Introduction
Peanut is a valuable commodity in the United States with approximately 634,800 ha harvested with an estimated value of $1.19 million USD (USDA 2016a; USDA 2016b). Approximately 7% of national peanut ha is in North Carolina (USDA 2016a) with the majority Virginia market type cultivars (Brown, 2016). Weeds compete with peanut for sunlight, moisture, and nutrients throughout the growing season (Wilcut et al., 1994). Peanut yield, quality, and economic value can be negatively affected by weeds (Everman et al., 2008; Walker et al., 1989). Season-long interference of combination of broadleaf and grass weeds can reduce peanut yield by 60 to 80% and reduced harvest efficiency in some instances (Everman et al., 2008; Wilcut et al., 1994). Common ragweed (Ambrosia artemisiifolia L.) (Clewis et al. 2001) and Palmer amaranth (Amaranthus palmeri L.) (Burke et al. 2007) at a density of one plant/m of row reduced peanut yield by 40 and 28%, respectively. The prostrate growth habit of peanut causes peanut to be vulnerable to interference from weeds throughout the season and requires effective season-long management strategies to protect yield and promote efficient digging and vine inversion (Walker et al., 1989; Wilcut et al., 1994).
Weed control programs often include preplant incorporated, preemergence, and postemergence (POST) herbicides to minimize interference of weeds with peanut and challenges with harvest (Clewis et al. 2007; Grichar and Dotray 2012; Henning et al. 1982; Jordan, 2016; Wilcut et al., 1994). However, in spite of these herbicide intensive programs weed escapes still occur. Herbicide options available for late-season weed control are limited because of injury potential and labeled pre-harvest intervals (Cahoon and Jordan, 2016; Jordan, 2016). While Palmer amaranth is the most troublesome and difficult to control weed in peanut in North Carolina, primarily due to resistance to acetolactate synthase (ALS)-inhibiting herbicides (Webster, 2013; Poirier et al., 2014), annual grasses and morningglory (Ipomoea spp.) are often found in fields late in the season (Jordan, 2016). Growers can apply diclosulam through the ground cracking stage in North Carolina (Anonymous, 2010) and up to 30 d after planting in other states to control Benghal dayflower (Commelina benghalensis L.) (Anonymous, 2004). Imazapic, lactofen, and 2,4-DB can be applied no closer to harvest than 90, 45, and 45 d, respectively (Anonymous, 2015; Anonymous, 2016; Anonymous, 2014a). Imazapic and lactofen are marginally effective on Palmer amaranth and morningglory that are large (Chahal et al., 2011; Lancaster et al., 2005 2007) or express resistance to ALS-inhibiting herbicides which is widespread in North Carolina (Poirier et al., 2014). Diclosulam suppresses several broadleaf weeds including annual morningglory, common ragweed, and Benghal dayflower when applied POST (Prostko, 2004; Lancaster et al., 2007). Of these herbicides 2,4-DB is applied late in the season more often to suppress Palmer amaranth and morningglory, although 2,4-DB is ineffective in controlling pitted morningglory (Ipomoea lacunosa L.) (Jordan, 2016). Determining efficacy of carfentrazone-ethyl and pyraflufen-ethyl herbicides against this weed and how peanut respond to these herbicides could lead to improved harvesting of peanut.
Carfentrazone-ethyl and pyraflufen-ethyl inhibit protoporphyrinogen oxidase (PPO) in sensitive plants including morningglory (Anonymous, 2011, Anonymous, 2014b; Dayan et al., 1997; Reed et al., 2004). However, carfentrazone-ethyl and pyraflufen-ethyl applied 28 to 51 d after planting injured peanut 62 to 48% when evaluated 14 d after treatment (Dotray et al., 2010). Grichar et al. (2010) reported 7 to 52% injury and 4 to 26% stunting of peanut when carfentrazone-ethyl and pyraflufen-ethyl were applied 35 to 56 d after planting. Peanut injury from these herbicides at these timings of application reduced pod yield of runner market types, but did not impact peanut market grade characteristics (Dotray et al., 2010; Grichar et al., 2010). Grichar et al. (2010) reported that peanut tolerance to carfentrazone-ethyl and pyraflufen-ethyl was cultivar dependent. Response of large-seeded Virginia market-type peanut to carfentrazone-ethyl and pyraflufen-ethyl is limited in the peer-reviewed literature. Therefore, research was conducted to determine peanut tolerance to these two herbicides when POST applied at different rates within 2 weeks prior to digging peanut pods and inverting vines and how peanut respond to these herbicide compared with other herbicides that have potential for late-season use.
Materials and Methods
Peanut response to carfentrazone-ethyl and pyraflufen-ethyl application rate and timing
Field experiments to compare herbicide rate and timings of carfentrazone-ethyl and pyraflufen-ethyl were conducted in North Carolina during 2012, 2013, and 2014 at the Peanut Belt Research Station located near Lewiston-Woodville (36.1323 N, -77.1705W) and at the Upper Coastal Plain Research Station (35.8942N, -77.68011W) located near Rocky Mount. Soil at Lewiston-Woodville was a Norfolk sandy loam (fine loamy, siliceous, thermic, Aquic Paleudalts) with organic matter ranging from 0.5 to 1.2% and pH 6.1. Soil at Rocky Mount was a Goldsboro loamy sand (fine loamy, mixed, semiactive, thermic, Typic Hapludults) with 1.5% organic matter and pH 5.9. The Virginia market type peanut cultivar 'Bailey' (Isleib et al., 2011) was used for these experiments.
Peanut response to carfentrazone-ethyl and pyraflufen-ethyl were evaluated in separate experiments. The experiment with carfentrazone-ethyl was conducted at Lewiston-Woodville and Rocky Mount during 2012 and 2013 and during 2014 only at Lewiston-Woodville. Carfentrazone-ethyl (Aim® EC, FMC Corporation, Philadelphia, PA) at 17.5 and 35 g ai/ha was applied 1 and 2 weeks prior to digging peanut pods and inverting vines (WBD). The experiment with pyraflufen-ethyl was conducted at Lewiston-Woodville during 2012, 2013, and 2014. Pyraflufen-ethyl (ET®, Nichino America, Inc., Wilmington, DE) at 1.8 and 3.6 g ai/ha was applied 1 and 2 WBD. The selected herbicide rates were within the registered rate of these herbicides in peanut (Anonymous, 2011; Anonymous, 2014). At the time of application, peanut was at the R8 growth stage with 65-70% of pod mesocarp color in orange, brown, and black categories (Boote, 1982).
Peanut response to carfentrazone-ethyl and pyraflufen-ethyl compared with diclosulam, lactofen, and 2,4-DB
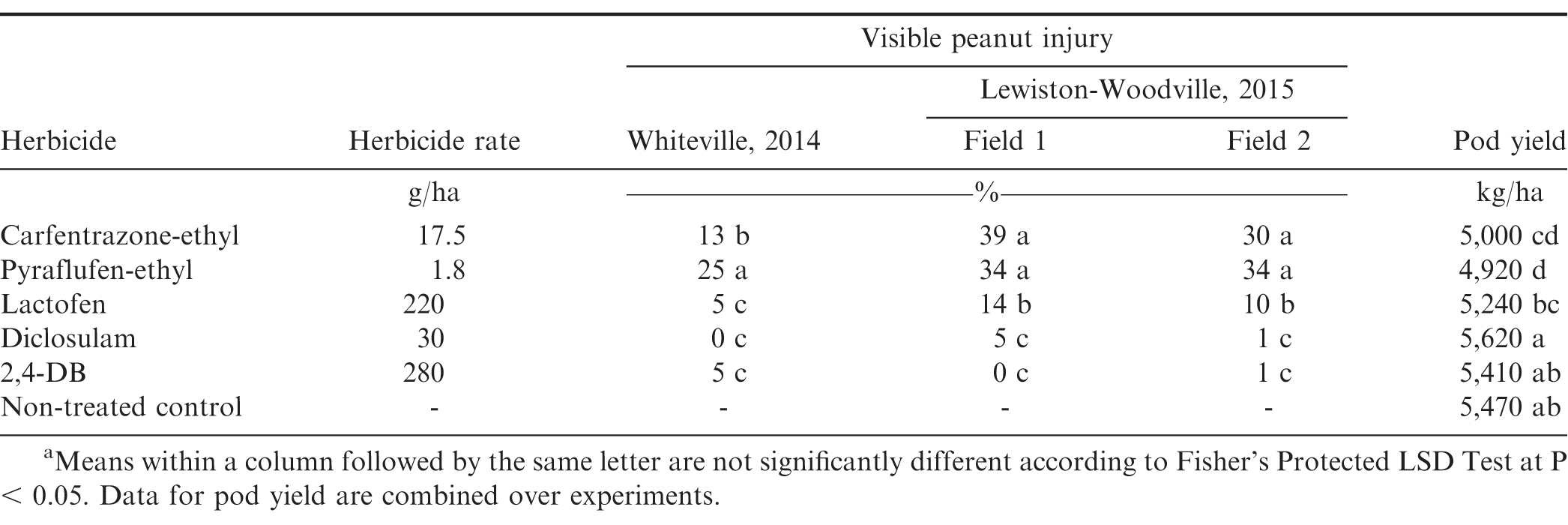
Another experiment was conducted in North Carolina during 2014 at the Border Belt Tobacco Research Station near Whiteville (34.4118 N, -78.7911W) on a Norfolk sandy loam soil and during 2015 in two different fields at Lewiston-Woodville to compare peanut response to carfentrazone-ethyl and pyraflufen-ethyl with diclosulam, lactofen, and 2,4-DB herbicides when applied 4 WBD. The cultivar 'Sullivan' (Isleib et al., 2016) was used for this experiment. Carfentrazone-ethyl at 17.5 g/ha, pyraflufen-ethyl at 1.8 g/ha, diclosulam (Strongarm®, DowAgrosciences, Indianapolis, IN) at 30 g ai/ha, lactofen (Cobra®, Valent USA Corp., Richmond, CA) at 220 g ai/ha, and 2,4-DB (Butyrac 200, Albaugh Inc., Ankeny, IA) at 280 g ai/ha were applied 4 WBD at the late R6 to early R7 growth stages corresponding to seed filling the pod cavity and pod mesocarp in the orange color, respectively (Boote, 1982) .
All experiments were conducted in conventionally-prepared, raised seedbeds in rows spaced 91 cm apart. Plot size was 2 rows by 6 m long. Peanut cultivar was planted at a seeding rate designed to provide a final in-row population of 12 plants/m. Fertilization, insect, and disease management practices other than specific treatments were standard for peanut production in North Carolina. Experiments were maintained weed free using soil-applied and postemergence herbicides during the season across the entire test area including non-treated plots. Pendimethalin (Prowl H2O herbicide, BASF Corp., Research Triangle Park, NC) at 1.1 kg ai/ha was applied preplant incorporated followed by S-metolachlor (Dual Magnum herbicide, Syngenta Crop Protection, Research Triangle Park, NC) at 1.1 kg ai/ha applied preemergence immediately after planting to the entire test area to prevent early season weed interference. To control weeds escaping herbicides applied at planting, acifluorfen plus bentazon (Storm herbicide, King of Prussia, PA) at 0.56 + 0.28 kg ai/ha plus 2,4-DB (Albaugh LLC, Ankeny, IA) at 0.28 kg/ha, and clethodim (Cleanse 2EC herbicide, Direct Ag Solutions LLC, Edora, IA) at 0.14 kg ai/ha were applied POST. Hand-removal of weeds supplemented herbicides to maintain plots free of weeds. Herbicides were applied at 145 L/ha aqueous solution, with nonionic surfactant at 0.24% (v/v) and crop oil concentrate at 1.0% (v/v) using a CO2-pressurized backpack sprayer set at 275 kPa. All experiments were conducted in a randomized complete block design with treatments replicated 4 times. A non-treated control was included in all the experiments.
Data collected and statistical analysis
In the experiment with carfentrazone-ethyl and pyraflufen-ethyl only, visible estimates of peanut injury were determined 1 and 2 week after treatment (WAT) using a scale of 0 to 100 where 0 =; no injury and 100 =; plant death. Peanut injury 2 WAT was only observed from carfentrazone-ethyl and pyraflufen-ethyl application that was made 2 WBD. Foliar chlorosis, necrosis, leaf defoliation, and plant stunting were considered when making the visible estimates. In the experiment with carfentrazone-ethyl and pyraflufen-ethyl compared with diclosulam, lactofen, and 2,4-DB, visible estimates of peanut injury were determined 2 WAT. Peanut pods were dug and vines inverted based on pod mesocarp color to obtain optimum yield (Williams and Drexler, 1981). Pod yield was determined 4-7 days after digging with final yield adjusted to 8% moisture.
Data were subjected to ANOVA using the PROC GLIMMIX procedure in SAS (SAS Institute Inc., Cary, NC) appropriate for the factorial treatment arrangement. Experiments were classified as specific combinations of year and/or location. Means of significant main effects and interactions were separated using Fisher's Protected LSD test at P ≤; 0.05. The non-treated control was included in peanut yield analysis but not in peanut injury.
Results and Discussion
Peanut response to carfentrazone-ethyl and pyraflufen-ethyl rate and timing
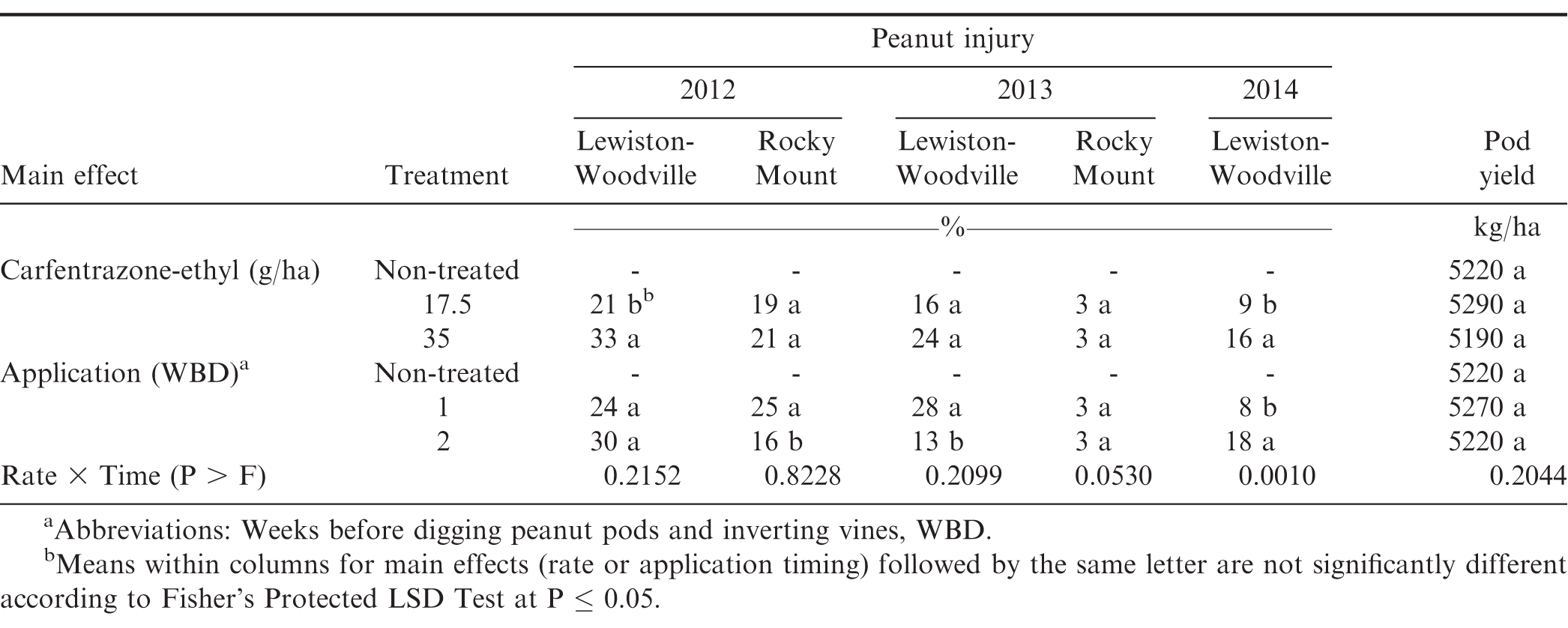
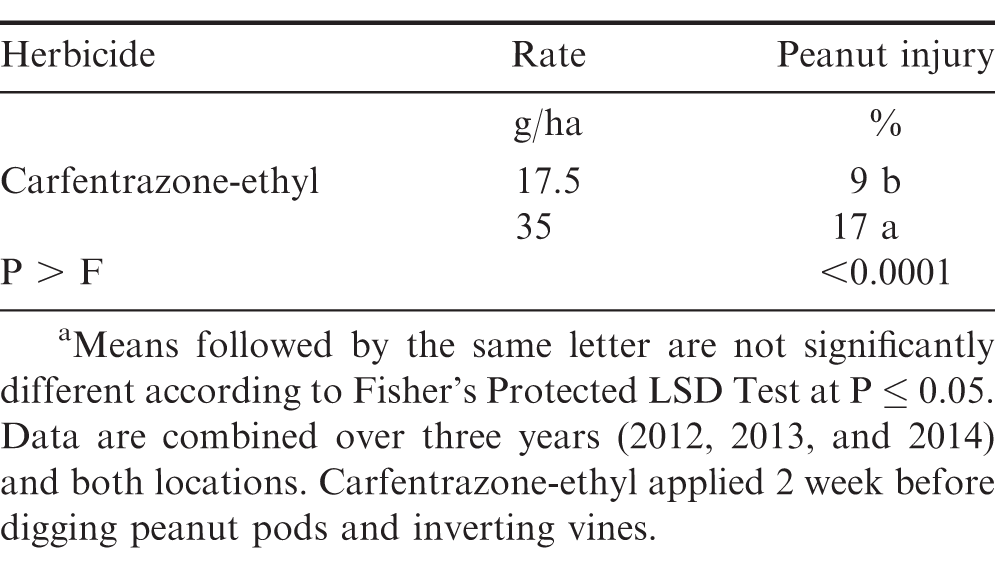
Peanut injury from carfentrazone-ethyl 1 WAT was influenced by the interaction of experiment by rate (P ≤; 0.0371) and experiment by application timing (P ≤ 0.0001); therefore data are presented by experiments. The interaction of carfentrazone-ethyl rate by application timing was significant for peanut injury 1 WAT for 2014 at Lewiston-Woodville (P =; 0.0010) but was not observed at Lewiston-Woodville and Rocky Mount during 2012 and 2013 (Table 1). However, at Lewiston-Woodville, the effect of carfentrazone-ethyl rate was significant for 2012 (P =; 0.0044), with injury increasing as the rate carfentrazone-ethyl increased (Table 1). The effect of application timing was significant for 2012 at Rocky Mount (P =; 0.0055) and 2013 Lewiston-Woodville (P =; 0.0035) and treatments applied 1 WBD caused higher injury (25 and 28%, respectively) when compared to treatments applied 2 WBD (16 and 13%) (Table 1). In 2014 at Lewiston-Woodville, carfentrazone-ethyl applied at 17.5 g/ha caused injury ≤ 10% regardless of application timing. However, increasing the carfentrazone-ethyl rate to 35 g/ha caused higher injury when applied 2 WBD than 1 WBD (25% versus 7%, data not shown in tables). The interaction of experiment by herbicide rate was not significant for peanut injury at 2 WAT (P =; 0.0570); therefore, data are combined over experiments. Carfentrazone-ethyl at 35 g/ha caused more peanut injury than when applied at 17.5 g/ha (17% versus 9%) (Table 2).
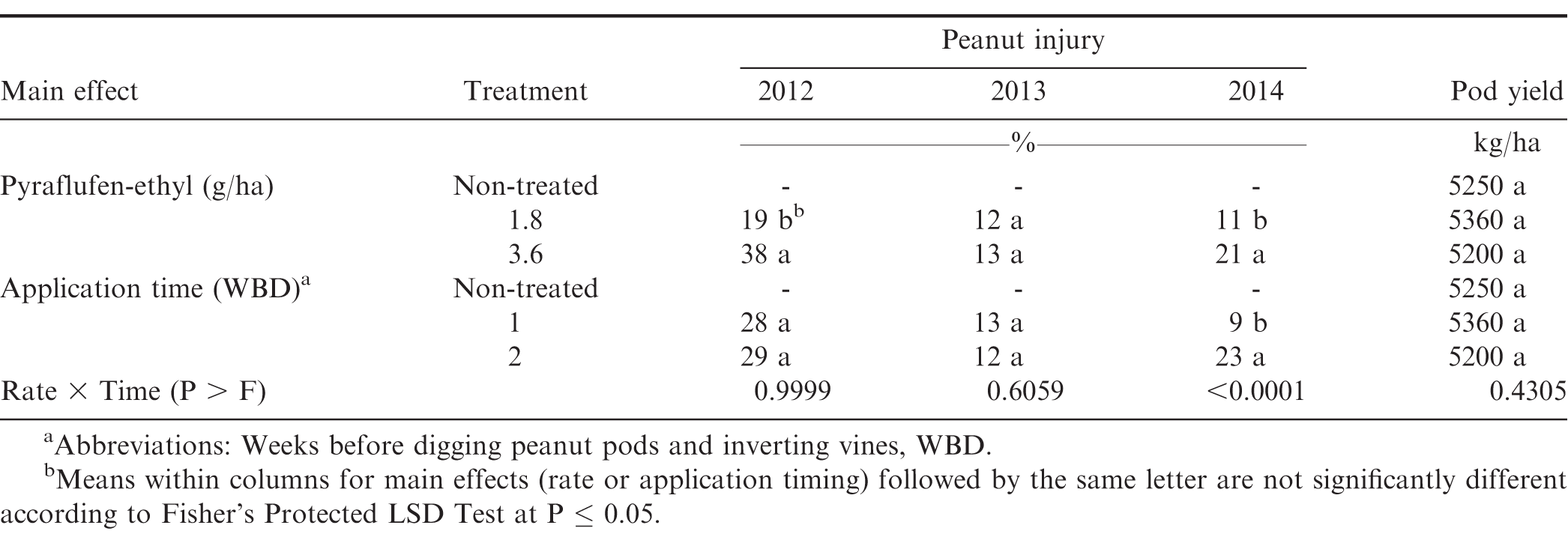
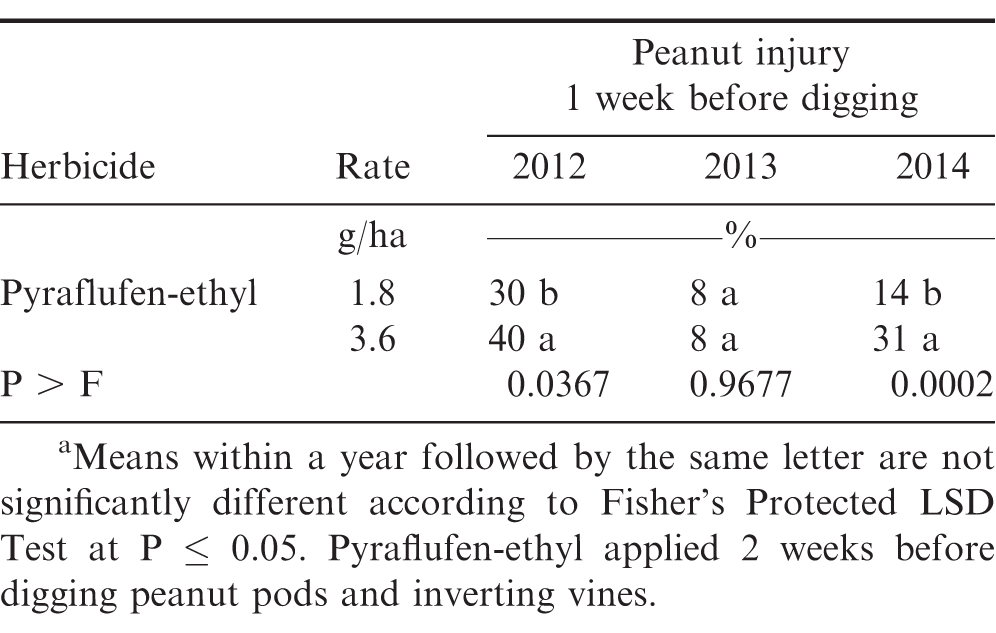
In the experiment with pyraflufen-ethyl, the interaction of experiment × application timing and experiment × herbicide rate were significant for peanut injury at 1 WAT (P ≤ 0.0001) and 2WAT (P = 0.0197). Therefore, data are presented by each year. Peanut injury at 1 WAT was not affected by the interaction of pyraflufen-ethyl rate × application timing for experiments during 2012 and 2013 (P > 0.05); this interaction was significant in 2014 (P 0.0001) (Table 3). Main effect of rate (P 0.0001) was significant for 2012. Peanut injury was higher when pyraflufen-ethyl was applied at 3.6 g/ha compared with to lower rate of 1.8 g/ha (Table 3). In 2014 at Lewiston-Woodville, pyraflufen-ethyl applied at 1.8 g/ha caused no more than 11% injury at both application timings. As was noted when comparing injury at the two timings, pyraflufen-ethyl at 3.6 g/ha injured peanut 33% at 2 WBD compared with only 8% at 1 WBD (data not shown in tables). At 2 WAT, the main effect of pyraflufen-ethyl rate was significant in 2012 (P ≤ 0.0367) and 2014 (P =; 0.0002), with the higher application rate of 3.6 g/ha causing more peanut injury compared with the rate of 1.8 g/ha (Table 4).
Peanut yield following carfentrazone-ethyl and pyraflufen-ethyl was not influenced by the main effect of herbicide rate or application timing and their interaction alone or with experiment. Peanut yield was similar to non-treated peanut regardless of herbicide rate or timing of application (Tables 1 and 3). Dotray et al. (2010) reported that carfentrazone-ethyl and pyraflufen-ethyl applied late POST 93 to 121 d after planting reduced peanut yield reduction at only at 1 of 6 locations. Peanut in our experiments was in the R8 stage of growth with 60-70% of pods in orange, brown, and black pod mesocarp classifications (Boote, 1982). It is postulated that herbicide phytotoxicity to foliage at this stage of growth and development did not affect foliage adequately to cause pod shed or the pod and seed maturation process.
Peanut response to carfentrazone-ethyl and pyraflufen-ethyl compared with diclosulam, lactofen, and 2,4-DB
The main effect of herbicide and the interaction of experiment × herbicide were significant for visible injury (P ≤ 0.0001). Although the main effect of herbicide was significant for pod yield, the interaction of experiment × herbicide was not significant (P = 0.3805). Therefore, data are combined over experiments for this interaction. The interaction of experiment × herbicide for visible injury was caused by lower peanut injury at Whiteville following carfentrazone-ethyl compared with pyraflufen-ethyl, and due to lack of differences in injury caused by lactofen and 2,4-DB compared with different results at Lewiston-Woodville (Table 5). At Lewiston-Woodville in both fields, no difference in injury was observed when comparing carfentrazone-ethyl and pyraflufen-ethyl. Visible injury at this location caused by lactofen exceeded that of diclosulam and 2,4-DB. Pod yield was 9% to 10% lower when carfentrazone-ethyl and pyraflufen-ethyl were applied compared with non-treated peanut (Table 5). Diclosulam, lactofen, and 2,4-DB did not negatively affect pod yield when compared with non-treated peanut. Other research (Lancaster et al., 2007) demonstrated no detrimental effect of diclosulam applied POST to peanut 7 to 35 d after planting prior to the R1 stage of growth (Boote, 1982). Diclosulam can be applied up to 30 d after planting with minimal concern for negative impact on peanut (Anonymous, 2004; Prostko, 2004). Although Baughman et al. (2002) reported no adverse impact of 2,4-DB on peanut yield when applied 30 to 120 d after planting which corresponds to pre-flowering to pod maturity, other research has shown that lactofen can negatively affect yield in some but not all instances (Ferrell et al., 2013; Grichar, 1997; Jordan et al., 1993). Results from these experiments indicate that the injury caused by carfentrazone-ethyl and pyraflufen-ethyl observed 4 WBD is more deleterious to peanut growth and development and subsequent yield than the same level of injury within 2 WBD.
Conclusions
Overall, these results demonstrate that variation in peanut injury can occur depending on rate of carfentrazone-ethyl and pyraflufen-ethyl and application timing. However, when applied within 2 weeks of digging, these herbicides will not reduce peanut yield and may have potential to minimize weed interference with digging and vine inversion.
Acknowledgements
The North Carolina Peanut Growers Association and FMC Corporation proved financial support for this research. Appreciation is extended to P. D. Johnson, A. T. Hare, T. Buck, and staff at the Border Belt Research Station, the Peanut Belt Research Station, and Upper Coastal Plain Research Station for technical assistance.
Literature Cited
Anonymous 2004 Supplemental label for Strongarm® herbicide. Dow AgroSciences, Indianapolis, IN 46268, EPA 24c Special Local Needs Reg. SLN GA-040010.
Anonymous. 2010 Specimen label for Strongarm® herbicide. Dow AgroSciences, Indianapolis, IN 46268 EPA Reg. Num, 62719-288.
Anonymous 2011 Specimen label for Aim® herbicide, FMC Corporation, Agricultural Products Group, Philadelphia, PA 19103. EPA Reg. Num. 279-3241.
Anonymous 2014 a Specimen label for Cadre Herbicide, BASF Corp., Research Triangle Park, NC. 27709. EPA Reg. No. 241-364.
Anonymous 2014 b Specimen label for ET® Herbicide, Nichino America, Inc., Wilmington, DE, 19808. EPA Reg. Num. 71711-7.
Anonymous 2015 Specimen label for Cobra® Herbicide. Valent Corp., Walnut Creek, CA, 94596 EPA Reg. No. 59639-34.
Anonymous 2016 Specimen label for Butyrac 200 Broadleaf Herbicide. Agri Star® by Albaugh,LLC, Ankeny, IA EPA Reg. No. 42750-38.
T. A., Baughman, W. J Grichar, and D. L Jordan (2002). Tolerance of virginia-type peanut to different application timings of 2,4-DB. Peanut Sci 29: 126- 128.
K. J Boote, (1982). Growth stages of peanut (Arachis hypogaea L.). Peanut Sci 9: 35- 40.
Brown, A. B 2016 Situation and outlook Pages 5- 6 in 2016 Peanut Information North Carolina Cooperative Extension Pub AG-331, College of Agriculture and Life Sciences, North Carolina State University, Raleigh, NC. 167 pages.
I. C., Burke, M Schroeder, W. E Thomas, and J. W Wilcut (2007). Palmer amaranth interference and seed production in peanut. Weed Technol 21: 367- 371.
Cahoon, C and D Jordan 2016 Weed control in peanuts Pages 41- 66 in Virginia Peanut Production Guide. Virginia Cooperative Extension Service Pub. AREC-117NP College of Agriculture and Life Sciences, Virginia Tech. 112 pages.
Chahal, G. S., D. L Jordan, A. C York, and E. P Prostko 2011 Palmer amaranth control with combinations of 2,4-DB and diphenylether herbicides J. Crop Management doi:10.1094/CM-2011-0802-01-RS.
S. B., Clewis, S. D Askew, and J. W Wilcut (2001). Common ragweed interference in peanut. Weed Sci 49: 768- 772.
S. B., Clewis, W. J Everman, D. L Jordan, and J. W Wilcut (2007). Weed management in North Carolina peanuts (Arachis hypogaea) with S-metolachlor, diclosulam, flumioxazin, and sulfentrazone systems. Weed Technol 21: 629- 635.
F. E., Dayan, S. O Duke, J. D Weete, and H. G Hancock (1997). Selectivity and mode of action of carfentrazone-ethyl, a novel phenyl triazolinone herbicide. Pestic. Sci 51: 65- 73.
P. A., Dotray, T. A Baughman, and W. J Grichar (2010). Peanut response to carfentrazone-ethyl and pyraflufen-ethyl applied postemergence. Peanut Sci 37: 52- 57
W. J., Everman, S. B Clewis, W. E Thomas, I. C Burke, and J. W Wilcut (2008). Critical period of weed interference in peanut. Weed Technol 22: 63- 67.
J. A., Ferrell, R. G Leon, B Sellers, D Rowland, and B Brecke (2013). Influence of lactofen and 2,4-DB combinations on peanut injury and yield. Peanut Sci 40: 62- 65.
J. L Grichar, (1997). Influence of herbicides and timing of application on broadleaf weed control in peanut (Arachis hypogaea). Weed Technol 11: 708- 713.
Grichar, W. J and P. A Dotray 2012 Weed control and peanut tolerance with ethalfluralin-based herbicide systems Int. J. Agron doi:10.1155/2012/597434.
W. J., Grichar, P. A Dotray, and T. A Baughman (2010). Peanut variety response to postemergence applications of carfentrazone-ethyl and pyraflufen-ethyl. Crop Protection 29: 1034- 1038.
Henning, R. J., A. H Allison, and L. D Tripp 1982 Cultural practices Pages 123- 138 in H. E Patteeand C.T Young, eds Peanut Science and Technology, Am. Peanut Res. Educ. Soc., Inc., Yoakum, TX. 825 pages.
T. G., Isleib, S. R Milla-Lewis, H. E Pattee, S. C Copeland, M. C Zuleta, B. B Shew, J. E Hollowell, T. H Sanders, L. O Dean, K. W Hendrix, M Balota, and J. W Chapin (2011). Registration of 'Bailey' peanut. J. Plant Reg 5: 27- 39.
T. G., Isleib, R. W Mozingo, J. B Graeber, J. E Hollowell, M. C Zuleta, P. W Rice, B. B Shew, H. E Pattee, S. R Milla-Lewis, and S Copeland (2016). Sullivan peanut. Technology No. 13236. North Carolina State University, Raleigh, NC: Technology Transfer. , .
Jordan, D. L 2016 Peanut weed management Pages 48- 86 in Peanut Information. North Carolina Cooperative Extension Pub. AG-331 College of Agriculture and Life Sciences, North Carolina State University, Raleigh, NC. 167 pages.
D. L., Jordan, J. W Wilcut, and C. W Swann (1993). Application timing of lactofen for broadleaf weed control in peanut (Arachis hypogaea). Peanut Sci 20: 129- 131.
S. H., Lancaster, D. L Jordan, A. C York, J. W Wilcut, R. L Brandenburg, and D. W Monks (2005). Interactions of late-season morningglory (Ipomoea spp.) management practices in peanut (Arachis hypogaea). Weed Technol 19: 803- 808.
S. H., Lancaster, J. B Beam, J. E Lanier, D. L Jordan, and P. D Johnson (2007). Weed and peanut (Arachis hypogaea) response to diclosulam applied postemergence. Weed Technol 21: 618- 622.
Poirier, A. H., A. C York, D. L Jordan, A Chandi, W. J Everman, and J. R Whitaker 2014 Distribution of glyphosate- and thifensulfuron-resistant Palmer amaranth (Amaranthus palmeri) in North Carolina J. International Agronomy , Volume 2014, Article ID 747810, 7 pages, doi.org/10.1155/2014/747810.
E. P Prostko, (2004). Strongarm applied postemergence in Georgia peanut. Proc. Am. Peanut Res. Educ. Soc 36: 30.
J. P., Reed, H. R Mitchell, and T Crumby (2004). Aim® (carfentrazone-ethyl) herbicide, a multiple use broadleaf herbicide for control and other crops. Proc. South. Weed Sci. Soc 57: 7- 8.
U.S. Department of Agriculture 2016 a Crop values 2015 summary, 12 Sept. 2016. http://usda.mannlib.cornell.edu/usda/current/CropValuSu/CropValuSu-02-24-2016.pdf.
U.S. Department of Agriculture 2016 b Crop production 2015 summary, 12 Sept. 2016. http://www.usda.gov/nass/PUBS/TODAYRPT/cropan16.pdf.
R. H., Walker, L. W Wells, and J. A McGuire (1989). Bristly starbur (Acanthospermum hispidium) interference in peanuts (Arachis hypogaea). Weed Sci 37: 196- 200.
T. M Webster (2013). Weed survey-southern states. Proc. South Weed Sci. Soc 66: 275- 287
J. W., Wilcut, A. C York, and G. R Wehtje (1994). The control and interaction of weeds in peanut (Arachis hypogaea). Rev. Weed Sci 6: 177- 205.
E. J Williams, and J. S Drexler (1981). A non-destructive method for determining peanut pod maturity, pericarp, mesocarp, color, morphology, and classification. Peanut Sci 8: 134- 141.
Notes
- First and second author: Postdoctoral Research Scholar and William Neal Reynolds Professor, Department of Crop and Soil Sciences, North Carolina State University, Raleigh, NC 27695. Third author: Assistant Professor, Department of Horticultural Sciences, North Carolina State University, Raleigh, NC 27695. [^] *Corresponding author's E-mail: david_jordan@ncsu.edu
Author Affiliations