunner™ '727', immature seed appeared to be more negatively impacted by immaturity by differences noted in emergence, flowering pattern, and yield impact. There is evidence of this variation among genotypes for the impact of maturity is present in other crop species. For example, Bellaloui et al. (2009) noted that for soybean, maturity had differing impact on seed composition for two separate sets of isolines, resulting in positive and negative linear relationships between protein and oil concentration and maturity (respectively) in the isoline Clark, while in the isoline Harosoy, maturity only affected oil concentration. Elias and Copeland (2001) also documented differences among canola cultivars and the acquisition of seed quality characteristics during maturity. This study also noted that seed germination capacity was attained prior to seed vigor and that the two characteristics became similar as seed matured (Elias and Copeland, 2001). Prior knowledge about levels of immaturity risk among different peanut cultivars could lead to improved management techniques that utilized enhanced maturity testing for those cultivars known to be more vulnerable to immaturity.
Conclusion
The main objective of this experiment was to compare critical characteristics of crop performance between mature and immature seed throughout the season. It was hypothesized that the immature yellow class would lag behind the mature brown/black class at the beginning of the season, but eventually match the performance of the mature seed. This study confirmed that the yellow class does indeed lag behind but disproved the catch-up hypothesis because plants from immature seed in most cases were not able to match the performance parameters of plants from the mature brown/black class. The mature brown/black classes of each cultivar were found to be consistently higher than the yellow classes in respect to every performance trait measured, with the exception of two leaf level processes and yield in 2015. The detrimental effects of immaturity were also found to be inconsistent among cultivars. A genotypic difference was quantified, with the yellow class of TUFRunner™ '727' performing lower than the yellow class of FloRun™ '107' as shown by traits such as emergence and the maturity ratio; despite it performing better in regard to flower production.
The data accumulated in this study during 2014 and 2015 indicated that mature seed, regardless of cultivar, performs greater in a field setting than immature seed. The amount of immature seed planted by growers each year could possibly be minimized by maintaining an optimum harvest date when growing for seed peanut. This is because the harvest date chosen by the grower will impact the percentage of immature seed at harvest, which is later moved to the shellers and incorporated into lots of seed saved for the following year's planting. This accurate harvest determination rests quite heavily on the grower because determining seed maturity by the time the crop reaches the sheller is difficult. This was particularly evident by the finding that grade was an inaccurate representation of maturity in this study.
Acknowledgements
The authors would like to acknowledge funding and encouragement from the National Peanut Board, Florida Peanut Producers Association, and Georgia Peanut Commission to continue research as a means to promote grower profitability.
Literature Cited
S.A Ajayi, and M.A.B Fakorede, (2000). Physiological maturity effects on seed quality, seedling vigour and mature plant characteristics of maize in a tropical environment. Seed Science and Technology 28: 301- 319.
Anonymous 2015 Farmers' Stock Peanuts Inspection Instructions United States Department of Agriculture https://www.ams.usda.gov/grades-standards/farmers-stock-peanut-inspection-instructions Accessed online 12 Feb 2019 .
N., Bellaloui, J.R., Smith, J.D., Ray, and A.M Gillen, (2009). Effect of maturity on seed composition in the early soybean production system as measured on near-isogenic soybean lines. Crop Science 49: 608- 620.
J.D Bewley, (1997). Seed germination and dormancy. The Plant Cell 9: 1055- 1066.
A.M., Borowski, V.A., Fritz, and L Waters, (1991). Seed maturity influences germination and vigor of two shrunken-2 sweet corn hybrids. Journal of the American Society for Horticultural Science 116: 401- 404.
S.C., Danésha, D.L., Jordan, L.C., Dharmasri, T.B., Sutton, R.L., Brandenburg, and M.G Burton, (2008). Peanut response to planting date and potential of canopy reflectance as an indicator of pod maturation. Agronomy Journal 100: 376- 380.
S.G., Elias, and L.O Copeland, (2001). Physiological and harvest maturity of canola in relation to seed quality. Agronomy Journal 93: 1054- 1058.
R.H Ellis, and C.P Filho, (1992). The development of seed quality in spring and winter cultivars of barley and wheat. Seed Science Research 2: 9- 15.
W.E Finch-Savage, and G.W Bassel, (2016). Seed vigour and crop establishment: extending performance beyond adaptation. Journal of Experimental Botany 67: 567- 591.
F., Forcella, R.L.B, Arnold, R., Sanchez, and C.M Ghersa, (2000). Modeling seedling emergence. Field Crops Research 67: 123- 139.
D.F Gilman, and O.D Smith, (1977). Internal pericarp color as a subjective maturity index for peanut breeding. Peanut Science 4: 67- 70.
B.W., Halliburton, W.G., Glasser, and J.M Byrne, (1975). An anatomical study of the pericarp of Arachis hypogaea, with special emphasis on the sclereid component. Botanical Gazette 136: 219- 223.
Hampton,
J.G
2000
Producing quality seed: the problem of seed vigour
In:
M.T
McManus,
H.A
Outred, and
K.M
Pollock
(eds.),
Current Research on Seeds in New Zealand, Agronomy Society of New Zealand, Christchurch.
F.R Hay, and R.J Probert, (1995). Seed maturity and the effects of different drying conditions on desiccation tolerance and seed longevity in foxglove (Digitalis purpurea L.). Annals of Botany 76: 639- 647.
D.L Ketring, and P.W Morgan, (1970). Physiology of oil seeds. I. Regulation of dormancy in virginia-type peanut seeds. Plant Physiology 45: 268- 273.
M.C., Lamb, and P.D Blankenship, (2005). The capacity and efficiency of official grade shellers. Peanut Science 32: 132- 135.
D.F., Miles, D.M., TeKrony, D.B Egli, (1988). Changes in viability, germination, and respiration of freshly harvested soybean seed during development. Crop Science 28: 700- 704.
R.W., Mozingo, T.A., Coffelt, and F.S Wright, (1991). The influence of planting and digging dates on yield, value, and grade of four Virginia-type peanut cultivars. Peanut Science 18: 55- 62.
H.E., Pattee, E.B., Johns, J.A., Singleton, and T.H Sanders, (1974). Composition changes of peanut fruit parts during maturation. Peanut Science 1: 57- 62.
T.A Pickett, (1950). Composition of developing peanut seed. Plant Physiology 25: 210- 224.
S., Qun, J., Wang, and B Sun, (2007). Advances on seed vigor physiological and genetic mechanisms. Agricultural Sciences in China 6: 1060- 1066.
J.A., Robertson, G.W., Chapmanand R.L Wilson, (1978). Relation of days after flowering to chemical composition and physiological maturity of sunflower seed. Journal of the American Oil Chemists' Society 55: 266- 269.
A.J., Robson, G Wright, and S Phinn, (2006). Using field spectroscopy and quickbird imagery for the assessment of peanut crop maturity and aflatoxin. Journal of Spatial Science 51: 151- 162.
D.L., Rowland, R.B., Sorensen, C.L., Butts, and W.H Faircloth, (2006). Determination of maturity and degree day indices and their success in predicting peanut maturity. Peanut Science 33: 125- 136.
K.S., Rucker, C.K., Kvien, G., Vellidis, N.S., Hill, and J.K Sharpe, (1991). A visual method of determining maturity of shelled peanuts. Peanut Science 21: 143- 146.
N.H., Samarah, N., Allataifeh, M., Turk, and A.R Tawaha, (2003). Effect of maturity stage on germination and dormancy of fresh and air-dried seeds of bitter vetch (Vicia ervilia L.). New Zealand Journal of Agricultural Research 46: 347- 354.
N.H., Samarah, N., Allataifeh, M., Turk, and A.R Tawaha, (2004). Seed germination and dormancy of fresh and air-dried seeds of common vetch (Vicia sativa L.) harvested at different stages of maturity. Seed Science and Technology 32: 11- 19.
R.U Schenk, (1960). Source of the inner brown color of the peanut shell. Botanical Gazette 121: 191- 192.
J.F., Spears, and G.A Sullivan, (1995). Relationship of hull mesocarp color to seed germination and vigor in large-seeded Virginia-type peanuts. Peanut Science 22: 22- 26.
D.M., TeKrony, T., Bustamam, D.B., Egli, and T.W Pfeiffer, (1987). Effects of soybean seed size, vigor, and maturity on crop performance in row and hill plots. Crop Science 27: 1040- 1045.
D.M., TeKrony, D.B., Egli, J., Balles, L., Tomes, and R.E Stuckey, (1984). Effect of date of harvest maturity on soybean seed quality and Phomopsis sp. seed infection. Crop Science 24: 189- 193.
B.L., Tillman, and D.W Gorbet, (2015). Registration of 'FloRun '107'' peanut. Journal of Plant Registrations 9: 162- 167.
V.K., Toole, W.K., Bailey, and E.H Toole, (1964). Factors influencing dormancy of peanut seeds. Plant Physiology 39: 822- 832.
L., Ventura, M. Doná, A., Macovei, D., Carbonera, A., Buttafava, A., Mondoni, G Rossi, and A Belestrazzi, (2012). Understanding the molecular pathways associated with seed vigor. Plant Physiology and Biochemistry 60: 196- 206.
Williams, E.J (2003) A simple, quick, inexpensive peanut blaster University of Georgia http://santarosa.ifas.ufl.edu/documents/ag_podblaster.pdf Accessed online 30 June 2015.
E.J., Williams, and J.S Drexler, (1981). A non-destructive method for determining peanut pod maturity. Peanut Science 8: 134- 141.
Notes
- Graduate research assistant, Professor, Professor, and Professor, Agronomy Dept., University of Florida, Gainesville, FL 32611; [^]
- Professor, Crop and Soil Sciences Dept., University of Georgia, Tifton, GA 31793; [^]
- Associate Extension Scientist, Entomology and Nematology Dept., University of Florida, Gainesville, FL 32611; [^]
- Associate Professor, Soil and Water Sciences Dept., University of Florida, Gainesville, FL 32611; [^]
- Assistant Professor, Agronomy Dept., National Chiayi University, Chiayi, Taiwan 60004 [^] *Corresponding author's E-mail: ethancarter@ufl.edu
Author Affiliations