Introduction
Peanut (Arachis hypogaea L.) is an important food and cash crop of Zambia. It is grown throughout the country and is rated as the second most widely cultivated crop among small-holder farmers (Central Statistical Office, 2014-2015 Post Harvest Survey). Nevertheless, peanut cultivation is constrained by a number of factors such as low-yielding cultivars, poor quality seed, weed pressure, damage by insects, diseases, poor soil health and drought stress. (Ross and De Klerk, 2012; Mukuka and Shipekesa, 2013). In most cases, a combination of two or more of these factors often accounts for the low productivity
Some of these production constraints such as poor soil health and drought stress are strongly associated with the reportedly high levels of aflatoxin contamination in harvested kernels (Njoroge et al., 2017). Aflatoxins refer to toxic metabolites of toxigenic molds, predominantly Aspergillus flavus and A. parasiticus (Richard and Payne, 2003). Aflatoxin accumulation in humans has been associated with stunting in children, suppression of the immune system, liver cancer and genetic mutations (Williams et al., 2004; Richard, 2008). In Zambia, stunting among children under the age of 5 yr has been associated with exposure to aflatoxin through consumption of contaminated food (Ismail et al., 2014).
Plant stress factors such as drought are linked to both productivity and aflatoxin contamination. Because drought stress is an important factor in the proliferation of Aspergillus spp. and the subsequent pre-harvest aflatoxin accumulation (Cole et al., 1985; Pitt et al., 2013), most pre-harvest interventions are centered on minimizing the effects of drought stress by conserving soil moisture during pod development, the most susceptible growth stage to aflatoxin formation in peanuts (Hill et al., 1983). Therefore, good agricultural practices such as soil water conservation during pod-development (Chalwe et al., 2016) should be encouraged to minimize pre-harvest aflatoxin accumulation. According to Cole et al. (1995) agronomic practices that reduce plant stress to maximize crop growth will in turn decrease pre-harvest aflatoxin accumulation.
Compost is relatively cheap and is a sustainable means of restoring soil health and promoting peanut productivity. Soil health is defined as the ability of the soil to function as a living system (Brady and Weil, 2010). Soil microbial respiration and the availability of soil water for plant use are as such important indicators of soil health. According to Waliyar et al (2008, 2013), farmyard manure was associated with up to 90% reduction of total pre-harvest aflatoxin concentrations in harvested peanuts kernels. The aim of the current study was to evaluate the effects of composted cattle manure on soil factors that relate to the growth of peanuts and the activity of Aspergillus spp. A field experiment was conducted to evaluate the effects of compost on PAW, soil microbial respiration, selected yield components, and pre-harvest aflatoxin accumulation in kernels as key peanut crop performance indicators.
Materials and Methods
Study site
The field experiment was conducted under rain-fed conditions at Kasisi Agricultural Training Centre, located at 15.2498 S, 28.4836 E in Chongwe District, Zambia. The site is located in agro-ecological region IIa, which has mean annual rainfall ranging from 800 to 1000 mm (Soil Survey Branch, 2002). The cropping season is from mid-December to end of April in the following year. The average annual rainfall recorded during the two cropping seasons for this experiment was 905 mm (SASSCAL Weather data, Kenneth Kaunda International Airport). The soil at the site was classified as a chromic luvisol on the Exploratory Soil Map of Zambia (Soil Survey Branch, 1991).
Soil sampling and characterization
A composite soil sample from the study site was constituted by mixing 8 random subsamples collected from the top 20 cm soil layer using an auger. The duly constituted composite sample was air dried, passed through a 2 mm sieve and then characterized using standard laboratory soil analysis procedures. To determine soil texture, the soil was first dispersed using 5% sodium hexametaphosphate (calgon) and then determined the particle size distribution using the hydrometer method (Day, 1965). Bulk density was determined using core samples according to (Blake, 1965). Soil organic matter was determined using the wet-oxidation method (Walkley and Black, 1934). Soil reaction (pH) was determined by equilibrating soil in 0.01 M CaCl2 in a 1: 2.5 soil to solution ratio and then measuring the exchangeable H+ concentration using a pH electrode (Van Reeuwijk, 1992). The exchangeable base cations K+, Mg2+ and Ca2+ were extracted from the soil using 1 M ammonium acetate at a neutral pH of 7 (Van Ranst et al., 1999) and then determined their concentrations using atomic absorption spectrophotometry (AAnalyst 400, PerkinElmer, USA). Available phosphorus was extracted using the Bray 1 extraction solution (Bray and Kurtz, 1945) and then measured the concentration using spectrophotometry at 882 nm. Total nitrogen was determined according to the modified Kjeldahl method (Bremner and Mulvaney, 1982).
Field Experiment
Treatments and experimental Design
Treatments for the field experiment were 6 levels of composted cattle manure (compost) applied to each experimental plot. The rates of application were 0, 4.5, 12.0, 19.5, 27.0 and 34.5 metric tons/ha. The application process involved; uniformly spreading the compost on the soil surface by hand and then mixing it with the soil in the top 10 cm using a hoe in a fine tillage operation. Compost was applied after the first continuous rains of the cropping season and was allowed to settle for one week before planting. Each rate of application was replicated 6 times resulting in 36 experimental plots. Treatments were laid out in a latin square experimental design. Each experimental plot measured 25 m2 with a 1 m border between plots. Thus, the experiment covered an area of about 0.12 ha. The experiment was conducted for two successive cropping seasons; December 2015 to April 2016 and December 2016 to April 2017. These two growing periods are hereafter referred to as 2016 and 2017 seasons, respectively.
Preparation and characterization of compost manure
The compost for this study was prepared conventionally in compost heaps consisting of cattle manure mixed with spoiled hay arranged in windrows. These windrows were moistened when necessary and turned regularly until the materials were decomposed to stable compost. The compost was characterized for selected chemical properties using standard laboratory methods. Some of the properties of the compost included a C/N ratio of 11, pH in 0.01 M CaCl2 of 7.2 and total phosphorus, potassium, calcium and magnesium levels of 1.2, 1.3, 5.4 and 1.6 mg/kg, respectively.
Field preparation, management, harvesting and drying of pods
Soil tillage was done by hand using a hoe. Thereafter, the ploughed area was levelled into a fine seedbed. A red-colored, virginia, bunch type peanut cultivar known as MGV 4, was then planted in rows at the recommended planting spacing of 75 cm x 10 cm, inter-row by intra-row spacing, respectively. Each plot had a total of 6 plant rows giving a net plot of 4 rows after subtracting the 2 border rows. Crop management practices were done manually and included regular weeding whenever weeds appeared and ridging at the on-set of the pegging stage. Major weeding was done four times per growing season. The crop was harvested by digging out plants with a hoe at physiological maturity, 130 d after planting. The pods were stripped from the plant by hand, packaged in polythene bags and then dried in an electric vacuum oven (D-6450 Hanau, Heraeus Instruments, Germany) set at 45 C to a gravimetric moisture content of about 10%, which took about 72 hr of continuous drying.
Determination of pod yield and preparation of laboratory samples
At harvest, the number and weight of mature grain-filled fresh pods per individual plant were determined from 6 representative plants randomly selected from the middle rows of each plot. After weighing, the pods were mixed with the other pods from plants in the middle rows for drying as described above and then shelling upon drying to approximately 10% w/w moisture content. Dry pods were shelled by hand and then temporarily stored in air-tight plastic jars at room temperature. Shelled kernels were sub divided into four equal subsample lots of about 500 g. Laboratory samples were collected by scooping 50 g samples per scoop for 4 times (200 g per 500 g subsample) from each quarter of the lots. A 120 mL plastic cup (1/2 standard cup) was used as a scooper. The samples were shaken before each scoop of sub-sample was taken. The scooped samples were added together and homogenized by shaking. The mixed sample constituted the laboratory sample, which was then ground into fine flour using an ordinary kitchen grinder (LM2211BM, Moulinex, China).
Determination of total aflatoxins in dry kernels
The total concentration of aflatoxins in dried kernels was determined using Neogen Afla Reveal® Q+ aflatoxin kit (Neogen Corporation, USA) within 1 wk after shelling. Ground samples were homogenized by thorough shaking. For each treatment 18 samples (6 replicates by 3 subsamples) each weighing 10 g were assayed for total aflatoxin using 30 ml of 65% ethanol (diluted from 95% ethanol, UN1170, Xilong Scientific Co., Shantou City, China) by shaking on a rotary shaker (ISO-9001-2000, Navyug, India) at a speed of 120 rpm for 3 min. The extract was then filtered through Whitman 42 filter paper. Five hundred uL of the diluent buffer solution was pipetted into a sample dilution cup using a standard 500 μL pipette and then thoroughly mixed with 100 μl of each extract using a clean sterile micro pipette. One hundred μL of the mixture was then transferred into a measuring cup into which one Afla Reveal® Q+ test strip per sample was placed and allowed to develop for 6 min. After 6 min, the aflatoxin content was read by placing the strip into the strip holder of a computer tablet (K011, ASUS Corporation, USA) installed with a mycotoxin reader application.
Determination of soil respiration and PAW
Soil microbial respiration and PAW were determined at 90 d (during pod-development stage) after planting as once-off soil health indicators in each of the two seasons. To determine soil respiration, composite soil samples each weighing 2 kg per plot were collected from 3 to 4 random sampling points in the top 10 cm of soil using a bucket soil auger. The samples were transported in air-tight plastic jars stored in a cooler box filled with ice blocks between jars containing soil samples. To determine carbon evolution due to microbial respiration, the evolved carbon dioxide was trapped in 1 M KOH (Landa and Fang, 1978) and quantified by titrating samples with 1 M HCl.
To determine plant-available-water, 3 undisturbed soil samples per plot were collected from the top 10 cm of soil using standard core rings. The samples were then placed in the pressure plate apparatus to determine water content at field capacity (FC) and permanent wilting point (PWP). The samples were subjected to -10 kPa and -1500 kPa pressure for FC and PWP, respectively.
Data management and statistical analysis
All the data collected in the experiment were managed in Microsoft Excel and SPSS version 20 statistical program. The effects of compost on PAW, soil microbial respiration, peanut pod and kernel yield and aflatoxin content in harvested kernels were determined year by year for the two cropping seasons. Each data set was checked for extreme outliers defined according to SPSS as data points with a magnitude of 3 times the inter-quartile range by first plotting box plots and removing flagged data points. There were no outliers in all the data sets. Scatter plots were used to establish whether or not there were linear relationships between the independent and the outcome variables. The central limit theorem was applied to assume normal distribution since all data sets had more than 30 observations. Simple linear regression analysis was then performed to estimate the response to each level of treatment.
Results and Discussion
Soil characteristics
The soil at the research site was a strongly acidic (pH = 4.22) sandy loam (19 % clay, 11.4 % silt and 69.6 % sand), with very low available phosphorus (0.56 mg/kg), low soil organic matter content (0.7 %) and low exchangeable calcium (0.06 cmol/kg). No measures were taken to correct neither the acidity nor nutrient deficiencies for the sole purpose of evaluating groundnut performance on marginal soils (control) common to local farmers.
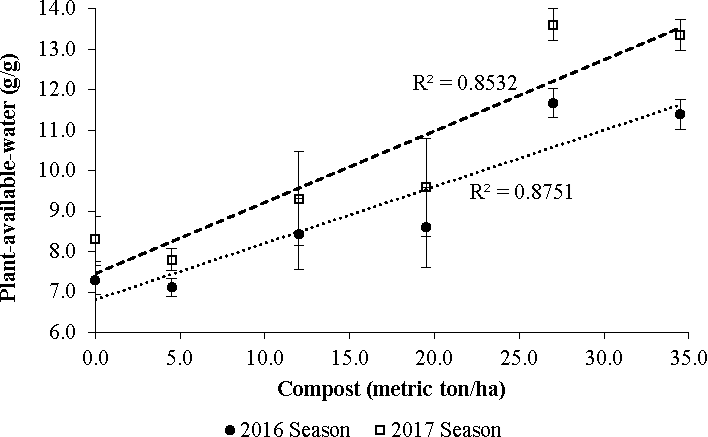
Effects of compost manure on plant-available-water
The plant-available-water (PAW) in soil treated with compost manure increased with increasing levels of compost manure applied (R2=0.86) (Fig. 1). Plant-available-water is the fraction of soil water between field capacity (FC) and the permanent wilting point (PWP). This is the water that is available for plant uptake after natural drainage (Brady and Weil, 2010). The increase in PAW can be attributed to the increase in soil moisture content at FC (-10 kPa suction pressure) at each level of compost applied to the soil (Fig. 2), while the moisture content at PWP (-15 000 kPa) did not vary with the level of compost manure (P > 0.05).
The role of organic matter on soil moisture retention capacity relates to its role on aggregate stability and soil structure. Aggregate stability relates to the capacity of the soil aggregate to maintain its physical structure/shape when subjected to a given pressure while soil structure relates to the distribution of the solid phase and the pore space (liquid and gaseous phases) in a given mass of soil. Stable aggregates tend to have more pore space and are able to hold more water than weak aggregates. According to Yazdanpanah et al. (2015) organic amendments were associated with higher aggregate stability and soil microbial respiration. In a related study, Ramos (2017) reported an improvement in structure and moisture holding capacity at different suction pressures with the addition of composted cattle manure at a rate of 40 Mg per ha. It is worth noting that the capacity of organic amendments to increase the soil's moisture holding capacity partly depends on the type of organic matter. Organic matter with high quantities of hydrophobic components such as humic substances tends to promote aggregate stability and hence good soil structure (Piccolo and Mbangwu, 1999). In this context, a more humified organic material such as compost would be more appropriate for moisture retention purposes than fresh organic wastes such as green manures and raw animal manure.
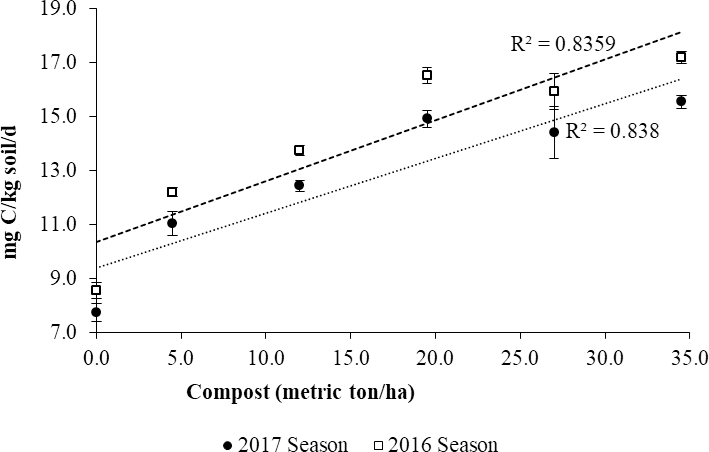
Effects of compost manure on soil microbial respiration
There was an increase in microbial respiration (R2=0.84) with increasing levels of compost (Fig. 3). The increase can be attributed to the addition of soil microorganisms contained in the manure and the activation of native soil microorganisms in the soil through the supply of nutrients. Soil microbial respiration is an important indicator of soil microbial activity, which also relates to the decomposition of soil organic matter.
Results similar to our study have been reported by several authors. For instance, Chaoui et al. (2002) evaluated the effects of earthworm casts and compost manure on nitrogen mineralization rates, soil microbial biomass and microbial respiration revealed that compost manure was superior in all the three aspects of the study. In a recent study, Yazdanpanah et al. (2015), observed higher mineralization rates and soil microbial respiration with the addition of organic matter. The same study also reported increased soil aggregate stability, which contribute to improved hydraulic conductivity and soil aeration. An active soil biota requires a good supply of nutrients and oxygen. A study on a black soil of Northeast China revealed that compost significantly contributed to the buildup of soil organic matter, increased electrical conductivity and the availability of major plant nutrients nitrogen, potassium, phosphorus and calcium (Yang et al., 2017). A 4-yr application of cattle manure in a commercial vineyard in Italy was associated with higher soil organic matter content and microbial biomass (Gaiottia et al., 2017).
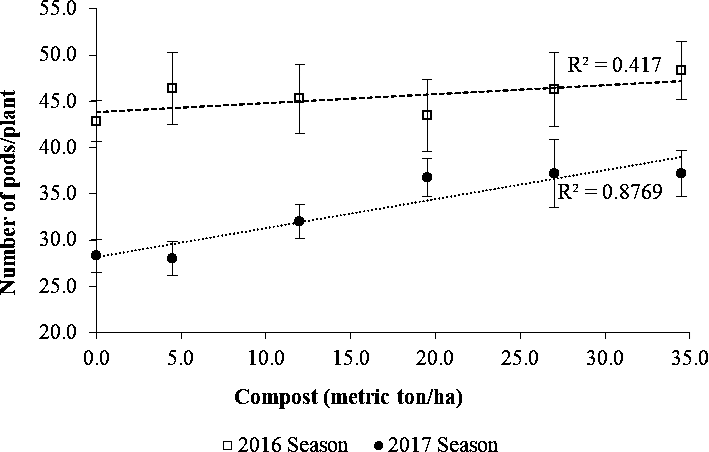
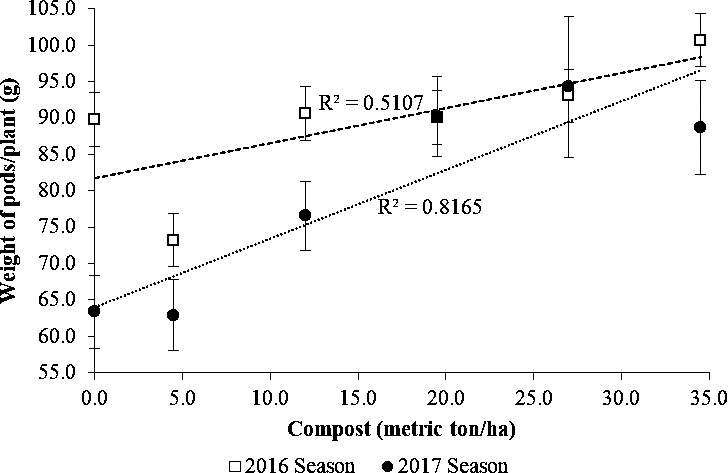
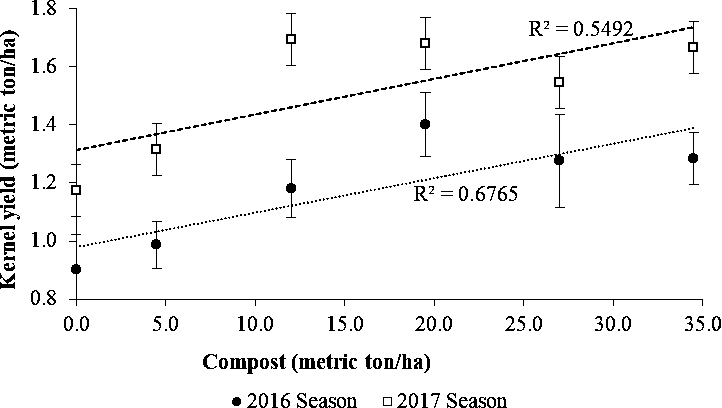
Effects of compost manure on pod and kernel yield
There were significant increases in pod (R2=0.65) and kernel yield (R2=0.61) with an increase in compost in each cropping season (Fig. 4, 5 and 6). However, a comparison of yield data between the two seasons showed that both pod and kernel yields in 2016 tended to be lower than in the 2017 season especially at lower levels of compost. As shown in Fig. 6, kernel yield was lower than the potential yield of 1.5 ton/ha for the MGV 4 cultivar across all levels of compost in 2016 while this was achieved in the second season at the rate 12 metric ton/ha and higher. The lower yield in the 2016 season could be attributed to poor rainfall distribution of 1 rainfall event per 2.5 days and lower total rainfall of 592.2 mm during the growing period compared with better rainfall distribution of 1 rainfall event per 1.6 days and more total rainfall of 916 mm received during the growing period in the 2017 season (SASSCAL weather data, Kenneth Kaunda International Airport).
Amending soils low in organic matter with compost is one of the sustainable means of improving soil fertility and crop productivity. Compost manure can enhance the growth of crops either by supplying plant nutrients or enhancing the supply and recycling of plant nutrients (Brady and Weil, 2010). The compost used in this study contained significant quantities of macro-nutrients in plant-available form and may have contributed to the improved yield. In a study in Senegal, the use of compost manure was associated with an increase in the effective cation exchange capacity and plant nutrients K and Mg and higher pod yield of groundnuts in amended soils (McClinton and Diop, 2005). A higher cation exchange capacity in a soil increases its nutrient retention capacity. At the same time, the increased charge enhances soil water retention, which contributes to nutrient availability by bringing dissolved elements into solution (Brady and Weil, 2010).
Effects of compost manure on pre-harvest aflatoxin contamination
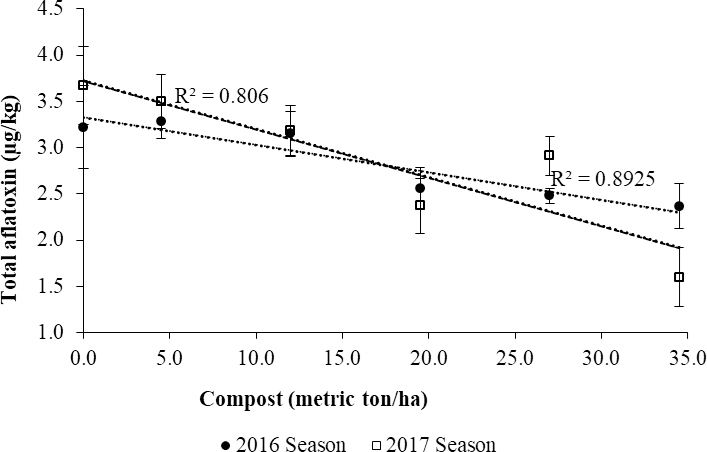
Total aflatoxin concentrations in kernels decreased with increasing levels of compost (Fig. 7). This result can be attributed to the observed increments in PAW (Fig 1) and soil microbial respiration (Fig. 3). According the R-square values of the fitted regression lines, the reduction in mean total aflatoxin concentrations was higher in the second cropping season (R2 = 0.89), than in the first one (R2 = 0.81).
The soil moisture content of the soil during pod-development in peanuts is not only crucial for minimizing pod colonization by Aspergillus flavus, but also promotes the formation of sound kernels. On the contrary, soil moisture deficit during pod development is strongly associated with higher levels of aflatoxin contamination in groundnut kernels (Hill et al., 1983; Cole et al., 1985; Pitt et al., 2014; Sibakwe et al., 2017). Although A. flavus and A. parasiticus, the two major toxigenic fungi are soil-borne and saprophytic in nature (Richard and Payne, 2003), their capacity to produce aflatoxins is influenced by the soil moisture status and temperature. Being xerophitic in nature, the Aspergilli species become active and produce aflatoxins under severe moisture deficits often associated with elevated soil temperature (Bowen and Hagan, 2015).
Adequate soil moisture is important to minimise soil temperature, an equally important factor influencing pre-harvest aflatoxin contamination in groundnuts (Hill et al., 1983; Dorner et al., 1989; Bowen and Hagan, 2015). According to Hill et al. (1983) soil temperatures lower than 25 C in the geocarposphere did not encourage aflatoxin contamination even under low soil moisture conditions. As such, enhancing soil moisture retention capacity using organic amendments, which often keeps the soil temperatures low, seems to override the effect of the high presence of Aspergillus flavus on aflatoxin production in soils that are rich in organic matter (Zablotowicz et al., 2007).
An increase in soil microbial respiration is indicative of improved microbial activity in the soil. Compost manure inoculates the soil with microorganisms and adds nutrients to the soil (Gaiottia et al., 2017). Adequate moisture and nutrients are essential for microbial activity. As reported by Cole et al., (1985) high microbial activity minimized pod colonization by the Aspergillus flavus, the causal agent for aflatoxin contamination in kernels. These results are consisted with studies by Waliyar et al. (2013) who reported 42% reduction in total aflatoxin levels following an application of farmyard manure at the rate of 2.5 metric ton/ha.
It is noteworthy that although there were significant differences in aflatoxin levels, the observed concentrations were markedly low for the warm climatic region in which the experiment was conducted. Soil temperature data from a weather station situated within 8 km southeast of the study site indicated average soil temperatures of 23.2 C in the last 6 wk to harvesting of the peanuts coupled with a fairly distributed average annual rainfall of 905 mm during the two growing seasons (SASSCAL Weather data, Kenneth Kaunda International Airport). The weather conditions were more favourable for plant growth and development than for pre-harvest aflatoxin development and hence the low levels of aflatoxin observed across treatments. According to Cole et al. (1995) agronomic practices that reduce plant stress and meant to maximise crop growth would in turn decrease mycotoxin occurrence. In both planting seasons, the field was free of weeds and diseases throughout the growing period and there were no physical signs of water stress such as wilting during the pod development stage of the crop.
Conclusions
Results from this study demonstrate the potential of compost manure to increase soil respiration, PAW, pod and kernel yield, and minimize aflatoxin development in peanut kernels at field level. There were significant increases in soil microbial respiration (R2 = 0.84) and PAW (R2 = 0.86) with increasing levels of compost. An improvement in soil microbial respiration is an important indicator for improved soil health. In terms of crop performance, compost had a strong positive correlation with kernel yield (R2 = 0.61) and a strong negative correlation with aflatoxin content in kernels (R2 = 0.85). With the potential yield achieved at the rate of 12 metric ton/ha, the study showed that compost can be used by local farmers for better crop performance. Additionally, the decline in aflatoxin levels with increasing levels of compost is an improvement in the quality of kernels. We therefore recommend the use of compost for better yield and lower pre-harvest aflatoxin content.
Acknowledgements
This study was funded by the U.S. Agency for International Development, under the terms of Award No. AID-ECG-A-00-07-0001 to The University of Georgia as management entity for the U.S. Feed the Future Innovation Lab on Peanut Productivity and Mycotoxin Control. We thank Idah Ngoma (University of Zambia) and Emmanuel Mulenga (Kasisi Agricultural Training Centre) for the technical support. We also acknowledge the Administration at Kasisi Agricultural Training Centre for availing the study site.
Literature Cited
G.R Blake (1965). Bulk density. In C.A Black et al. (ed.) Methods of soil analysis, Part 1. Agronomy 9: 374- 390.
K.L Bowenand A.K Hagan (2015). Temperature and moisture conditions that affect aflatoxin contamination of peanuts. Peanut Science 42: 121- 127
Brady N.C and R.R Weil 2010 Elements of the nature and properties of soils. 3rd ed Prentice Hall, Upper Saddle River, New Jersey, USA.
R.H Brayand L.T Kurtz (1945). Determination of total, organic and available phosphorus in soils. Soil Science 59: 39- 45.
Bremner D.C and J.M Mulvaney 1982 Total Nitrogen In Page A.L., Miller R.H., Keeney R.D (eds) Methods of soil analysis part 2: Chemical and microbiological properties, 2nd Ed American Society of Agronomy, 9 (2), Madison, Wisconsin, USA.
H., Chalwe A.M Mweetwa, O.I Lungu, E Phiri, S.M.C Njorogeand R.L Bradenburg (2016). Reducing pre-harvest aflatoxin content in groundnuts through soil water management. RUFORUM Working Document Series , 14: 921- 926.
H.I., Chaoui L.M Zibilskeand T Ohno (2003). Effects of earthworm casts and compost on soil microbial activity and plant nutrient availability. Soil Biology and Biochemistry 35: 295- 302.
Cole R.J., J.W Dornerand C.C Holbrook 1995 Advances in mycotoxin elimination and resistance Pages 456- 474 In Advances in Peanut Science American Peanut Research and Education Society, Stillwater OK.
R.J., Cole T.H Sanders, R.A Hill, and P.D Blankenship (1985). Mean geocarposphere temperatures that induce preharvest aflatoxin contamination of peanuts under drought stress. Mycopathologia 91: 41- 46.
Central Statistical Office 2014-2015 Post Harvest Survey Report Republic of Zambia, Central Statistical Office, P. O. Box 31908, Lusaka- Zambia https://www.zamstats.gov.zm/index.php/publications/.../12-agriculture?...2014-2015.
P.R Day (1965). Particle fractionation and particle-size analysis. In C.A Black et al. (ed.) Methods of soil analysis, Part 1. Agronomy 9: 545- 567.
J.W., Dorner R.J Cole, T.H Sandersand P.D Blankenship (1989). Interrelationship of kernel water activity, soil temperature, maturity, and phytoalexin production in preharvest aflatoxin contamination of drought-stressed peanuts. Mycopathologia 105: 117- 128.
F., Gaiottia P Marcuzzoa, N Belfiorea, L Lovata, F Fornasierband D Tomasia (2017). Influence of compost addition on soil properties, root growth and vine performances of Vitis vinifera cv Cabernet sauvignon. Scientia Horticulturae 225: 88- 95.
R.A., Hill P.D Blankenship, R.J Coleand T.H Sanders (1983). Effects of soil moisture and temperature on pre-harvest invasion of peanuts by the Aspergillus flavus group and subsequent aflatoxin development. Applied and Environmental Microbiology 45: 628- 633.
Ismail S., J Shindano, D.B Nyirenda, R Bandyopadhyayand J Akello 2014 Does exposure to aflatoxin constrain efforts to reduce stunting in Zambia? In J Harris et al . (eds) IDS special collection ( p 34- 38). Brighton: Institute of Development Studies Brighton BN1 9RE, UK http://hdl.handle.net/10568/87933.
E.R Landaand S.C Fang (1978). Effect of mercuric chloride on carbon mineralization in soils. Plant and Soil 49: 179- 183.
N.C McClintockand A.M Diop (2005). Soil Fertility Management and Compost Use in Senegal's Peanut Basin. International Journal of Agricultural Sustainability 3: 79- 91.
Mukuka R.M and A Shipekesa 2013 Value chain analysis of the groundnuts sector in the Eastern province of Zambia Indaba Agricultural Policy Research Institute, Working paper No. 78 Indaba Agricultural Policy Research Institute, Plot No. 26A, Middleway Road, Kabulonga, Postnet 99 Kabulonga, Lusaka, Zambia www.bdsknowledge.org/dyn/bds/docs/.../GroundnutsSector_EasternZambia_IAPRI.p.
S.M.C., Njoroge L., Matumba K., Kanenga M., Siambi F., Waliyar J., Maruwo N., Machinjiri E. S Monyo (2017). Aflatoxin B1 levels in groundnut products from local markets in Zambia. Mycotoxin Research and Springer , 33: 113- 119
A Piccoloand J.S.C Mbagwu (1999). Role of hydrophobic components of soil organic matter in soil aggregate stability. Soil Science Society of America Journal 63: 1801- 1810.
J.I., Pitt M.H Taniwakiand M.B Cole (2013). Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement of Food Safety Objectives. Food Control 32: 205- 215.
M.C Ramos (2017). Effects of compost amendment on the available soil water and grapeyield in vineyards planted after land leveling. Agricultural Water Management 191: 67- 76.
Richard J.L and G.A Payne 2003 Mycotoxins: Risks in plant, animal, and human systems Task Force Report No. 139 Council for Agricultural Science and Technology Ames, Iowa, USA
J.L Richard (2008). Discovery of aflatoxins and significant historical features. : Toxin Reviews 27: 171- 201
S Rossand M De Klerk (2012). Groundnut Value Chain and Marketing Assessment in Eastern Province of Zambia. Conservation Farming Unit. 23B Twin Palm road, Kabulonga, Lusaka, Zambia, http://www.conservationagriculture.org.
SASSCAL-Southern African Science Service for Climate Change and Adaptive Land Management WeatherNet, University of Zambia, Lusaka. Weather data accessed online on 7th September, 2017 http://www.sasscalweathernet.org.
C.B., Sibakwe T.K Donga, S.M.C Njoroge, W.A.B Msuku, W.G Mhango, R.L Brandenburgand D.L Jordan (2017). The role of drought stress on aflatoxin contamination in groundnuts (Arachis hypogaea L.) and Aspergillus flavus population in the soil. Modern Agricultural Science and Technology 3: 22- 29.
Soil Survey Branch, 1991 Exploratory Soil Map of Zambia. Republic of Zambia, Ministry of Agriculture Mt. Makulu Research Station, Department of Agriculture, Private Bag 7, Chilanga, Zambia.
Soil Survey Branch, 2002 Agro-ecological Map of Zambia. Republic of Zambia, Ministry of Agriculture, Mt Makulu Research Station, Department of Agriculture, Private Bag 7, Chilanga, Zambia.
Van Ranst, E., M Verloo, A Demeyerand J.M Pauwels 1999 Manual for the Soil Chemistry and Fertility Laboratory. International Training Centre for Post-graduate Soil Scientists, Krijgslaan 281/58, B-9000, Gent, Belgium (ISBN 90-76603-01-4).
L.P Van Reeuwijk (2002). Procedures for soil analysis. 6th ed. International Soil Reference and Information Center (ISRIC), (Technical Paper/ISRIC. ISSN 0923-3792: no 9) P.O. Box 353, 6700 AJ Wageningen, The Netherlands, https://www.isric.org/sites/default/files/ISRIC_TechPap09.pdf
Waliyar F., M Osiru, H.K Sudiniand S.M.C Njoroge 2013 -Reducing Aflatoxins in Groundnuts through Integrated Management and Biocontrol In L Unnevehr and D Grace (Eds) Aflatoxins: Finding solutions for improved food safety International Food Policy Research Institute, 2033 K Street, NW, Washington, DC 20006-1002, USA.
Waliyar F., P.L Kumar, A Traoré, B.R Ntare, B Diarraand O Kodio 2008 Pre- and postharvest management of aflatoxin contamination in peanuts In Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade ( p 209- 218) Cromwell Press, Trowbridge, United Kingdom www.cabi.org. ISBN-13 : 978 1 84593 082 0.
A Walkleyand I.A Black (1934). An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Science 37: 29- 38.
J.H., Williams T.D Phillips, P.E Jolly, J.K Stiles, C.M Jollyand D Aggarwal (2004). Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. American Journal of Clinical Nutrition 80: 1106- 1122.
W., Yang Y Guo, X Wang, C Chen, Y Hu, L Cheng, S Guand X Xu (2017). Temporal variations of soil microbial community under compost addition in black soil of Northeast China. Applied Soil Ecology 121: 214- 222.
N., Yazdanpanah M Mahmoodabadiand A Cerdà (2015). The impact of organic amendments on soil hydrology, structure and microbial respiration in semiarid lands. Geoderma 266: 5- 65.
R.M., Zablotowicz, H.K Abbasand M.A Locke (2007). Population ecology of Aspergillus flavus associated with Mississippi Delta soils. Food Additives and Contaminants 24: 1102- 1108.
Notes
- University of Zambia, Department of Soil Science, P. O. Box 32379, Lusaka, Zambia [^]
- International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Chitedze Agricultural Research Station, P. O. Box 1096, Lilongwe, Malawi [^]
- North Carolina State University, Department of Entomology and Plant Pathology, Box 7613, Raleigh, North Carolina, USA [^]
- North Carolina State University, Department of Crop and Soil Sciences, Box 7620, Raleigh, North Carolina, USA. [^] *Corresponding author's E-mail: hendrix.chalwe@unza.zm
Author Affiliations