Introduction
Peanut (Arachis hypogaea L.) is an economically important crop in the Virginia-Carolina region of the U.S. that is harvested from over 70,000 ha annually with a value of over $150 million (www.nass.usda.gov). The predominant market type grown in this region is the Virginia-type which is desirable for the gourmet market due to its large pod and kernel size. Breeding programs have developed numerous Virginia-type cultivars adapted to the region that have desirable agronomic characteristics as well as good yield and quality (Branch et al., 2014). In the 1980s, the high-oleic fatty acid seed oil trait was identified in peanut (Norden et al., 1987) and since then several high-oleic peanut cultivars have been released (Isleib et al., 2015). Peanut processers prefer high-oleic peanuts due to their increased shelf life (Mozingo, 2004), so incorporating this trait into new cultivars is an aim of many peanut breeding programs (Isleib et al., 2015). However, despite the advantages of the high-oleic trait for the peanut processing industry, the agronomic performance of these new cultivars must meet or exceed that of currently grown cultivars that do not have the high-oleic trait.
A major constraint to peanut production in terms of both yield and profitability is the susceptibility of the peanut crop to several fungal diseases. The most common foliar diseases of peanut in the Virginia-Carolina region include early leaf spot, caused by Cercospora arachidicola S. Hori, and late leaf spot, caused by Cercosporidium personatum (Berk. & M. A. Curtis) Deighton. The most wide-spread and economically important soilborne diseases of peanut in the region are Cylindrocladium black rot (CBR), caused by Cylindrocladium parasiticum Crous, Wingefield, and Alfenas; Sclerotinia blight, caused by Sclerotinia minor Jagger; and stem rot, caused by Sclerotium rolfsii Sacc. It is estimated that fungal diseases account for 5-10% peanut yield loss on an annual basis (Mehl, 2014), and management of these diseases to minimize crop losses typically requires costly fungicide inputs. A variety of effective fungicide chemistries are available for control of these diseases, but if disease pressure is high and a peanut cultivar is susceptible to disease, four or more fungicide applications may be required to minimize yield loss (Woodward et al., 2013). Thus, disease management costs can greatly reduce the profitability of peanut production.
Incorporation of disease resistance traits into peanut cultivars is an important part of peanut breeding programs (Wynne et al., 1991; Chapin et al., 2010). As new cultivars with partial disease resistance are developed and released, the need for costly fungicide inputs to produce acceptable yields has decreased (Monfort et al., 2004; Cantonwine et al, 2006). The Virginia-type cultivar Bailey was released in 2011 (Isleib et al., 2011), and the partial resistance of this cultivar to diseases including late leaf spot, CBR, and Sclerotinia blight, as well as good yield and quality characteristics, has made this the cultivar of choice for growers in the Virginia-Carolina region. Along with longer rotations out of peanut, the partial disease resistance of Bailey has reduced the need for soil fumigation to control CBR in the region (Phipps, 1990), and CBR can typically be managed with fungicides applied to the seed furrow at planting (Brenneman et al., 2011). Use of resistant cultivars is an effective management approach for minimizing yield losses to disease, but when disease pressure is high, it must be combined with chemical control methods with efficacy against target organisms.
Integrated disease management, which incorporates cultivar resistance, good cultural practices, and judicious use of fungicides, has been demonstrated to reduce overall fungicide costs and increase the profitability of peanut production (Phipps, 1993; Cantonwine et al, 2006; Woodward et al., 2010, 2014). Weather-based disease advisory programs have reduced the number of fungicide sprays required for control of peanut diseases, thereby reducing total fungicide costs in peanut production, and leaf spot and Sclerotinia advisories for peanut are frequently utilized by peanut producers in the Virginia-Carolina region (Cu and Phipps, 1993; Phipps et al., 1997; Langston et al., 2002). Some studies have found that reduced-input fungicide programs can effectively manage diseases in cultivars with partial disease resistance or tolerance and result in overall greater profitability compared to standard, high-input fungicide programs (Monfort et al., 2004; Cantonwine et al, 2006; Woodward et al., 2014). Ultimately, weather-based fungicide advisories may need to be revised to account for the higher levels of disease resistance in newer peanut cultivars, and there may be potential to further reduce fungicide inputs in peanut production.
New peanut cultivars with improved disease resistance and desirable agronomic traits (e.g. high-oleic) are continuously being developed. Cultivar and fungicide selection impact disease and resulting peanut yields, but the extent to which partial disease resistance reduces the need for overall fungicide inputs needs to be evaluated as new cultivars are released. The objectives of this study were to 1) evaluate Virginia-type peanut cultivars for disease resistance/tolerance, yield, and quality when grown under different fungicide programs and in locations varying in disease pressure; 2) compare currently grown Virginia-type cultivars without the high-oleic trait to recently released high-oleic cultivars; and 3) assess the overall profitability of different fungicide programs for cultivars varying in disease resistance under variable amounts of disease pressure.
Materials and Methods
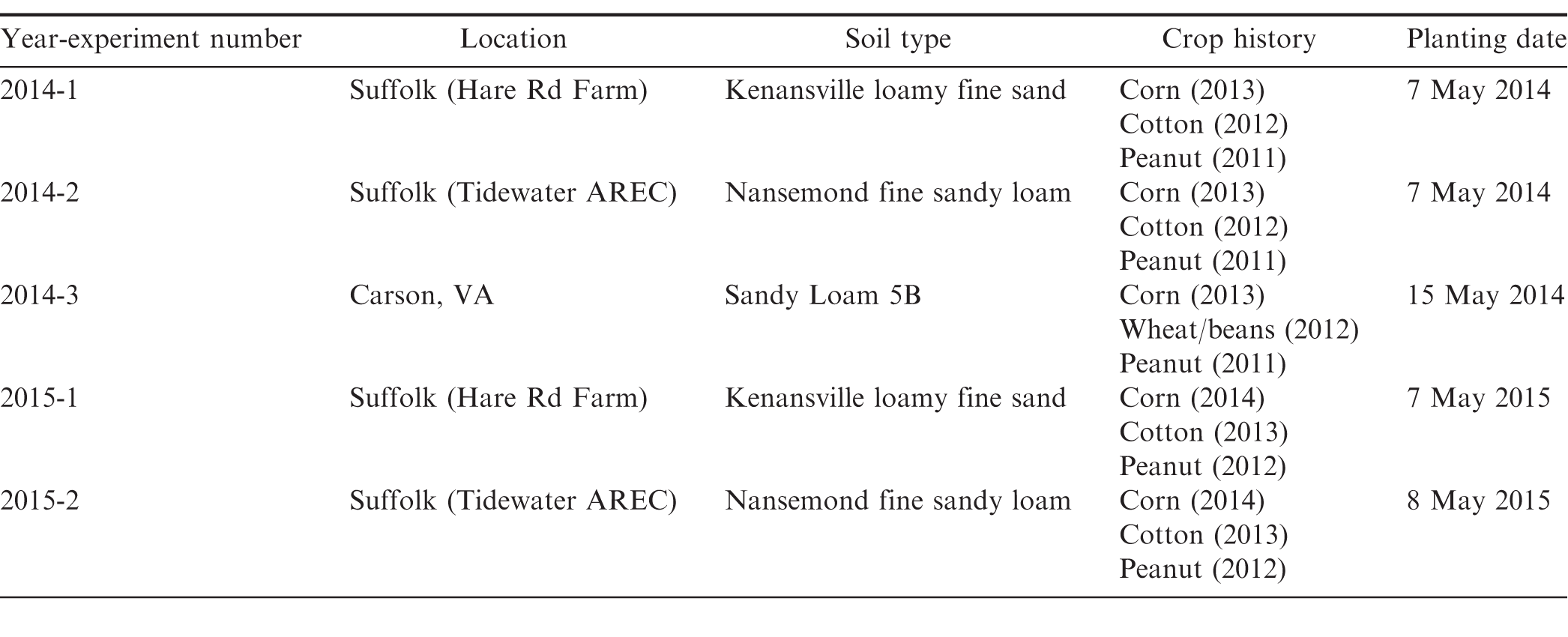
Experiments evaluating different peanut cultivar-fungicide program combinations were conducted at a total of five field locations in southeast Virginia during the 2014 (3 locations) and 2015 (2 locations) growing seasons. Selected fields varied in prior disease pressure, rotation, and soil type. Location, soil type, crop history, and planting date are indicated in Table 1. Since the main objective of this work was to evaluate the impact of fungal pathogens on cultivars varying in disease susceptibility and not to assess yield potential across field environments, experimental fields were primarily selected based on variable levels of disease pressure rather than factors such as soil type and cropping history.
Four Virginia-type cultivars were selected for evaluation based on seed availability, agronomic characteristics, and known levels of disease resistance/susceptibility. These included disease susceptible (CHAMPS) and tolerant (Bailey) cultivars without the high-oleic trait and two recently released high-oleic cultivars, Sullivan and Wynne (Isleib et al., 2015). Bailey (Isleib et al., 2011) is a widely grown cultivar with desirable agronomic traits and a disease resistance package including partial resistance to early and late leaf spots, CBR, and Sclerotinia blight. CHAMPS (Mozingo et al., 2006), which is highly susceptible to these three diseases, was included as a disease susceptible check. Seed with a standard seed treatment (Dynasty [azoxystrobin + fludioxonil + mefenoxam], Syngenta Crop Protection, Greensboro, NC) was planted at a rate of 13 seed m-1 of row on the dates indicated in Table 1.
Three different fungicide programs plus a no fungicide (untreated) control were evaluated in each of the experiments. All fungicide programs included foliar applications for leaf spot control beginning at R3 (beginning pod) and thereafter according to the Virginia Peanut Leaf Spot Advisory Program (http://webipm.ento.vt.edu/infonet) until R7 (beginning maturity); in both years this resulted in a total of four leaf spot fungicide applications. Growth stages were determined using the definitions described by Boote (1982). The first three leaf spot fungicide applications consisted of 0.11 kg ai ha-1 of prothioconazole + tebuconazole (Provost 433SC, Bayer CropScience, Research Triangle Park, NC) and the fourth and final application was 1.26 kg ai ha-1 of chlorothalonil (Bravo Weather Stik, Syngenta Crop Protection, Greensboro, NC). The first fungicide program included applications for leaf spot control only. The second and third fungicide programs included the leaf spot applications indicated above with the addition of a fungicide to control CBR and Sclerotinia blight, respectively. The second fungicide program included 0.20 ai ha-1 prothioconazole (Proline 480SC, Bayer CropScience, Research Triangle Park, NC) applied to the seed furrow at planting with a microtube in a volume of 46.8 L ha-1 for CBR suppression. The third fungicide program included a foliar application of 0.58 ai ha-1 fluazinam (Omega 500F, Syngenta Crop Protection, Greensboro, NC) for Sclerotinia blight control when disease risk was high according to the Virginia Sclerotinia Blight Advisory (http://webipm.ento.vt.edu/infonet). All foliar applications were made with three, D323 nozzles per row delivering 139 L ha-1. Production practices other than fungicide applications were based on the Virginia Cooperative Extension peanut production guidelines and recommendations (Balota et al., 2015). Cultivars and fungicide programs were arranged in a split-plot design with fungicide treatments in 16-row main plots and cultivars in 4-row subplots. Treatments were arranged in four randomized complete blocks separated by 3-m alleys between blocks. Plots consisted of 10.7-m rows spaced 0.9 m apart. Environmental data were collected with a Spectrum Watchdog weather station located near the experimental fields at the Tidewater Research Farm in Suffolk, VA.
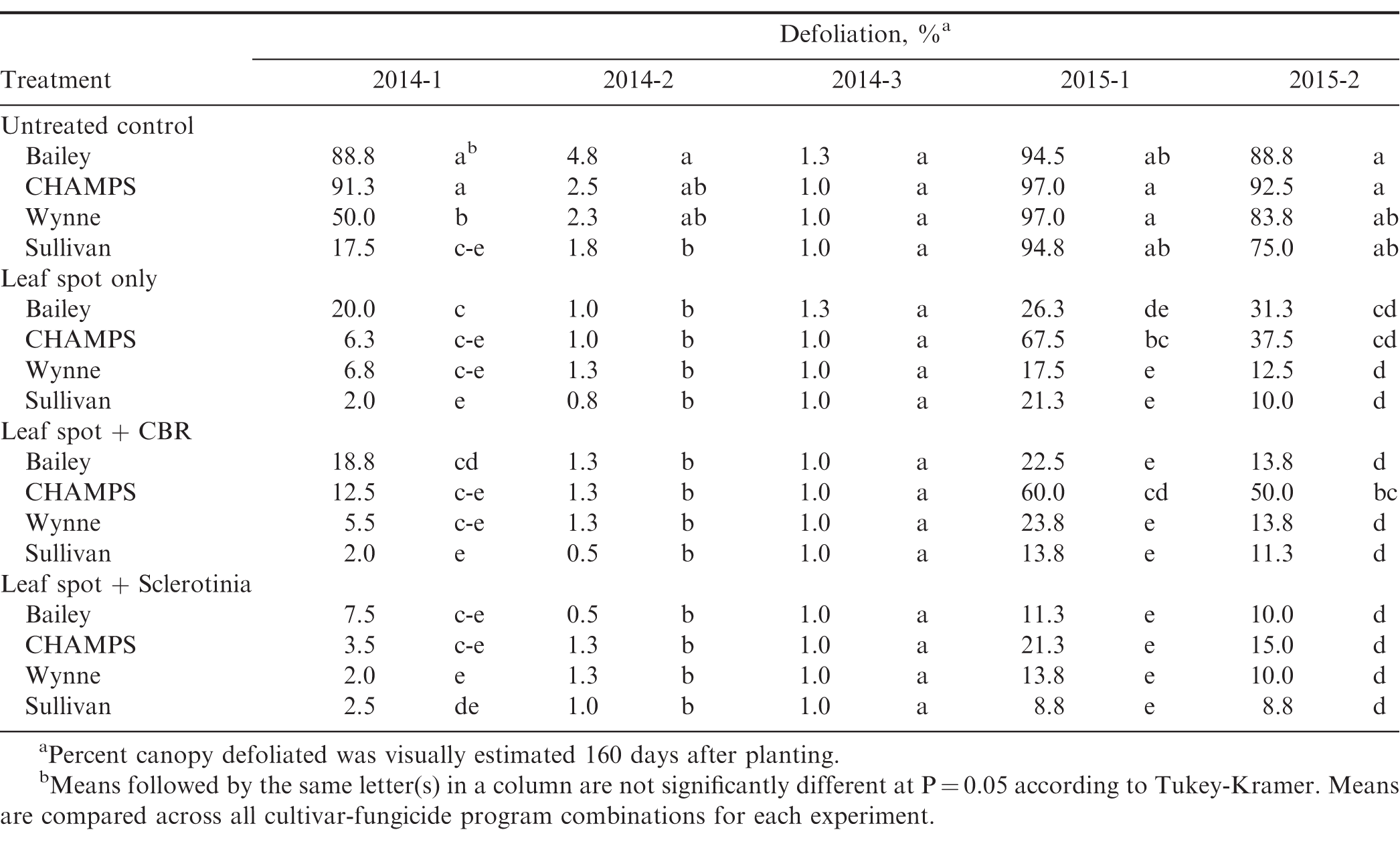
Diseases were monitored throughout the growing season, and following the first observation of disease, plots were evaluated for soilborne disease incidence, leaf spot severity, and defoliation approximately every two weeks. In both years, disease did not develop until late in the season, so only the final disease ratings described below were analyzed. Final ratings for soilborne diseases and leaf spot were made at or just prior to the R7 (beginning maturity) growth stage or approximately 130 days after planting. Ratings for defoliation resulting from leaf spot were made just prior to harvest or approximately 160 days after planting. Soilborne disease incidence was rated by counting infection points in the two center rows of each plot or a total of 21 meters of row. An infection point was identified as a plant with symptoms and/or signs of disease and 15 cm on either side of that plant. Soilborne disease incidence was converted to a percentage of the row with disease by dividing the number of infection points by the total row length and multiplying by 100 percent. Leaf spot severity was evaluated as a visual estimate of the percent of the total leaflets in the canopy with one or more leaf spot lesions. Defoliation was rated as a visual estimate of the total percent of the canopy with leaf drop due to early or late leaf spot.
Peanuts were dug in October approximately 160 days after planting. Pod blasting was conducted on a subsample of each cultivar prior to harvest to assess maturity. On average, CHAMPS matures five days earlier than the other cultivars, but all cultivars were similarly mature based on pod color when harvested in this study. Inverted plants were allowed to dry in windrows and harvested one week later using conventional peanut harvest equipment. Pod yield was measured from each plot and weights were adjusted to 7% seed moisture. A 500 g pod sample from each plot was evaluated for grade characteristics including foreign material (FM), loose shelled kernels (LSK), extra-large kernels (ELK), sound splits (SS), sound mature kernels (SMK) and damaged kernels (DK). Gross value was calculated from the federal formula which is based on a combination of grade factors determined by the Federal Inspection Service guidelines (USDA-AMS, 2015), pod yield, and the current loan rate. Extra-large kernels are kernels that do not pass a 25.4 × 8.5 mm screen, and there is a 0.039 ¢/kg premium received for each % ELK. Sound mature kernels are kernels that do not pass a 25.4 × 6 mm screen, and there is a 0.5 ¢/kg premium received for each % SMK. Sound splits are undamaged kernel halves and there is no penalty for up to 4 % SS. Damaged kernels (DK) include kernels that are inedible due to decay, mold, insect damage, sprouting, freeze damage, or skin discoloration, and when DK exceeds 2.4% seed is considered segregation 2 and growers receive 35% of the gross value for that portion of the peanut crop. The cost of each of the three fungicide programs evaluated was estimated based on chemical product prices from pesticide dealers in southeast Virginia. The cost of each fungicide program was subtracted from the gross value of the peanuts harvested from each plot to determine the net crop value.
Data were analysed using PROC GLIMMIX of SAS v. 9.2 (SAS Institute, Cary, NC) to evaluate the effects of cultivar and fungicide program on disease, yield, grade factors, and crop value. Initial analyses indicated variation among experiments, so each individual site-year was analysed separately. Percentage data were arcsine transformed prior to analysis, but untransformed means are presented. The level of significance was set to 𝜶 = 0.05 and the Tukey-Kramer test was used to compare treatments. When no interaction between cultivar and fungicide program was detected, only main effects were compared. When cultivar by fungicide interactions were detected, comparisons were made between all cultivar-fungicide combinations.
Results and Discussion
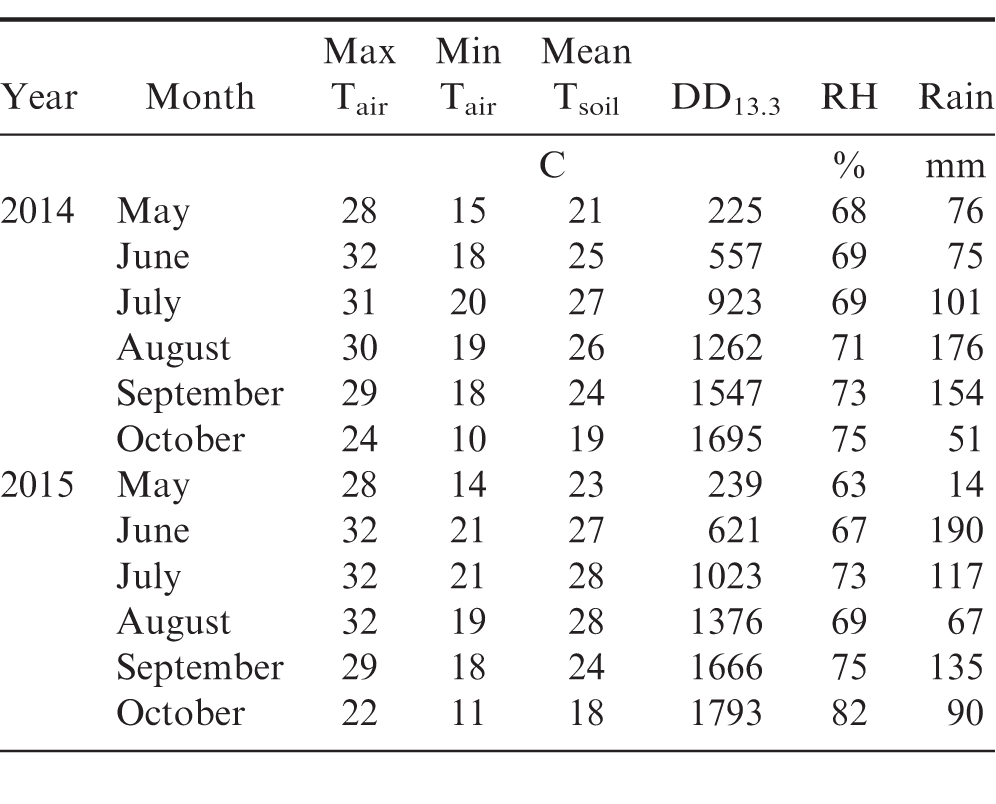
Disease pressure and yield varied among locations and between growing seasons. Variation between years may have been due in part to different weather conditions in 2014 compared to 2015 (Table 2). Though overall rainfall during the growing season (May through October) was similar in both years, 2015 had a wetter early summer and drier mid to late summer compared to 2014. Though peanuts are not typically irrigated in commercial fields in Virginia, experiment 2015-1 was irrigated to encourage disease development when conditions became dry mid-season. Approximately 2.5 cm irrigation was applied to the field on 27 July, 31 July, and 1 September. Monthly average maximum and minimum temperatures for June through August were 1 to 5 degrees C higher in 2015 compared to 2014, resulting in faster accumulation of peanut heat units in 2015. Higher temperatures in 2015 compared to 2014 may account for the overall lower incidence of Sclerotinia blight in 2015, which is favored by moderate to cool temperatures (Langston et al., 2002), and the absence of stem rot in 2014, which is favoured by warm to hot temperatures (Rideout et al., 2008).
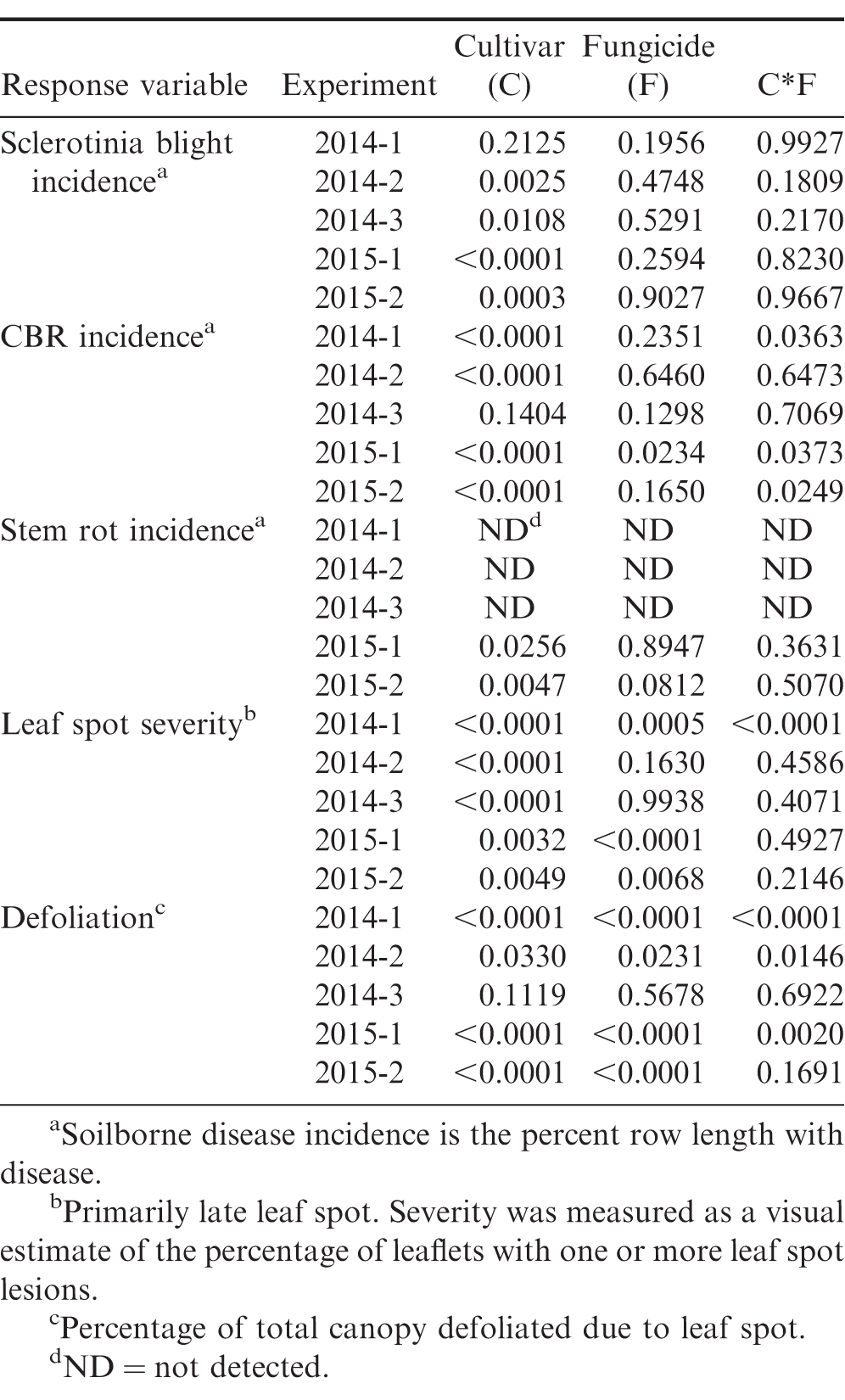
Incidence and severity of diseases varied among cultivars in all five experiments (Table 3, 4, 5). Sclerotinia blight, CBR, and late leaf spot were observed in both years, but stem rot was detected only in 2015. A fungicide program specifically targeting stem rot control was not included in the experimental design since this disease is not always observed in Virginia, but the Provost in the leaf spot program and the Omega applied for Sclerotinia blight do have activity against S. rolfsii. In addition, Provost suppresses CBR, so the leaf spot program likely provided some control of soilborne diseases. A fungicide program consisting of only chlorothalonil (Bravo) would have exclusively targeted control of leaf spots, but the Provost/Bravo leaf spot program was selected since it reflects a standard fungicide program for the region. Though all fungicide programs in the study should have provided some control of soilborne diseases, Sclerotinia blight, CBR, and stem rot incidence did not vary among fungicide programs with the exception of one experiment in 2015 (2015-1). In this experiment, untreated CHAMPS had a lower incidence of CBR compared to fungicide treated CHAMPS, but these results may have been confounded by the high levels of leaf spot and defoliation in the untreated plots of this cultivar (data not shown). A lack of differences in disease incidence with fungicide treatments targeting CBR and Sclerotinia blight were likely due to difficulties with season-long control of these soilborne diseases (Woodward et al., 2013). The fungicides used in this study have been demonstrated to effectively suppress Sclerotinia and CBR (Brenneman et al., 2011; Langston et al., 2002), but if disease pressure is high, signs and symptoms of the disease will still be observed in the field. However, suppression of diseases during pod development can protect yields and application of the fungicides may result in a yield benefit even if late-season disease incidence is high (Phipps et al., 2010). Furthermore, data represent the frequency with which signs and symptoms of disease were observed in the plot and do not necessarily represent the overall severity of the disease during the growing season.
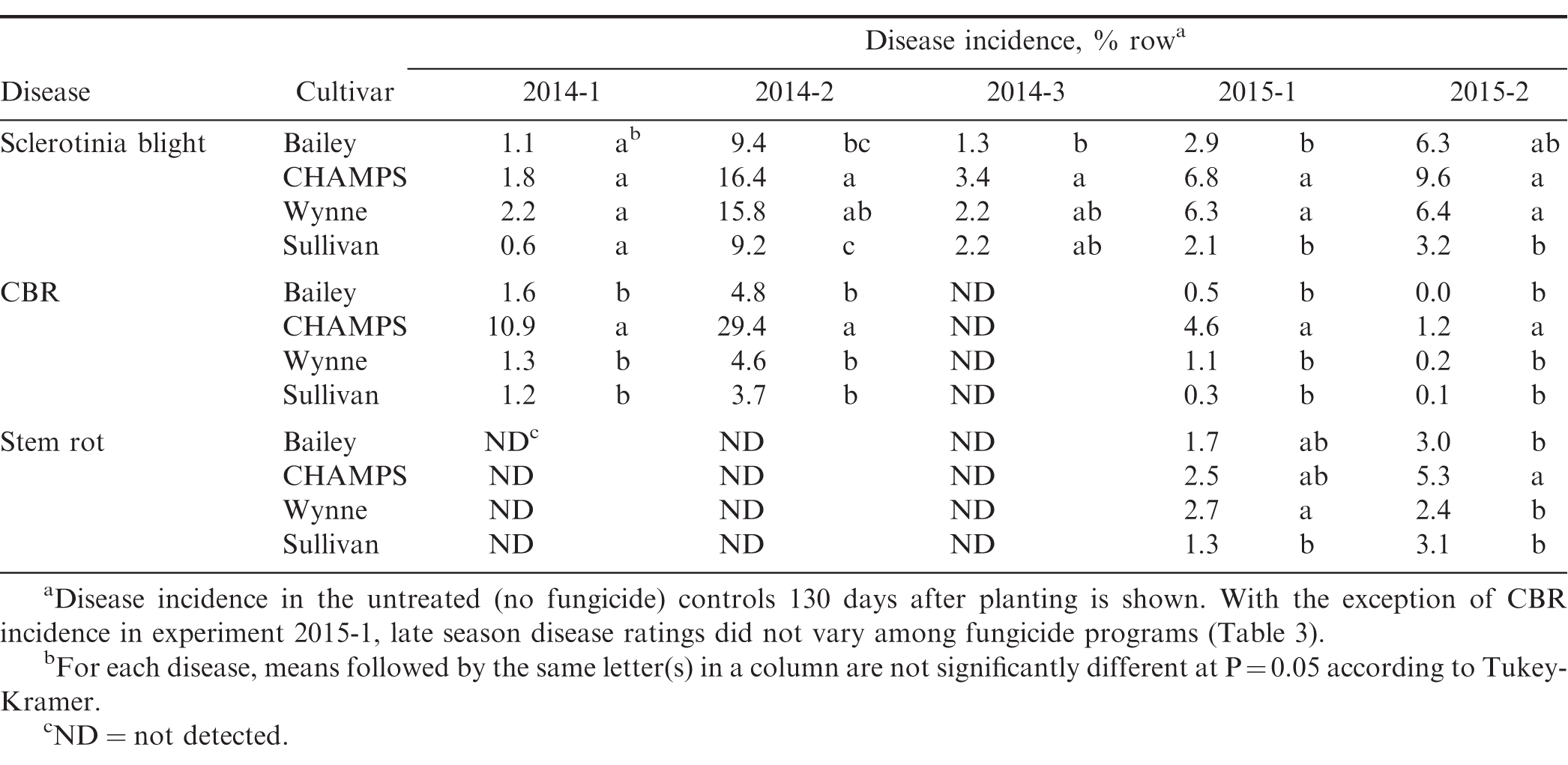
Mean Sclerotinia blight incidence in the untreated plots ranged from 1.8 to 19.7% and varied significantly among cultivars in all but one experiment (2014-1) where incidence was low (Table 3, 4). CHAMPS and Wynne had the highest and Sullivan and Bailey the lowest incidence of Sclerotinia blight supporting previous observations of both Sullivan and Bailey being partially resistant to the disease. Mean CBR incidence in untreated plots ranged from <1% to 33% of the plot row length, and CHAMPS had significantly higher symptoms of CBR than the other three cultivars except in experiment 2014-3 where overall incidence was less than 1% (Table 3, 4). A major weakness of the CHAMPS cultivar is its high susceptibility to CBR as demonstrated in this study, but other Virginia-types including Bailey and the high-oleic cultivars Wynne and Sullivan are able to yield well in CBR infested fields. Southern stem rot was present in 2015, but overall incidence was low and ranged from 1.5 to 6.2% in untreated plots (Table 4).
Though trace amounts of early leaf spot were observed, late leaf spot was the predominant foliar disease at all locations in both years. Leaf spot severity and defoliation due to leaf spot infection varied among cultivars, fungicide programs, and in some experiments there was a significant cultivar by fungicide program interaction (Table 3, 5). In experiments where leaf spot severity exceeded 5%, disease was significantly reduced by all fungicide programs (P<0.05, data not shown). In three of the five experiments, the interaction between cultivar and fungicide program impacted defoliation due to late leaf spot. In experiment 2014-3, only a trace amount of leaf spot and defoliation was detected so data are not presented; mean defoliation for all cultivar-fungicide program combinations in the other four experiments are presented in Table 5. Overall, Bailey and CHAMPS had higher levels of defoliation compared to Wynne and Sullivan. For Sullivan and Wynne, defoliation was reduced similarly among the three fungicide programs, but for Bailey and CHAMPS, the leaf spot + Sclerotinia program provided additional leaf spot control over the leaf spot program alone (Table 5). Though not considered a leaf spot product, this indicates the Omega (fluazinam) applied for Sclerotinia blight was providing additional leaf spot control.
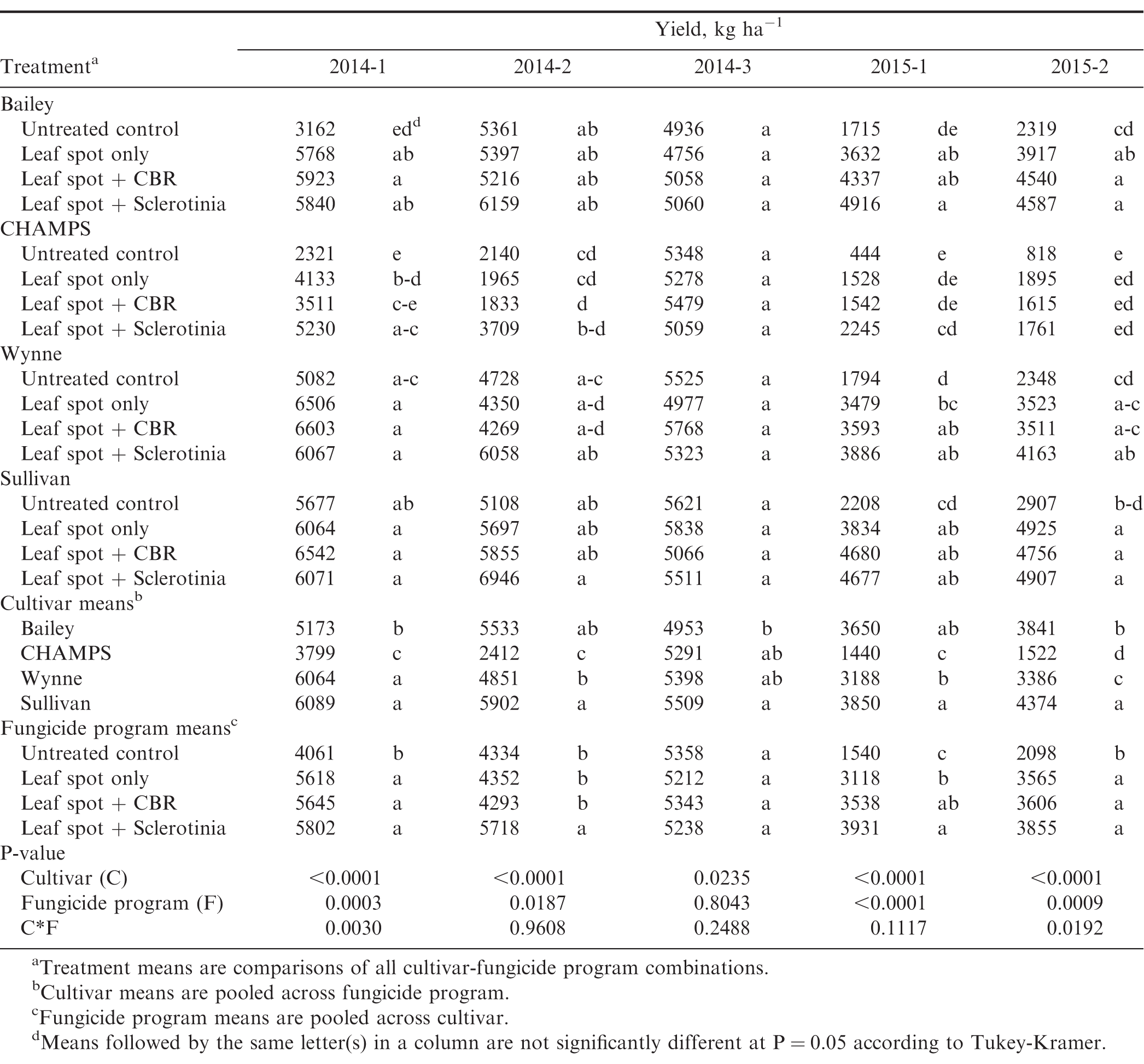
Peanut yield varied among cultivars and fungicide programs (Table 6). Sullivan had the numerically highest crop value in all five experiments, and CHAMPS had the lowest crop value in all experiments except 2014-3 where disease pressure was low. Wynne had higher yields than Bailey when Sclerotinia blight pressure was low (experiments 2014-1 and 2014-3), but Bailey yielded higher than Wynne when Sclerotinia blight incidence was high (Table 4, 6). When both Sclerotinia blight and CBR pressure were low, CHAMPS had higher yield and crop value than Bailey which is partially resistant to both diseases. This demonstrates the importance of disease susceptibility in determining whether or not a cultivar can achieve its yield potential in a particular environment. Fungicide applications increased yield in all experiments except 2014-3 where disease pressure was low. In experiment 2015-1, there was not a cultivar by fungicide program interaction, and for all cultivars the leaf spot + Sclerotinia fungicide program resulted in the highest yields. Experiments 2014-1 and 2015-2 had a significant cultivar by fungicide program interaction. In experiment 2014-1, the leaf spot + Sclerotinia program resulted in the highest yield For CHAMPS, but all three fungicide programs resulted in similar yields for the other cultivars. In experiment 2015-2, yield increases were similar with all fungicide programs for CHAMPS and Sullivan. However, the CBR and Sclerotinia fungicide programs had higher yields compared to the leaf spot program alone for Bailey, and the Sclerotinia program had higher yields compared to the other two fungicide programs for Wynne (Table 6).
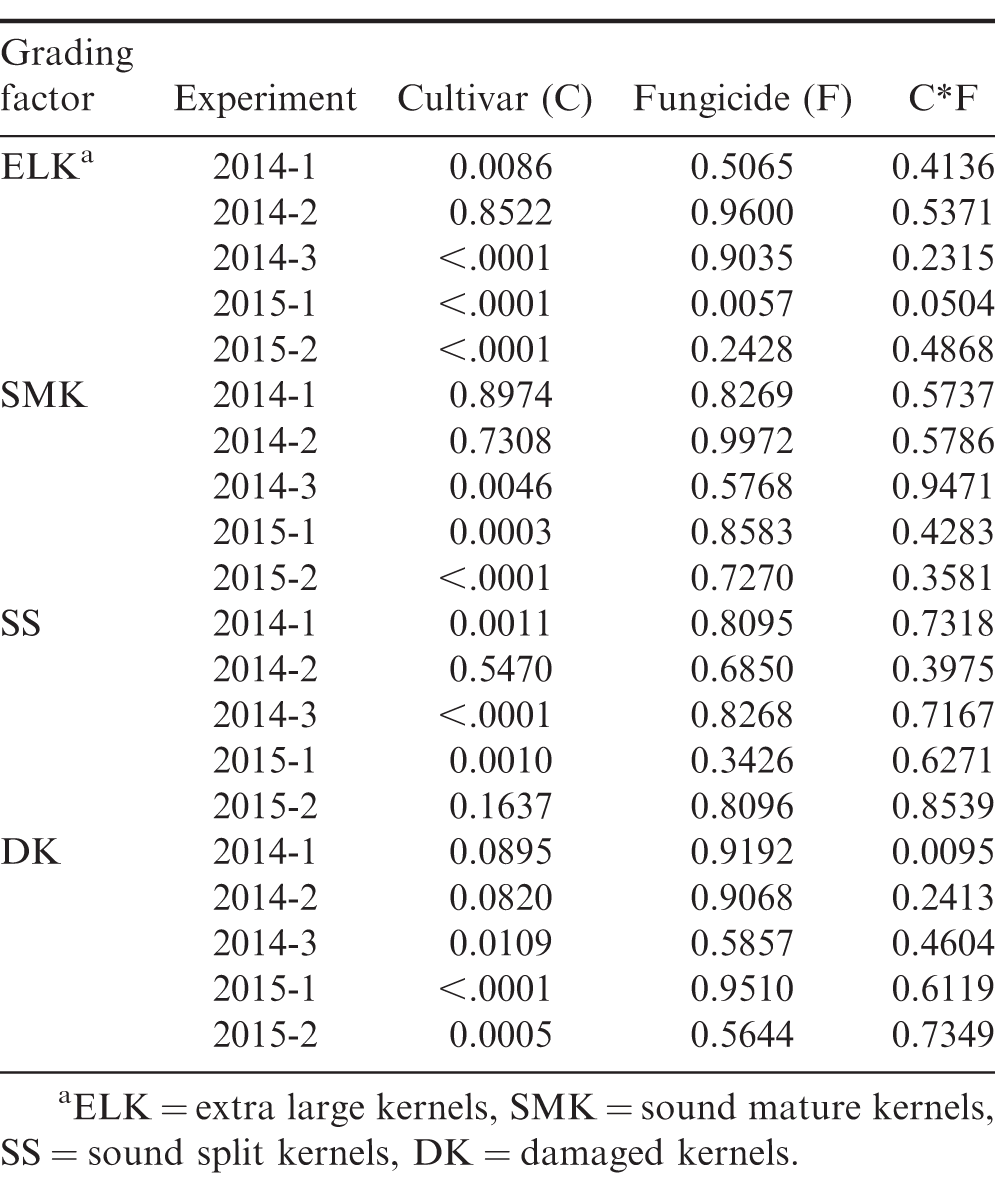
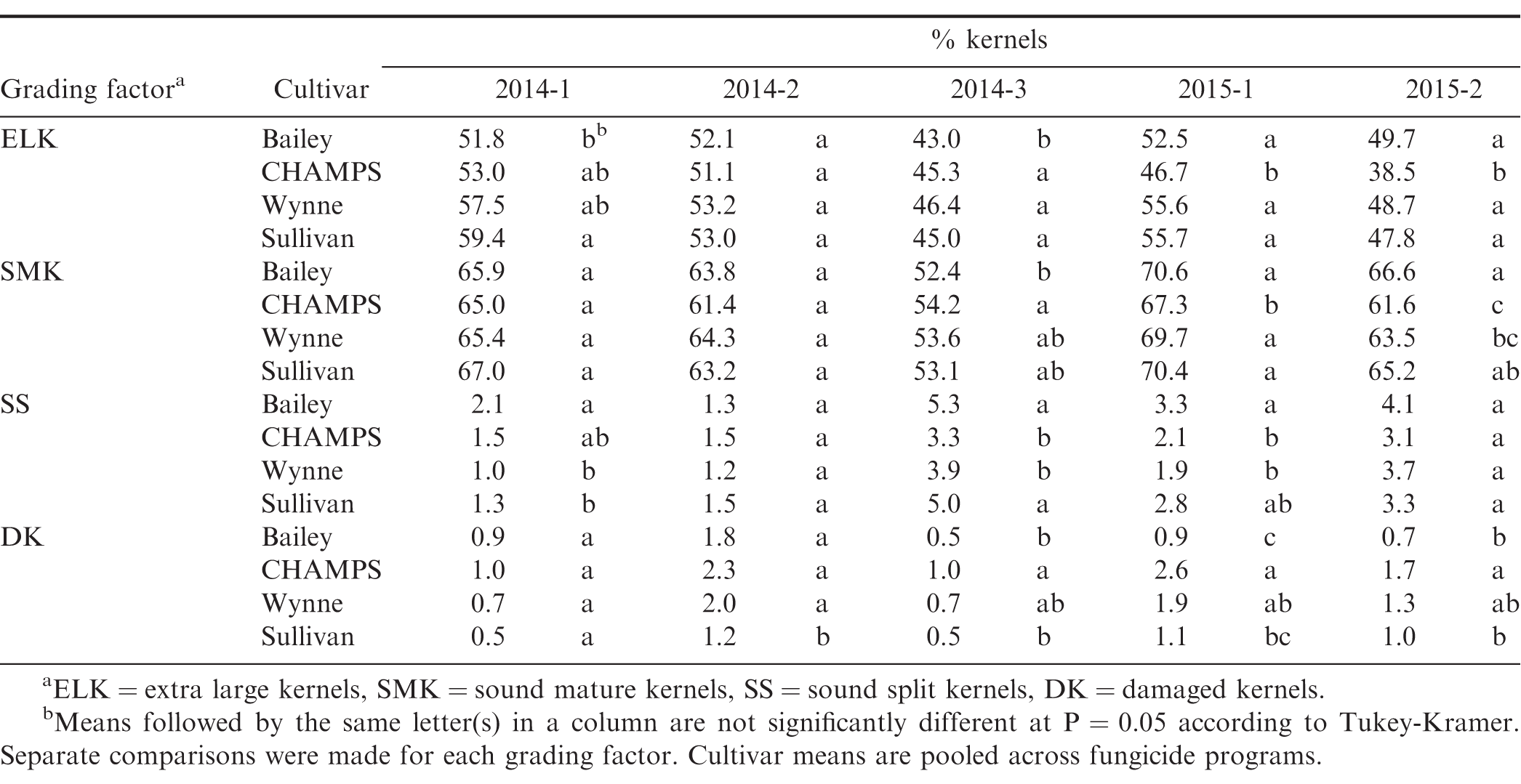
Though yield was impacted by fungicide program in four of the five experiments, with only one exception, fungicide program did not influence measured grade factors (P>0.05), but all grade factors differed among cultivars in three or more of the experiments (Table 7, 8). In experiment 2015-1, the percentage of extra-large kernels (ELK) was significantly greater in the treatments that included a leaf spot fungicide program compared to the untreated control (53.9% vs 48.8%). This is likely due to the relatively high leaf spot pressure at this location in 2015 (Table 5) which reduced the ability of the crop to provide photosynthates to the developing pods (Nutter and Littrell, 1996). Since fungicide program did not impact grading factors in the other experiments, only comparisons among cultivars are presented in Table 8. Percentages of ELK and SMK varied among experiments, but Sullivan consistently had one of the highest ELK and SMK percentages among the four cultivars. A premium is paid for both ELK and SMK, so cultivars that consistently rate high for these grade factors are desirable. Sullivan also had the overall lowest percentage of damage kernels, but in one of the experiments, the sound split kernels exceeded 4% which results in a price penalty. CHAMPS had the overall highest percentage of damaged kernels due to seed decay from CBR and other fungi. When damaged kernels exceed 2.4%, the seed is considered segregation 2 and the grower receives only 35% of the value for the crop.
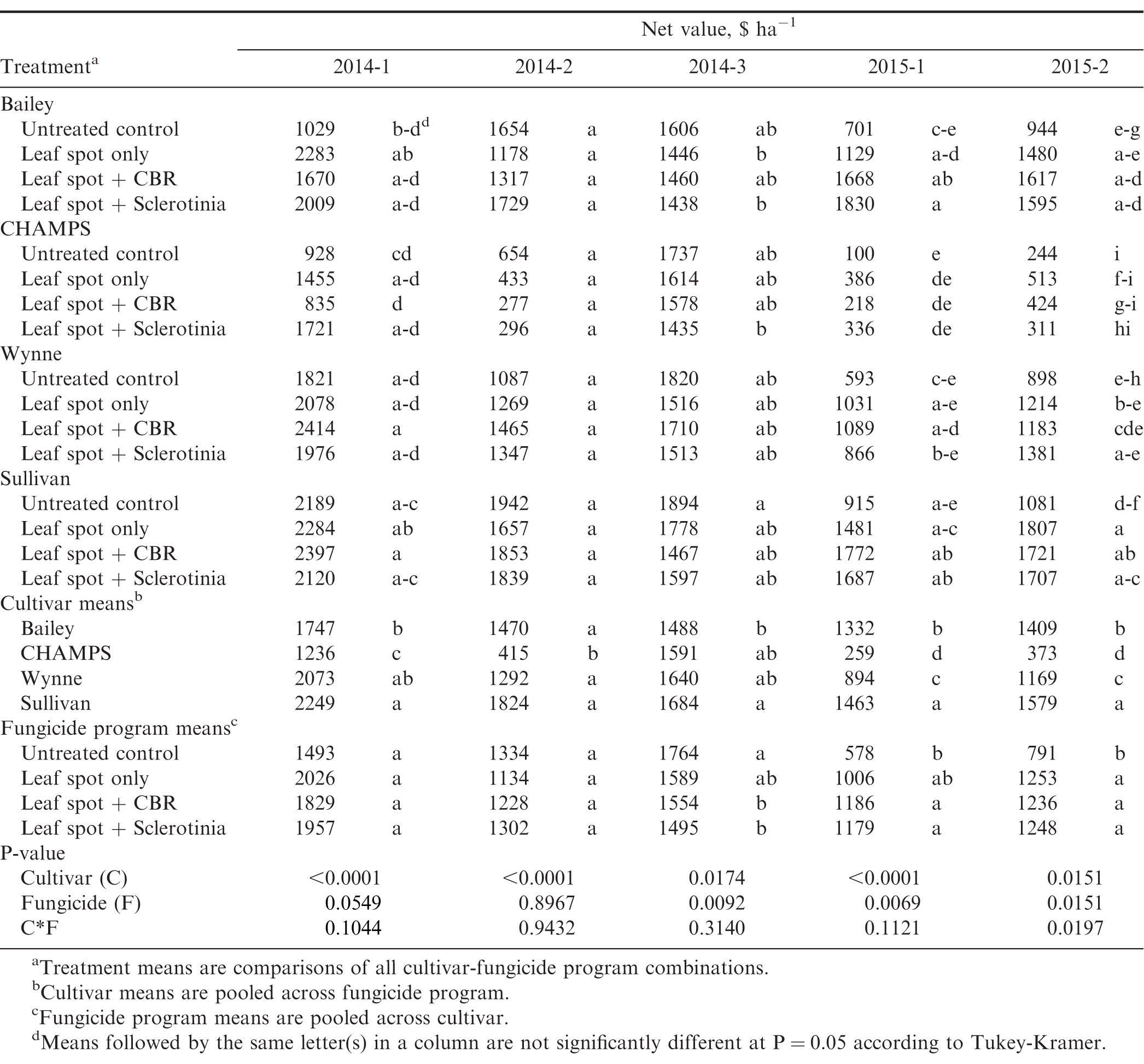
The three fungicide programs evaluated in this study varied in overall cost. Based on the current price of the chemicals and not taking into account any other costs associated with fungicide application, the cost of the leaf spot only, leaf spot + CBR, and leaf spot + Sclerotinia fungicide programs are estimated to be $126, $183, and $229 per treated hectare, respectively. Table 9 gives the net value of the crop for each cultivar-fungicide program combination calculated by subtracting the fungicide program costs from the gross crop values based on yield and grading factors. The influence of cultivar and fungicide program on net crop value varied among experiments. In experiments 2014-1 and 2014-2, net crop value was similar among fungicide programs including the untreated control. This indicates that fungicide programs increased the value of the crop but only enough to offset the cost of the fungicide treatments. In experiment 2014-3 the untreated control provided a greater value than the treatments including fungicide applications. In this field, incidence and severity of all diseases was low, so fungicide applications did not increase yields (Table 6) and resulted in an average loss of $175 to $269 per hectare (Table 9). In both of the experiments conducted in 2015 where late leaf spot pressure was high, the leaf spot fungicide program increased the net value of the crop by over $420 per acre when averaged across all cultivars and net crop value for the other fungicide programs was similar. Net value varied among cultivars at all locations, and overall Sullivan had the greatest value ($1759 ha-1) followed by Bailey ($1489 ha-1) and Wynne ($1413 ha-1), and CHAMPS had the lowest net crop value among the four cultivars ($775 ha-1) (Table 9).
Summary and Conclusions
In this study, four Virginia-type peanut cultivars including two recently released high-oleic cultivars were evaluated under different levels of disease pressure with different fungicide programs for disease control. Bailey has been the highest acreage cultivar in the Virginia-Carolina region for many years due to its high yield, quality, and strong disease resistance package. The high-oleic trait, which is not in Bailey, is desirable for the peanut processing industry due to the increased shelf-life of the product. However, as new cultivars with the high oleic trait such as Sullivan and Wynne are developed and released, growers expect them to meet or exceed the performance of Bailey. Across a variety of environments with varying disease pressure, Sullivan and Bailey were equally resistant to Sclerotinia blight, and Sullivan, Wynne, and Bailey were equally resistant to CBR. Sullivan was more tolerant of late leaf spot than Bailey and thus it may be possible to delay and reduce the overall number of fungicide applications for leaf spot control in Sullivan. Under some conditions, Wynne and Bailey yielded similarly well, but Wynne did not yield as well as Bailey when Sclerotinia blight pressure was high. Due to slightly higher disease tolerance compared to Bailey, good agronomic characteristics, high yield and quality under a variety of growing environments, and the presence of the high-oleic trait, Sullivan is an excellent cultivar for Virginia-type peanut production in the Virginia-Carolina region. A lack of yield response to higher-input fungicide programs for Sullivan and Bailey demonstrates the value of incorporating disease resistance/tolerance in peanut breeding programs (Wynne et al., 1991; Monfort et al., 2004; Cantonwine et al, 2006; Chapin et al., 2010). Furthermore, high yields and net returns of Sullivan regardless of fungicide program suggests further reducing fungicide inputs during production of this and other disease resistant/tolerant cultivars may be possible under low to moderate disease pressures as suggested by previous studies (Phipps, 1993; Cantonwine et al, 2006; Woodward et al., 2010, 2014). Delayed fungicide applications are already recommended for Bailey (Balota et al., 2015), but fungicide advisories, including the weather-based advisories for leaf spot and Sclerotinia blight fungicide applications in Virginia (Cu and Phipps, 1993; Phipps et al., 1997; Langston et al., 2002), need to be re-evaluated for new cultivars with greater levels of disease tolerance.
Acknowledgements
This work was supported with funding from the Virginia Peanut Growers Association and the National Peanut Board. The author would like to thank Linda Byrd-Masters, Steve Byrum, Ed Hobbs, Kelsey Vasser, and Joseph Opoku for assistance with planting and maintaining field experiments, application of treatments, collecting and tabulating data from field plots, and grading peanuts.
Literature Cited
Balota, M., R Grisso, D.A Herbert, D Jordan, H.L Mehl, and M Roberts 2015 Virginia peanut production guide 2015 Virginia Tech and Virginia Cooperative Extension, Publication AREC-117NP. http://www.pubs.ext.vt.edu/AREC/AREC-117/AREC-117.html
K.J Boote, (1982). Growth stages of peanut (Arachis hypogaea L.). Peanut Sci 9: 35- 40.
Branch, W.D., M Balota, T.G Isleib, W.S Montfort, J.P Bostick, B.L Tillman, M.D Burow, M Baring, and K.D Chamberlin 2014 Uniform Peanut Performance Tests, 2013, Univ. Georgia Coastal Plain Exp. Stn. Prog. Rep. No. 4-143, 25 p.
T., Brenneman, H Young, and K Rucker (2011). Early emergence application of prothioconazole for management of Cylindrocladium black rot of peanut. Phytopathol 101: S19.
E.G., Cantonwine, A.K Culbreath, K.L Stevenson, R.C Kemerait, T.B Brenneman, N.B Smith, and B.G Mullinix, (2006). Integrated disease management of leaf spot and spotted wilt of peanut. Plant Dis 90: 493- 500.
J.W., Chapin, J.S Thomas, T.G Isleib, F.M Shokes, W.D Branch, and B.L Tillman (2010). Field evaluation of Virginia-type peanut cultivars for resistance to Tomato Spotted wilt Virus, late leaf spot, and stem rot. Peanut Sci 37: 63- 69.
R.M Cu, and P.M Phipps (1993). Development of a pathogen growth response model for the Virginia peanut leaf spot advisory program. Phytopathology 83: 195- 201.
T.G., Isleib, H.E Pattee, R.S Tubbs, T.H Sanders, L.O Dean, and K.W Hendrix (2015). Intensities of sensory attributes in high-and normal-oleic cultivars in the uniform peanut performance test. Peanut Sci 42: 83- 91.
T.G., Isleib, S.R Milla-Lewis, H.E Pattee, S.C Copeland, M.C Zuleta, B.B Shew, J.E Hollowell, T.H Sanders, L.O Dean, K.W Hendrix, M Balota, and J.W Chapin (2011). Registration of 'Bailey' peanut. J. Plant Registrations 2011 , 5: 27- 39.
D.B., Langston, P.M Phipps, and R.J Stipes (2002). An algorithm for predicting outbreaks of Sclerotinia blight of peanut and improving the timing of fungicide sprays. Plant Dis 86: 118- 126.
Mehl, H.L 2015 Applied research on field crop disease control 2014. Virginia Tech and Virginia Cooperative Extension Publication AREC-126NP.
W.S., Monfort, A.K Culbreath, K.L Stevenson, T.B Brenneman, D.W Gorbet, and S.C Phatak (2004). Effects of reduced tillage, resistant cultivars, and reduced fungicide inputs on progress of early leaf spot of peanut (Arachis hypogaea). Plant Dis 88: 585- 564.
R.W., Mozingo, S.F O'Keefe, T.H Sanders, and K.W Hendrix (2004). Improving shelf life of roasted and salted inshell peanuts using high oleic fatty acid chemistry. Peanut Sci 31: 40- 45.
R.W., Mozingo, T.A Coffelt, P.M Phipps, D.L Coker, S Machado, and S.E Petrie (2006). Registration of 'CHAMPS' peanut. Crop Sci 46: 2711- 2712.
A.J., Norden, D.W Gorbet, D.A Knauft, and C.T Young (1987). Variability in oil quality among peanut genotypes in the Florida breeding program. Peanut Sci 14: 7- 11.
F.W., Nutter, and R.H Littrell (1996). Relationships between defoliation, canopy reflectance and pod yield in the peanut-late leafspot pathosystem. Crop Protection 15: 135- 142.
P.M Phipps, (1990). Control of Cylindrocladium black rot of peanut with soil fumigants having methyl isothiocyanate as the active ingredient. Plant Dis 74: 438- 441.
P.M Phipps, (1993). IPM in peanuts: Developing and delivering working IPM systems. Plant Dis 77: 307- 309.
P.M., Phipps, D.P Telenko, and G.H Musson (2010). Control of Cylindrocladium black rot of peanut with Propulse, a 1: 1 mixture of prothioconazole and fluopyram. Phytopathology 100: S100- 101.
P.M., Phipps, S.H Deck, and D.R Walker (1997). Weather-based crop and disease advisories for peanuts in Virginia. Plant Dis 81: 236- 244.
S. L., Rideout, T.B Brenneman, A.K Culbreath, and D.B Langston (2008). Evaluation of weather-based spray advisories for improved control of peanut stem rot. Plant Dis 92: 392- 400.
USDA-AMS 2015 Farmers' Stock Peanuts Inspection Instructions Fruit and Vegetable Div., Fresh Products Branch, Washington, D.C.
Woodward, J.E., T.B Brenneman, and R.C Kemerait 2013 Chemical control of peanut diseases: targeting leaves, stems, roots, and pods with foliar-applied fungicides InTech, DOI:10.5772/51064.
J.E., Woodward, T.B Brenneman, R.C Kemerait, A.K Culbreath, and N.B Smith (2010). Management of peanut diseases with reduced input fungicide programs in fields with varying levels of disease risk. Crop Protection 29: 222- 229.
J.E., Woodward, T.B Brenneman, R.C Kemerait, A.K Culbreath, and N.B Smith (2014). On-farm evaluations of reduced input fungicide programs in peanut fields with low, moderate, or high levels of disease risk. Peanut Sci 41: 50- 57.
J.C., Wynne, M.K Beute, and S.N Nigam (1991). Breeding for disease resistance in peanut (Arachis hypogaea L.). Ann. Rev. Phytopathol 29: 279- 303.
Notes
- Assistant Professor, Virginia Tech Tidewater Agricultural Research and Extension Center, Suffolk, VA 23437 [^] *Corresponding author's E-mail: hlmehl@vt.edu
Author Affiliations