Introduction
Peanut (Arachis hypogaea L.) is an important crop to Georgia and many other southern states of the US. Georgia produces the most peanut in the US, with 314,400 ha harvested in 2015 (Anonymous, 2016). Peanut is regularly grown near cotton (Gossypium hirsutum L.) and soybean [Glycine max (L.) Merr.] across the southeast (Lassiter et al., 2007; Prostko et al., 2011; Prostko et al., 2013). A large percentage of cotton and soybean cultivars have herbicide resistance technology to assist in weed management, and the increased use of glyphosate and glufosinate herbicides throughout the growing season increased the occurrence of accidental injury to peanut crops (Grey and Prostko, 2010; Johnson et al., 2012a; Lassiter et al., 2007). When spray systems are not properly cleaned prior to other applications to different crops, crop injury can occur from herbicide residue remaining in the spray system (Grey and Prostko, 2010; Prostko et al., 2011).
Peanut injury can also occur when the herbicide becomes suspended in air, never reaching the target site, then moving onto nearby peanut (Auch and Arnold, 1978; Grey and Prostko, 2010). Herbicide drift causes and severity can be attributed to many different factors: nozzle type, boom height, pressure, wind speed, spray formulation, volume per area, sprayer speed, and other environmental variables (Wolf et al., 1993). Grover et al. (1978) reported that herbicide drift can be 1 to 8% of the spray solution, and Wolf et al. (1993) indicated off-target spray drift can reach 16% when no shielding is used.
When applied 75, 90, and 105 d after planting (DAP), 240, 320, and 470 g ai ha--1 of glyphosate caused peanut yield reductions of 12 to 36% (Grey and Prostko, 2010). In another study, glyphosate applied to peanut at 560 g ai ha-1, 4 weeks after planting, resulted in yield loss greater than 50% and peanut injury was directly correlated with yield loss (Lassiter et al., 2007). Glufosinate trials indicated yield loss to peanut when less than normal rates of 538 g ai ha-1 were applied. Glufosinate at 135 and 269 g ai ha-1 applied approximately three weeks after emergence (WAE) reduced peanut yield 14 and 51% respectively in North Carolina (Jordan et al., 2011). A significant negative correlation with visual estimates of peanut injury and peanut yield was reported.
The frequency of herbicide-resistant weeds, especially weeds with resistance to acetolactate synthase inhibitor (ALS) (Wise et al., 2009) and glyphosate herbicides (Culpepper et al., 2006) have increased to the extent that some weed management programs are now less effective. Thus, there is an interest in developing other types of herbicide-resistant crops (Johnson et al., 2012b; Subramanian et al., 1997). Agricultural seed and chemical companies have reacted by developing alternative weed management systems that use 2,4-Dichlorophenoxyacetic acid (2,4-D) postemergence (POST) on 2,4-D-resistant crops including cotton and soybean (Egan et al., 2014; Johnson et al., 2012b; Leon et al., 2014). 2,4-D-resistant crops have been genetically altered so that they metabolize the 2,4-D herbicide more readily, thus no injury occurs from application (Johnson et al., 2012b). The proposed 2-4-D-resistant crops will also include herbicide-resistance technology for other herbicide mechanisms of action (MOA) (Johnson et al., 2012b). This benefit will permit the use of several MOA as a means of reducing the selection pressure on a single MOA (Vencill et al., 2012), as well as assist in management of current herbicide-resistant weeds.
2,4-D is known to cause significant injury to sensitive broadleaf crops when off-target exposure occurs as a result of tank contamination, herbicide drift, and movement due to volatilization (Egan et al., 2014; Grover et al., 1972; Johnson et al., 2012b). There is great concern with the introduction of 2,4-D-resistant crops, as the use of 2,4-D in amplified quantities throughout the growing season would likely increase the occurrence of accidental crop injury to sensitive broadleaf species grown in the same vicinity, including peanut (Egan et al., 2014; Grover et al., 1972; Johnson et al., 2012a; Leon et al., 2014). It is important to note, herbicide drift rates up to 16% of the spray solution have been reported to move off-target (Wolf et al., 1993). The 2,4-D and 2,4-D choline registrations for use in common and Enlist-Duo® crops of the southeast can be approximately 1066 g ae ha-1, and 16% of that rate is 171 g ha-1. If 171 g ha-1 of 2,4-D can move off target in a controlled experiment, higher rates of 2,4-D could drift off-target if applicators did not follow all herbicide label directions. Exposure rates of 2,4-D from tank contamination could range considerably.
Prostko et al. (2003) reported that 2,4-D applied to peanut preplant or preemergence (PRE) at 250 to 1000 g ha-1 caused peanut injury of 8% or less for all applications and tillage systems with no yield loss. Leon et al. (2014) reported that peanut treated at 21 and 42 DAP had injury of 0 to 35% when 2,4-D was applied at 70 to 1120 g ha-1, respectively, and 1120 g ha-1 caused 41% maximum yield loss. Merchant et al. (2012) applied 2,4-D to peanut at 30, 60, and 90 DAP at rates of 105 to 1680 g ha-1 and reported yield loss of 7 to 24%. The earlier applications (30 and 60 DAP) were more injurious than the 90 DAP treatments.
These studies are of great benefit to growers that face the possibility of peanut crop injury from accidental 2,4-D exposure, because in such an unfortunate situation the grower, extension agent, and crop consultant would be able to make an informed decision about the appropriate plan of action (Leon et al., 2014; Prostko et al., 2011). In an effort to link previous data, it is necessary to quantify the injury and yield response of peanut to 2,4-D across various rates applied at preemergence up to beginning bloom (R1) (Boote, 1982). Therefore, research was conducted to determine the sensitivity of peanut to 2,4-D at four early-season application timings during the vegetative growth stages, and to establish correlations between visual estimates of injury, canopy diameter, and peanut yield.
Materials and Methods
Field trials were conducted during 2012 and 2013 at three locations that represent the peanut growing regions in Georgia. The first location was the University of Georgia (UGA) Southwest Georgia Research and Education Center in Plains, GA, which had a Greenville sandy loam (fine, kaolinitic, thermic Rhodic Kandiudult) soil with 3.8% organic matter (OM), 60% sand, 10% silt, and 30% clay; the second location was the UGA Coastal Plains Experiment Station (Tifton campus) Ponder Farm in Ty Ty, GA, which had a Tifton loamy sand (fine-loamy, kaolinitic, thermic Plinthic Kandiudult) soil with 1% OM, 90% sand, 6% silt, and 4% clay; and the third location was the UGA Attapulgus Research and Education Center in Attapulgus, GA, which had an Orangeburg loamy sand (fine-loamy, kaolinitic, thermic Typic Kandiudult) soil with 1.5% OM, 86% sand, 6% silt, and 8% clay. Soil pH was 5.56, 5.63, and 6.0, respectively. The soil was prepared using a tillovator to loosen the soil and make it more suitable for planting. Peanut cultivar 'Georgia-06G' (Branch, 2007) was planted when soil conditions were warm enough for proper germination (around the 2nd week of May in Georgia). Specific planting dates are shown in Table 1. Peanuts were planted with 91 cm row spacing, two rows per plot, using vacuum style planters adjusted to 20 seed m-1 row-1. Plots were 1.8 m wide and 7.6 m or 9.1 m long with the same size border on either side of each plot.
Preemergence herbicides were applied to suppress weeds early in the season, and included 1066 g ai ha-1 of pendimethalin, 71 g ai ha-1 of flumioxazin, and 27 g ai ha-1 of diclosulam. Irrigation was applied to incorporate the herbicide into the soil and to firm up the soil around the peanut seed. Applications of POST herbicides were used as needed throughout the season which included acifluorfen, clethodim, and imazapic (Anonymous 2013). Hand-weeding was used as necessary. Supplemental irrigation was applied throughout the growing season as needed, which is shown in Table 1 with monthly rainfall received. Fertilizer, fungicides, and insecticides were applied by on-site farm management for the duration of the trials based on University of Georgia recommendations (Anonymous, 2013).
Trials were conducted using a randomized complete block design with 4 treatment timings and 6 herbicide rates, which included a non-treated control. 2,4-D (Weedar® 64, dimethylamine salt, 456 g ae L-1, Nufarm Inc., 150 Harvester Drive, Burr Ridge, IL) at rates of 0, 67, 133, 266, 533, and 1066 g ae ha-1 were applied preemergence (PRE), 10, 20, and 30 DAP. Treatments were replicated 4 times at Plains and Ty Ty and 3 times at Attapulgus. All treatments were applied using a CO2 pressurized backpack sprayer calibrated to deliver 140 L ha-1 at 152 kPa using TeeJet® XR8002VS nozzles (TeeJet® Technologies, Spraying Systems Company, Wheaton facility, P.O. Box 7900, Wheaton, IL).
PRE treatments were applied within three d of planting. The 10, 20, and 30 DAP treatments coincided with V2, V3, and V5 vegetative growth stages respectively (Boote, 1982). The treatments will be discussed as PRE, V2, V3, and V5 treatments. When the V5 treatments were applied, approximately 25% of the peanut plants had blooms, making them very close to what is considered beginning bloom for a population of peanut plants or the first reproductive stage (R1) (Boote, 1982).
Peanut plant populations were recorded 20 d after planting. Visual estimates of peanut injury were determined based on a combination of plant chlorosis, necrosis, stem epinasty, leaf cupping, plant stunting, and lack of emergence with a scale of 0 (no injury relative to NTC to 100% (plant death). Crop injury was evaluated throughout the growing season every 10 d until 80 DAP. Canopy diameter measurements were documented (3 per plot) every 10 d until 80 DAP.
At 130 DAP, non-2,4-D-treated sections of peanut were checked for maturity using the hull-scrape method (Williams and Drexler, 1981). Based on maturity tests, peanuts were dug and inverted. Digging dates and harvest dates are presented in Table 1. Peanuts were partially dried in bright sunlight for 5 to 10 d, and then each peanut plot crop was harvested with a two-row peanut harvester, bagged, and weighed. Moisture content was recorded and adjusted to 10% for final yield determination. Yield data were not collected for the Ty Ty 2013 site year due to animal damage late in August of that season. Peanut pod samples (500 g) from each plot were stored in small paper or mesh bags. Within three wk of harvest, each plot sample was shelled with a mechanical peanut sheller, and the weight of 100 randomly selected whole seed was measured from each sample.
Data were subjected to ANOVA using PROC GLMMIX (SAS Institute, Inc. 2 012) to determine interactions between main factors (𝜶 = 0.05). Location, treatment timing, and herbicide rate were considered fixed effects, whereas random effects included years, repetitions and the associated interactions. Visual estimates of peanut injury, crop population density, peanut canopy diameter, peanut yield, and kernel weight were analyzed. Linear and nonlinear regression models were evaluated to determine associations between herbicide rate and all dependent variables. Pearson correlation analysis was conducted to define relationships between peanut injury, canopy diameter, and yield.
Results and Discussion
Location effects indicated no interactions with peanut response by herbicide rate or treatment timing (P = 0.30). Treatment timing by herbicide rate interactions were highly significant (P 0.0001) for peanut injury, canopy diameter as a percentage to the NTC, and yield as a percentage NTC. Peanut plant population at 20 d after treatment (DAT) was not affected by herbicide rate (P = 0.87), and peanut kernel weights indicated no mass reduction (P = 0.78) (data not shown).
Preemergence treatments
All visual estimates of injury and canopy diameter measurements reported were observed 20 DAT as compared to the NTC. This timeframe from application coincides with the amount of time a grower may need when determining the outcome of an injured peanut crop. Additionally, if a peanut crop was exposed to an auxin herbicide, waiting 20 d after the initial injury would give indications of which herbicide caused the injury. 2,4-D injury on peanut has been reported to be higher at 1 week after treatment (WAT) when compared to 3 WAT, and dicamba injury on peanut indicated to increase up to 3 WAT (Leon et al., 2014).
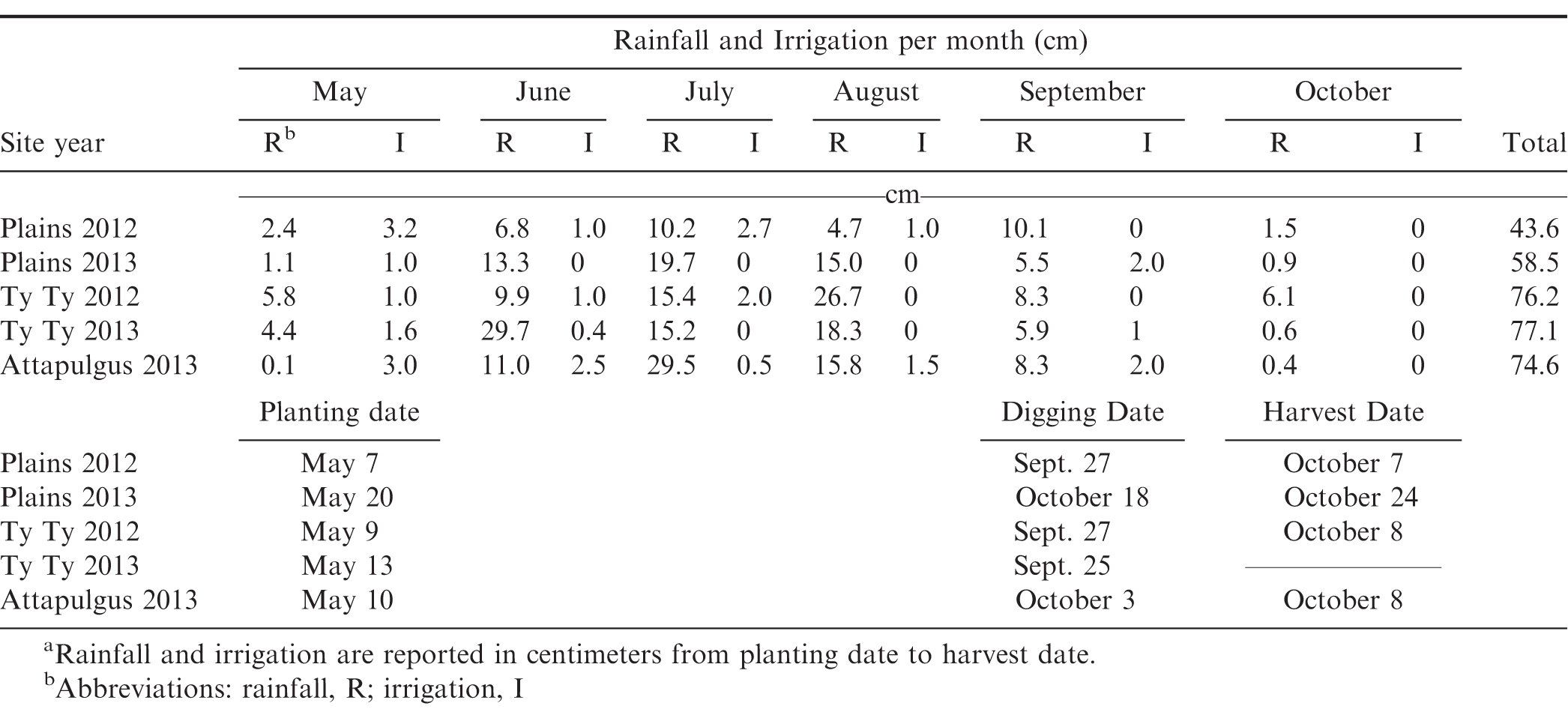
Linear regression indicated minimal peanut injury for PRE 2,4-D treatments across all rates (Figure 1a). When compared to the NTC, peanut injury estimates were 10% at the highest rate of 2,4-D (1066 g ae ha-1), but injury was transient and not observed later in the season (data not shown). As previously mentioned, peanut plant stand at 20 DAT were not different than the NTC, but a short delay in emergence was noted at 10 DAT from 2,4-D at 533 and 1066 g ha-1 (data not shown). Peanut canopy diameter was stunted 8 to 10% with all rates of 2,4-D (67 to 1066 g ha-1) relative to the NTC, but stunting was transient.
Peanut injury 20 DAT (a) and yield (b) as a percentage compared to the nontreated control by 2,4-D rate. Plains, Ty Ty, and Attapulgus data are combined by treatment timing. Treatment timings are combined if there were no differences in response to herbicide rate. Data points represent the observed means with standard error.
Injury, PRE treatments y = 0.01x - 1.1, R2 = 0.53
Injury, V2 treatments y = 0.02x + 1.9, R2 = 0.58
Injury, V3 and V5 treatments y = 0.03x + 6.8, R2 = 0.61
Yield, V3 and V5 treatments y = - 0.04x + 101, R2 = 0.39
When 2,4-D is applied PRE it quickly dissipates in the soil and has negligible residual activity. Data indicates that 2,4-D degrades more rapidly in well drained soils; where sandy loam soils had 2,4-D half-lives of 3 to 7 d (Smith, 1980). It has a low binding affinity in mineral soils and sediment, with half-lives averaging 6.2 d according to the EPA (2005). Therefore, the lower concentrations of 2,4-D, applied PRE, degraded before injury occurred to peanut, but higher concentrations resulted in noticeable leaf cupping and stem epinasty. Within a few d after initial peanut injury, there was a quick recovery, due to peanut tolerance to low doses of 2,4-D. Since peanut grew out of the initial injury from PRE treatments, there was no evidence of yield loss when compared to the NTC for any 2,4-D rate (Figure 1b). In a similar study, 2,4-D was applied preplant or PRE at 250 to 1000 g ha-1 causing peanut injury of 8% or less for all applications and tillage systems, with no yield losses (Prostko et al., 2003).
V2 growth stage treatments
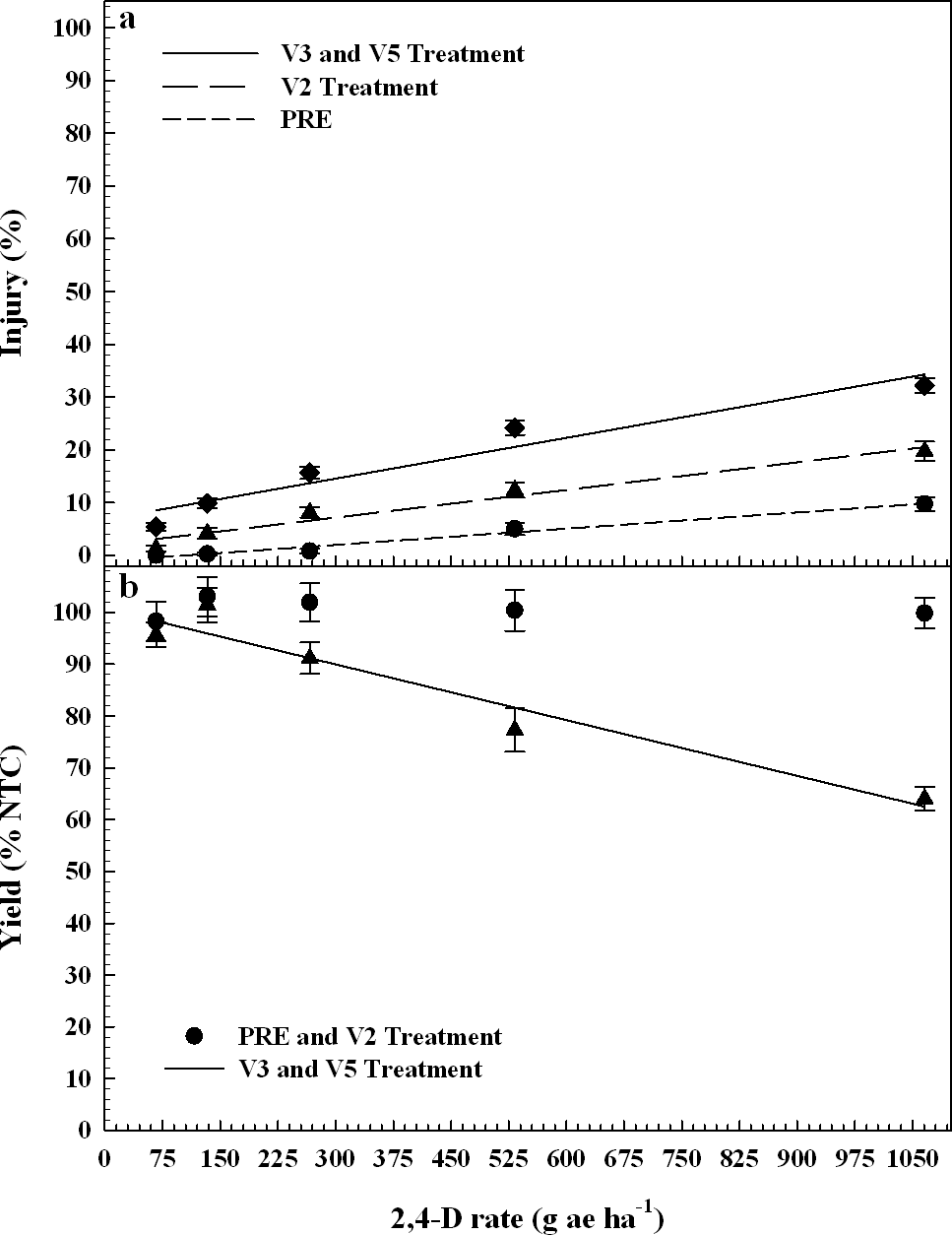
Peanut injury estimates 20 DAT were higher for V2 growth stage (Boote, 1982) treatments when compared to PRE treatments (Figure 1a). Peanut plants received a direct application of 2,4-D for V2 treatments but was soil applied for PRE treatments. Peanut injury for V2 treatments was 1 to 20% from 2,4-D rates of 67 to 1066 g ha-1. When studying Virginia Bunch peanuts, Rawson (1963) reported that visible crop injury to be lowest on peanuts treated at 1 week after emergence (WAE) with rates up to 680 g ha-1 of 2,4-D as compared to treatments of 1 to 16 WAE. In the present study, V2 treatments were less injurious to peanut when compared to the later treatments of 2,4-D at V3 to V5 growth stages (Figure 1). The majority of injury at 20 DAT was attributed to overall peanut stunting relative to the NTC and old necrotic tissue, but at that time peanut plants had new healthy growth after the initial 2,4-D injury (leaf cupping, stem epinasty, etc). Peanut canopy diameter was stunted 4 to 24% at 2,4-D rates of 67 to 1066 g ha-1 when applied at the V2 growth stage (Data not shown). After correlation analysis, peanut canopy diameter as a % NTC and injury estimates as compared to the NTC, were highly significant (P 0.0001) with a correlation coefficient of - 0.56 (Table 2), which is evidence that peanut canopy diameter can be a useful parameter when determining injury to peanut from 2,4-D.
Visual estimates of injury and canopy diameter stunting were not indicators of peanut yield loss when 2,4-D was applied at the V2 growth stage. With a maximum of 20% injury and 24% peanut canopy diameter stunting from 1066 g ha-1 of 2,4-D, the peanut recovered to have no yield loss at harvest as compared to the NTC. Thus, peanut yield data were combined for the PRE treatments and for the V2 treatments since both treatment timings resulted in no yield loss (Figure 1). This result is another indicator that peanut has the ability to tolerate high levels of 2,4-D up to V2 growth stage, while having enough time to recover before harvest.
V3 to V5 growth stage treatments
Since there were no differences in response of peanut to 2,4-D treatments made at V3 and V5 growth stages (P = 0.86), data were combined and will be referred to as V3 and V5 treatments. Linear regression indicated peanut injury was 5 to 32% from 2,4-D rates of 67 to 1066 g ha-1 after V3 and V5 treatments (Figure 1), which increased by 2,4-D rate. Johnson et al. (2012a) reported that 2,4-D treatments made to peanut approximately 3 WAE, planted 15 to 20 cm wide, caused 30 to 40% injury at 1 and 2 WAT, respectively. Leon et al. (2014) indicated that peanut injury, averaged over three rating dates, from 2,4-D at 70, 140, and 280 g ha-1 applied 21 and 42 DAP was not different when compared to the NTC, but rates of 560 and 1120 g ha-1 caused 15 to 35% injury, which is similar to previously described results. The present study indicated peanut canopy diameter was stunted 5 to 35% from 67 to 1066 g ha-1 of 2,4-D relative to the NTC (Figure 2). After correlation analysis, peanut canopy diameter 20 DAT as a % NTC and visual estimates of injury 20 DAT relative to the NTC indicated to be highly significant (P 0.0001) with a correlation coefficient of - 0.76 (Table 2).
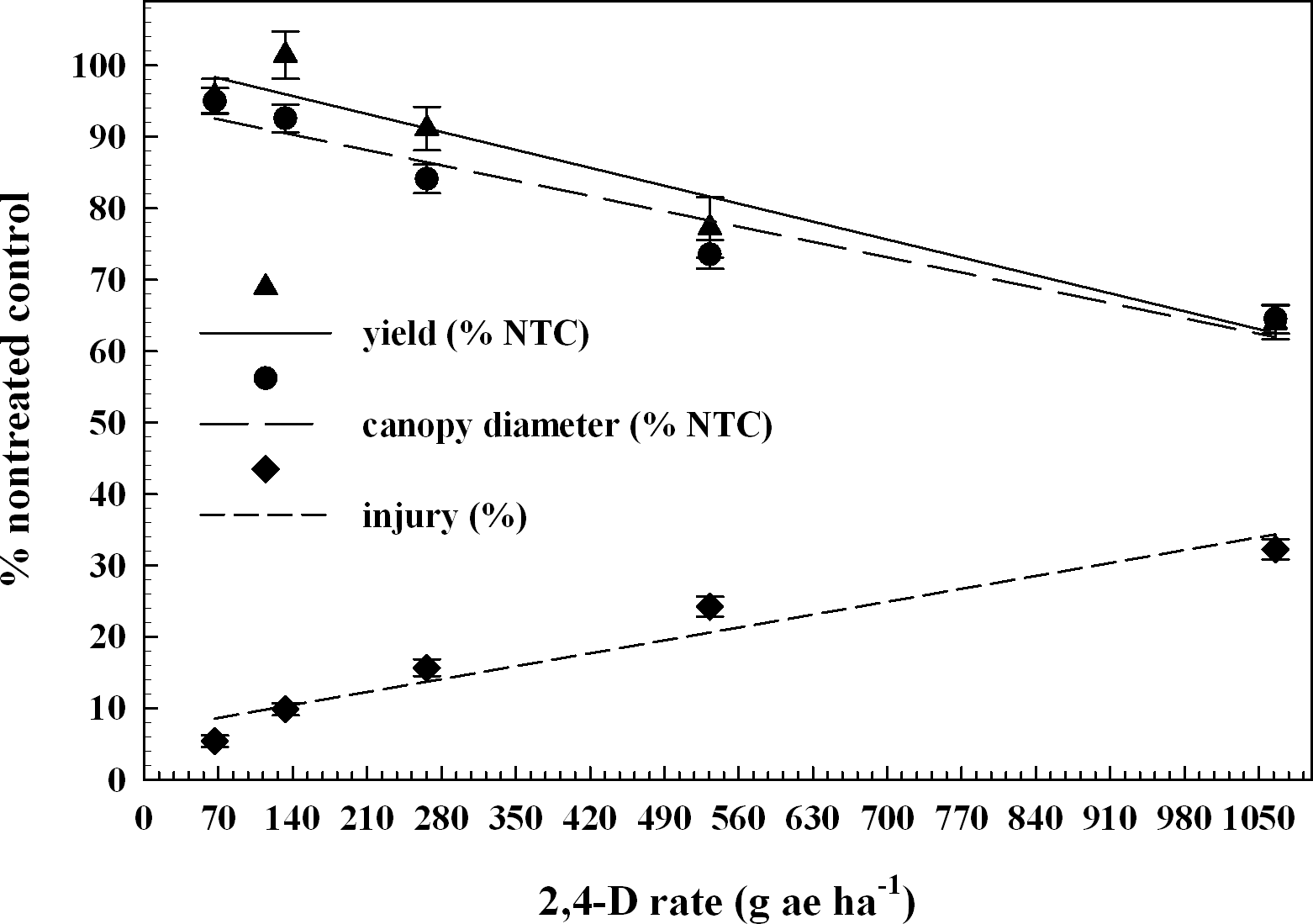
Peanut yield, canopy diameter 20 DAT, and injury 20 DAT as a percentage compared to the nontreated control by 2,4-D rate. Plains, Ty Ty, and Attapulgus data are combined by V3 and V5 treatment timing. Data points represent the observed means with standard error.
Yield y = - 0.04x + 101, R2 = 0.39
Canopy diameter y = - 0.03x + 95, R2 = 0.45
Injury y = 0.03x + 6.8, R2 = 0.61
When peanut was treated at the V5 growth stage, peanut was in 25% bloom (data not shown). According to Boote (1982), the first reproductive growth stage is beginning bloom (R1), which begins when 50% of the peanut population has blooms, or had a bloom. Thus, the peanut trials had not entered the R1 growth stage at the time of treatments, but did within 10 d. Peanut had greater injury and increased canopy diameter stunting after V3 and V5 treatments of 2,4-D, and since reproductive tissues were forming, there was increased yield loss when compared to V2 treatments. Peanut pod yield loss was 9, 23, and 36% at 266, 533, and 1066 g ha-1 of 2,4-D respectively (Figure 1) after V3 and V5 treatments. Johnson et al. (2012a) reported that peanut had considerable tolerance to sublethal rates of 2,4-D, but at 269 g ha-1 yield loss occurred at one location in 2009. Leon et al. (2014) reported peanut yield loss of 11 to 41% at 70 to 1120 g ha-1 of 2,4-D when applied 21 and 42 DAP. V3 and V5 treatments resulted in significant correlations (P 0.0001) of peanut yield as % NTC with injury estimates 20 DAT and canopy diameter 20 DAT as % NTC; having correlation coefficients of -0.48 and 0.51 respectively (Table 2).
This research and similar studies were conducted to provide growers and extension agents with usable information. In the unfortunate situation of off-target exposure or accidental treatments of 2,4-D due to tank contamination, growers need to know how the injury will affect peanut yield. As shown in Figure 2, the previously described correlations indicate that the peanut injury estimates and canopy diameter measurements obtained 20 d after 2,4-D exposure can be very helpful when determining yield loss to peanut when injured at V3 to V5 growth stages.
Summary and Conclusions
Peanut injury, canopy diameter, and peanut yield loss varied due to 2,4-D treatments made at different peanut growth stages. PRE and V2 growth stage treatments injured peanut, but peanut recovered to have no yield loss. There were significant increases in peanut injury as 2,4-D rate increased, especially after V3 and V5 treatments. Generally, 2,4-D was more injurious to peanut when applied closer to beginning bloom (approximately 30 DAP); the first reproductive growth stage (R1). Linear regression models were accurate representations of injury estimates and peanut yield as % NTC by herbicide rate. Visual estimates of injury and canopy diameter measurements of peanut in the present study provided practical and easily obtained information that a grower could do with little to no cost. Visual estimates of peanut injury at 20 DAT were correlated with peanut yield when 2,4-D was applied at V3 to V5 growth stages. Highly significant correlations (P 0.0001) were observed for peanut injury 20 DAT, canopy diameter 20 DAT, and peanut yield % NTC.
2,4-D resistant crops will likely have widespread use in the near future, so great care should be taken to prevent 2,4-D drift, sprayer contamination, and volatilization. Applicators should always read the herbicide label and follow all directions and restrictions. Due to the sensitivity of peanut to 2,4-D, growers and applicators should avoid applying 2,4-D in the proximity of peanut during V3 to V5 growth stages and early reproductive growth stages. In the unfortunate situation where peanut injury from accidental 2,4-D exposure occurs, these data could assist the grower in determining peanut yield loss estimates and a possible plan of action.
Acknowledgements
This research was partially supported through a grant provided by the Georgia Peanut Commission. Thanks are given to Sidney Cromer, Fritz Turpin, Brittney Cromer, Steve Li, Jody Thompson, Haydon Davis, and all the UGA farm crews for their technical assistance.
Literature Cited
Anonymous 2016 Crop production 2015 summary National Agricultural Statistics Service: Online at https://www.nass.usda.gov/Statistics_by_State/Georgia/index.php Accessed: Feburary 16, 2017.
Anonymous 2013 Georgia Pest Management Handbook - commercial edition: College of Agricultural & Environmental Sciences Pp. 848. Online at http://www.caes.uga.edu Accessed 20 March 2017.
D.E., Auch W.E Arnold (1978). Dicamba use and injury on soybeans (Glycine max) in South Dakota. Weed Sci 26: 471- 475.
K.J Boote (1982). Growth stages of peanut (Arachis hypogaea L.). Peanut Sci 9: 35- 40.
Branch W.D 2007 Registration of 'Georgia-06G' peanut J. Plant Reg.:120.
A.S., Culpepper A.C York, A.M Brown, W.W Hanna, J.W Davis, W.K Vencill, T.L Grey, T.M Webster, J.M Kichler (2006). Glyphosate-resistant Palmer amaranth (Amaranthus palmeri) confirmed in Georgia. Weed Sci 54: 620- 626.
J.F., Egan K.M Barlow, D.A Mortensen (2014). A meta-analysis on the effects of 2,4-D and dicamba drift on soybean and cotton. Weed Sci 62: 193- 206.
Environmental Protection Agency, EPA 2005 2,4-D RED Facts Pesticides: Reregistration Online at https://archive.epa.gov/pesticides/reregistration/web/html/24d_fs.htmlAccessded 20 Mar 2017.
T.L., Grey E.P Prostko (2010). Physiological effects of late season glyphosate applications on peanut seed development and germination. Peanut Sci 37: 124- 128.
R., Grover J Maybank, K Yoshida (1972). Droplet and vapor drift from butyl ester and dimethylamine salt of 2,4-D. Weed Sci. 20: 320- 324.
R., Grover K Yoshida, J Maybank (1978). Spray drift from agricultural pesticide applications. J. Air Pol. Cont. Assoc 28: 1009.
Johnson V.A., L.R Fisher, D.L Jordan, K.E Edmisten, A.M Stewart, A.C York 2012 a Cotton, peanut, and soybean response to sublethal rates of dicamba, glufosinate, and 2,4-D Weed Technol 26: 195- 206.
Johnson W.G., S.G Hallett, T.R Legleiter, F Whitford, S.C Weller, B.P Bordelon, B.R Lerner 2012b. 2,4-D and dicamba-tolerant crops- some facts to consider Purdue Extension Report ID-453-W Online at https://www.extension.purdue.edu/extmedia/id/id-453-w.pdf Accessed 20 Mar 2017.
Jordan D.L., J.A Johnson, L.R Fisher 2011 Peanut response to simulated drift rates of glufosinate. Crop Manage CM-2011-0802-02-RS.
B.R., Lassiter I.C Burke, W.E Thomas, W.A Pline-Srnic, D.L Jordan, J.W Wilcut, G.G Wilkerson (2007). Yield and physiological response of peanut to glyphosate drift. Weed Technol 21: 954- 960.
R.G., Leon J.A Ferrell, B.J Brecke (2014). Impact of exposure to 2,4-D and dicamba on peanut injury and yield. Weed Technol 28: 465- 470.
R.M., Merchant E.P Prostko, P.M Eure, T.M Webster (2012). Peanut response to simulated drift rates of 2,4-D. Pages 36 APRES, American Peanut Res. Educ. Soc. Raleigh, NC, .
E.P., Prostko T.L Grey, W.C Johnson, D.L Jordan, W.J Grichar, B.A Besler, K.D Brewer, E.F Eastin (2003). Influence of preplant applications of 2,4-D, dicamba, tribenuron, and tribenuron plus thifensulfuron on peanut. Peanut Sci 30: 18- 22.
E.P., Prostko T.L Grey, M.W Marshall, J.A Ferrell, P.A Dotray, D.L Jordan, W.J Grichar, B.J Brecke, J.W Davis (2011). Peanut yield response to dicamba. Peanut Sci 38: 61- 65.
E.P., Prostko T.M Webster, M.W Marshall, R.G Leon, T.L Grey, J.A Ferrell, P.A Dotray, D.L Jordan, W.J Grichar, B.J Brecke (2013). Glufosinate application timing and rate affect peanut yield response. Peanut Sci 40: 115- 119.
J.E Rawson (1963). The susceptibility of virginia bunch peanut to post-emergence application of 2,4-D and related herbicides. Queensland J. Agri. Sci 20: 463- 467.
SAS Institute I (2012) SAS/STAT® 9.2 User's Guide: World Headquarters SAS Institute Inc.100 SAS Campus Drive, Cary, NC.
A.E Smith (1980). Persistence studies with [14C] 2,4-D in soils previously treated with herbicides and pesticides. Weed Res 20: 355- 359.
M.V., Subramanian J Tuckey, B Patel, P.J Jensen (1997). Engineering dicamba selectivity in crops: a search for appropriate degradative enzyme(s). Ind. Microbiol. Biotechnol 19: 344- 349.
W.K., Vencill R.L Nichols, T.M Webster, J.K Soteres, C.M Smith, N.R Burgos, W.G Johnson, M.R McClelland (2012). Herbicide resistance: Toward an understanding of resistance development and the impact of herbicide-resistant crops. Weed Sci 60: 2- 30.
E.J., Williams J.S Drexler (1981). A non-destructive method for determining peanut pod maturity. Peanut Sci 8: 134- 141.
A.M., Wise T.L Grey, E.P Prostko, W.K Vencill, T.M Webster (2009). Establishing the geographical distribution and level of acetolactate synthase resistance of Palmer amaranth (Amaranthus palmeri) accessions in Georgia. Weed Technol 23: 214- 220.
T.M., Wolf R Grover, K Wallace, S.R Shewchuk, J Maybank (1993). Effect of protective shields on drift and deposition characteristics of field sprayers. Canadian J. Plant Sci 73: 1261- 1273.
Notes
- Former Graduate Research Assistant, Professor and Professor, respectively. Department of Crop and Soil Sciences, The University of Georgia, 2360 Rainwater Drive, Tifton, GA 31793; [^]
- Professor, Department of Crop and Soil Sciences, The University of Georgia, 3111 Miller Plant Sciences, Athens, GA 30602 [^]
- Research Agronomist, USDA-ARS - Crop Protection and Management Research Unit, Tifton, GA 31794. [^] *Corresponding author Email: tgrey@uga.edu
Author Affiliations