Introduction
The most rapidly expanding sector of the U.S. peanut industry is the organic peanut market (Lambb, 2007; Parker, 2007). In North Carolina, organic peanut buyers obtained most of their organic peanut from New Mexico peanut producers. Organic peanuts were paid a price premium of $1.76 to $3.30 per kg, which was more than twice the price for conventional peanut (Guerena and Adam, 2008). Peanut producers are unable to meet the demand for organic peanut. In 2005, there was an unmet need for almost 4000 metric tons of organic peanut in the U.S. (Culbreath, 2005). One of the biggest challenges to organic peanut production is weed control (Organic Farming Research Foundation, 2001).
Without herbicides as a management option, organic producers must rely on a diversity of tactics to reduce weed populations and subsequent interference (Liebman and Gallandt, 1997). Utilization of more competitive peanut cultivars may improve weed management in addition to tactics such as cultivation, plant population, and planting pattern. Cultivar selection is an important component of disease (Shew, 2009; Wynne et al., 1991a), and insect (Sharma et al., 2003) management programs in both organic (Branch and Culbreath, 2008) and conventional systems (Shew, 2009). A more competitive peanut cultivar could be useful for conventional peanut producers interested in reducing reliance on herbicides.
Genotypic differences in competitiveness for weeds have been identified for several crops including corn (Zea mays L.) (Wooley and Smith, 1986), cowpea (Vigna unguiculata L.) (Remison, 1978), rice (Oryza sativa L.) (Haefele et al. 2004), wheat (Triticum aestivum L.) (Ramsel and Wicks, 1988), and several other crops (Callaway, 1992). Genetic differences have been report among peanut genotypes in tolerance to weed interference (Agostinho et al., 2006; Hiremath et al., 1997) and ability to suppress weed growth (Fiebig et al., 1991). However, peanut genotype differences in competitiveness with weeds have rarely been investigated within virginia market type peanut genotypes grown in the mid-Atlantic U.S.
Objectives
The objective of this investigation was to determine if differences exist among virginia market type peanut genotypes in weed competitiveness. Specific objectives are:
-
To determine if differences among genotypes exist in early-season canopy ground cover.
-
To determine if differences among genotypes exist in reduction of weed biomass.
-
To determine if differences among genotypes exist in reduction of peanut biomass due to weeds.
-
To determine if differences among genotypes exist in pod yield due to weed competition.
Materials and Methods
Experiments were conducted in North Carolina during 2007 and 2008 at the Upper Coastal Plain Research Station located near Rocky Mount on a Goldsboro sandy loam soil (fine-loamy, siliceous, subactive, thermic Aquic Paleudults). Peanut was planted in mid-May in conventionally-tilled raised seedbeds in rows spaced 91 cm apart at a seeding rate designed to achieve an in-row population of 13 plants/m. Two non-treated peanut rows separated two row experimental units. Plot length was 6 m. Aldicarb1 was applied at 1.1 kg ai/ha in-furrow to control thrips (Frankliniella spp.). With the exception of weed control treatments, other management practices common for the region were utilized (Brandenburg, 2009; Jordan, 2009; Shew, 2009).
Eight peanut genotypes including the cultivars NC 10C (Wynne et al., 1991b), NC-V 11 (Wynne et al., 1991c), NC 12C (Isleib et al., 1997), Phillips (Isleib et al., 2006), and VA 98R (Mozingo et al., 2000) and the breeding lines N99027L, N01013T, and N02020J (Table 1) were compared under weedy and weed-free conditions. These cultivars were chosen based on variation in seed size and growth habit within virginia market type genotypes. Weed-free subplots were maintained with an early-postemergence application of paraquat2 at 0.14 kg ai/ha plus diclosulam3 at 24 g ai/ha plus metolachlor4 at 1.42 kg ai/ha plus nonionic surfactant5 at 0.125% (v/v) 8 days after planting followed by clethodim6 at 0.14 kg ai/ha plus crop oil concentrate7 at 1.0% (v/v) in mid June. Additionally, weed escapes were removed weekly by hand throughout the season. Natural weed infestations were left unmanaged in weedy subplots where weed and peanut biomass were determined. Weedy plots where peanut pod yield was measured were treated with clethodim at 0.14 kg/ha plus crop oil concentrate at 1.0% (v/v) in mid June to limit natural weed infestations to broadleaf weeds. The decision to eliminate grass weeds in the weedy subplots designated for yield measurement was made to avoid an overwhelming weed infestation which could preclude a mechanical pod harvest process. Herbicides were applied using a CO2–pressurized backpack sprayer calibrated to deliver 145 L/ha using regular flatfan nozzles8 at 214 kPa.
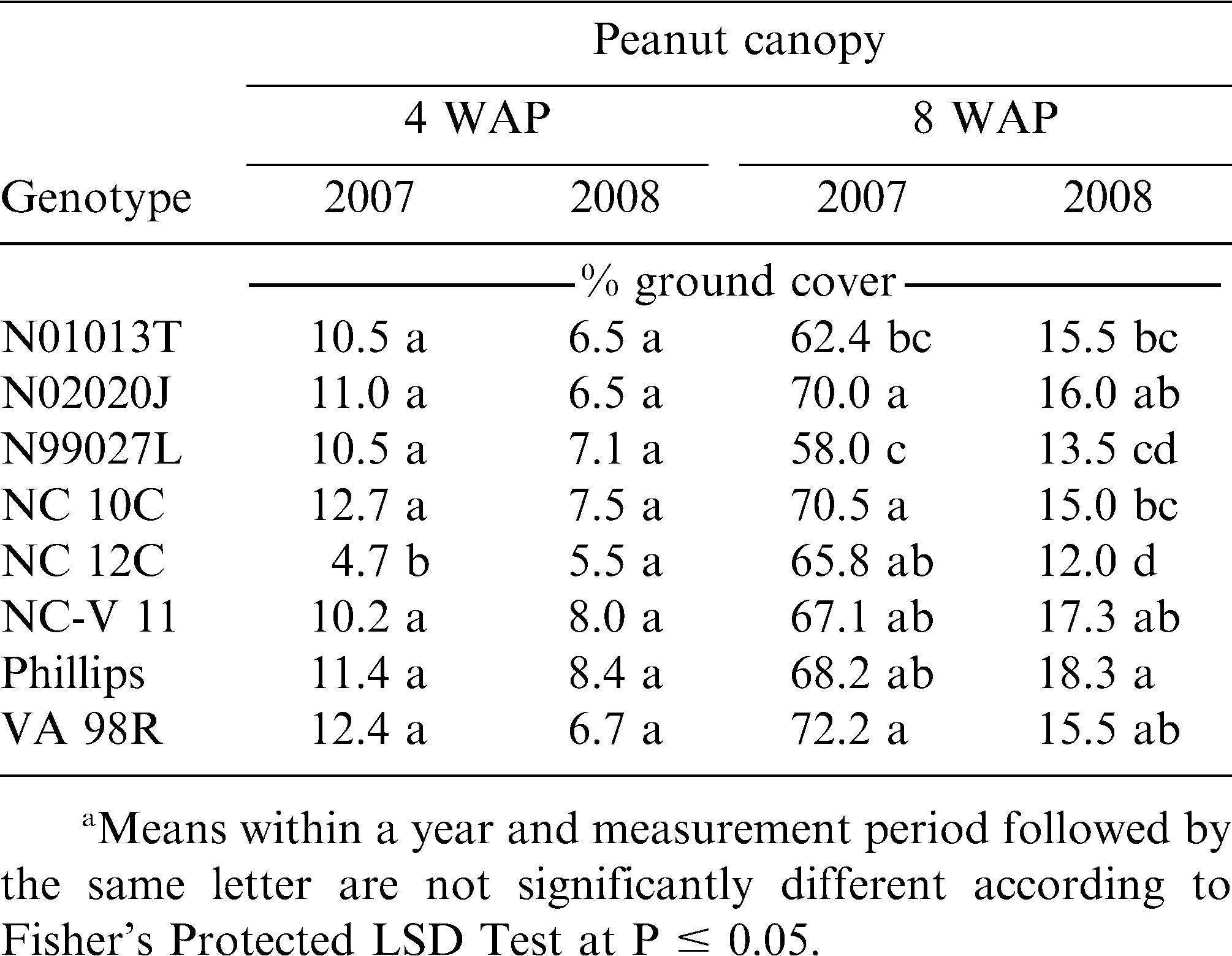
Estimations of canopy cover were recorded with overhead photography 4 and 8 weeks after planting (WAP) in weed-free plots. Images were recorded with a Canon PowerShot A360 Digital Camera9 (8.0 mega pixels) using a custom built camera stand to center and level the camera 2 m above the row of each weed-free plot. Plots were shaded during photography to avoid excessive shadow effects in image processing. The digital image size was 1.37 m wide and 1.87 m of the row, capturing approximately 23 plants in the row. The digital images were processed with Adobe Photoshop 5.010 to convert peanut canopy to black pixels and visible ground to white pixels. A Javascript pixel counting software11 as described by Stewart et al., (2007) was utilized to estimate the percent canopy coverage from the ratio of black pixels to the total number of black and white pixels. Weedy peanut biomass, weed-free peanut biomass, and weed biomass were harvested by hand at 10 WAP in the first experiment. Fresh weight was determined, subsampled and oven dried at 70 C for 5 days to determine total dry biomass. Differences in weed biomass between genotypes were measured with the hypothesis that less weed biomass indicated a more competitive peanut genotype. Weed species density data were recorded 12 WAP in weedy yield subplots. Because of the species variation in the weed population, a predicted percent yield loss due to weeds was estimated using the Herbicide Application Decision Support System12 (HADSS) (Bennett et al., 2003). HADSS predicted yield loss is based on the population density and competitive index of the weed species (Table 2). This HADSS predicted yield loss provided an overall weed competitive estimate through the predicted peanut pod percent yield loss estimate. Dominant weed species in 2007 and 2008 were common lambsquarters (Chenopodium album L.), ivyleaf morningglory [Ipomoea hederacea (L.) Jacq.], jimsonweed (Datura stromonium L.), and Palmer amaranth (Amaranthus palmeri S. Wats.) at a density of 0.5 to 2 plants/m2, 0.5 to 1 plants/m2, 0.5 to 2 plants/m2, 0.5 to 3 plants/m2 respectively. Peanut were dug and vines inverted in early October for all test sites. Peanut were dug based on mesocarp color (Williams and Drexler, 1981). The entire subplot width was dug and inverted. Peanut pods and vines were air dried for approximately 1 week before threshing. Final pod yield was adjusted to 8% moisture. The HADSS yield loss estimation was compared with actual percent yield loss for each genotype. Differences between estimated yield loss and actual yield loss were determined with the hypothesis that a genotype resulting in less actual yield loss than predicted was indicative of a genotype with a greater tolerance to weed interference.
HADSSa (Bennett et al., 2003) individual competitive indices (CI) for dominant weeds counted at 12 weeks after planting.
The experimental design was a split plot arrangement in a randomized complete block with eight replications. Main plots consisted of 2 rows 15.3 m in length for each peanut genotype. Subplots treatments, which were randomly assigned in position within the main plot, included a weed-free section for peanut pod yield measurement (6.1 m of 2 rows), a weedy section for peanut pod yield measurement (6.1 m of 2 rows), a weed-free section for digital photographic estimations of canopy coverage at 4 and 8 WAP and peanut biomass measurement at 10 WAP (3.05 m of 1 row), and a weedy section for peanut and weed biomass measurement at 10 WAP (3.05 m of 1 row).
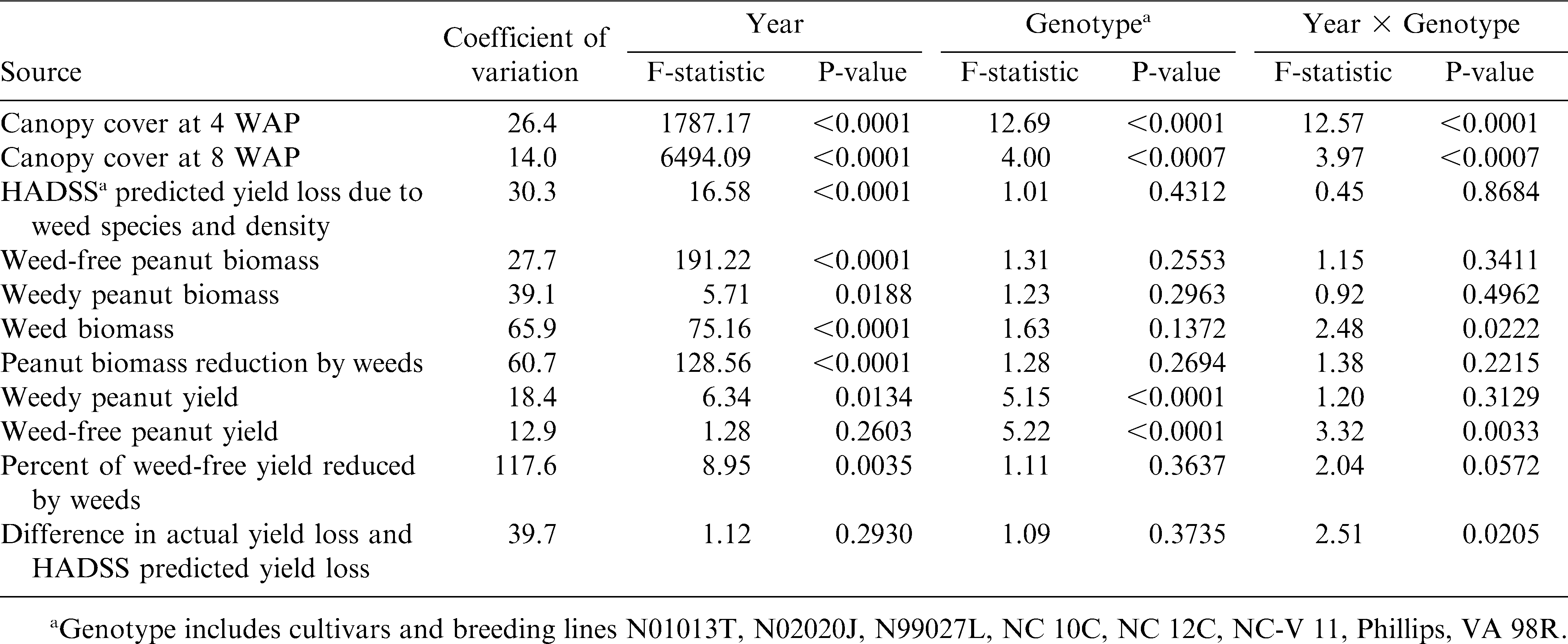
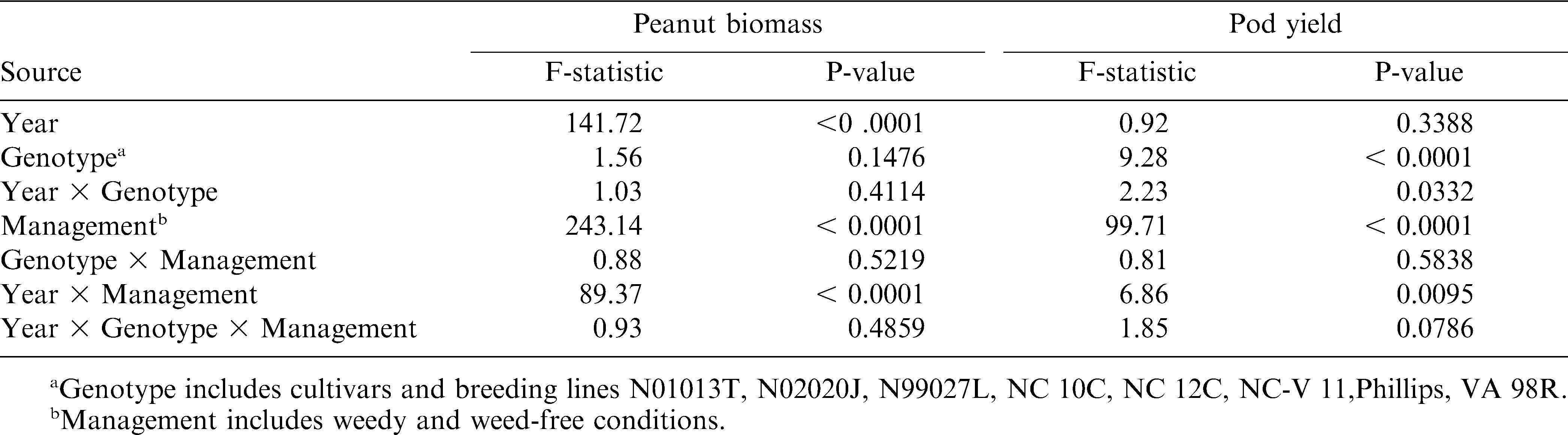
Statistical analyses were performed using the SAS13 statistical analysis software package. Results were reported separately for 2007 and 2008 for canopy cover at 4 and 8 WAP, HADSS predicted yield loss due to weed species and density, weed-free peanut biomass, weedy peanut biomass, weed biomass, peanut biomass reduction by weeds, weedy peanut yield, weed-free peanut yield, percent of weed-free yield reduced by weeds, and the difference in actual yield loss and HADSS predicted yield loss due to weeds. Separate year reporting was due to several significant year by genotype interaction effects on analyzed measurements (Table 3). Estimates of percent ground canopy cover at 4 and 8 WAP, HADSS predicted yield loss due to weeds, weedy and weed-free peanut biomass, weed biomass, peanut pod yield in weedy and weed-free conditions, actual percent of yield loss due to weeds, and differences in HADSS predicted losses vs. actual percent yield losses due to weeds were factors measured only in weed-free or weedy conditions and therefore only analyzed for the effect of year, genotype, and year by genotype interactions (Table 3). Peanut biomass and peanut pod yield (Table 4) were measured in both weedy and weed-free conditions and were analyzed for the effect of management (weedy and weed-free conditions) and interactions of year and genotype with management. Means of significant main effects and interactions were separated using Fisher's protected LSD at P ≤ 0.05.
Analysis of variance for the effect of year, genotype, and year × genotype on peanut canopy cover, peanut biomass, HADSS (Bennett et al., 2003) estimated yield loss due to weeds, peanut biomass, weed biomass, peanut yield in weedy and weed-free conditions, percent of weed-free peanut yield reduced by weeds, and difference in actual peanut yield loss and HADSS predicted yield loss at Rocky Mt., NC during 2007 and 2008.
Results and Discussion
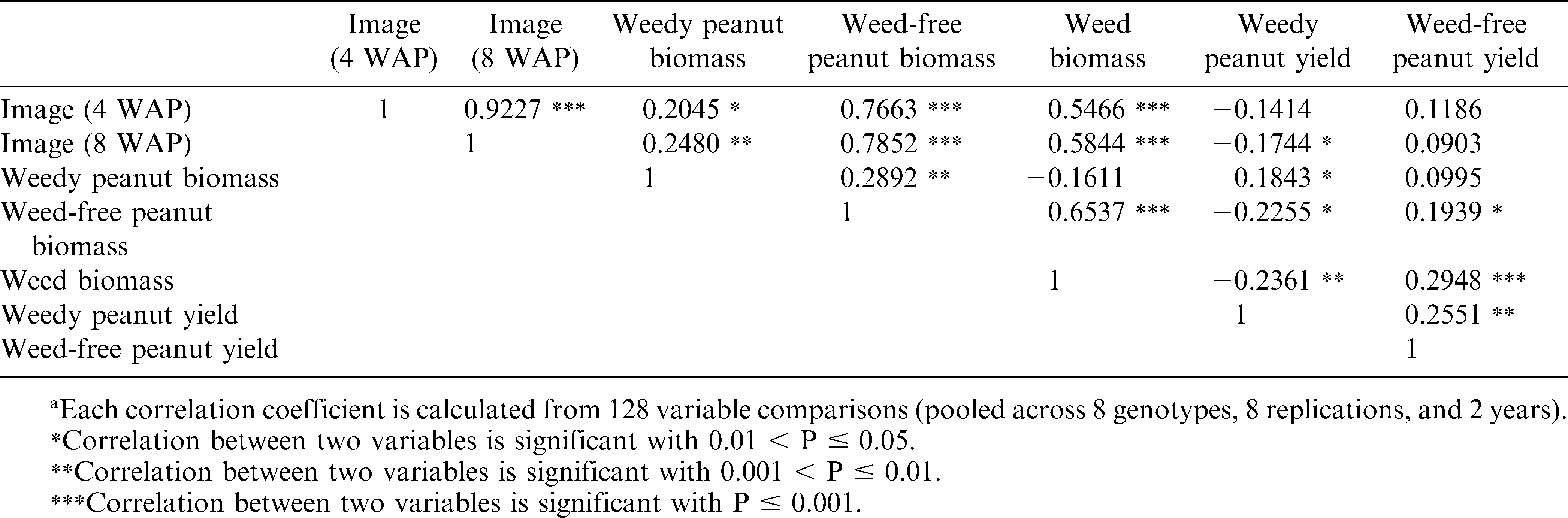
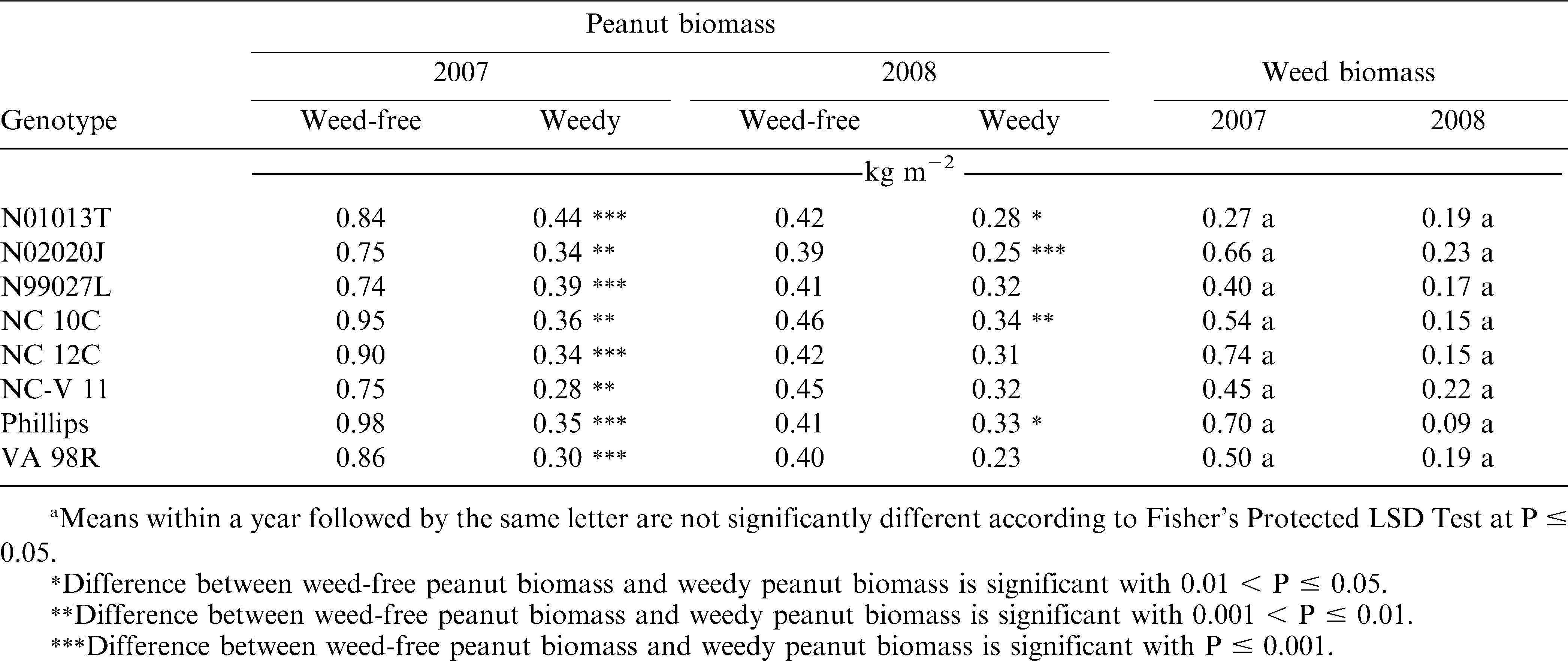
Differences in peanut canopy among genotypes were detected with overhead photography estimations of percent ground cover by canopy (Table 5). Canopy growth in 2008 at 4 and 8 WAP were severely reduced compared to growth in 2007 due to drier conditions early in the season and reduced ability to irrigate. The only early (4 WAP) difference in canopy coverage was detected in 2007. The smaller percent ground cover by NC 12C may have been due to delayed germination and the effect dissipated by 8 WAP. Variability in percent ground cover among genotypes was more pronounced later in the season (8 WAP). Genotypes N02020J, NC-V 11, Phillips, and VA 98R were in the largest canopy coverage group in both years. Stewart et al. (2007) found that overhead photography was well correlated with leaf area index (R2 = 0.74), a critical canopy characteristic for light competition (Gibson et al., 2003). However, increased canopy cover did not translate to improved weed suppression (estimated by weed biomass harvested at 10 WAP). The positive correlation between individual canopy coverage estimations and weed biomass suggests that weed biomass was increasing with more canopy coverage (Table 6). However, no significant correlation was detected between genotype mean values for canopy cover and weed biomass (data not shown). A similar pattern was present between weed-free peanut biomass and weed biomass with a strong correlation (0.65, P <.001) at the plot level (Table 6), but not with mean values (data not shown). This pattern may be influenced by the inescapable consequence that plots with soil conditions capable of supporting more peanut canopy growth also may have supported more weed growth. Both 2007 and 2008 were drier than average years, potentially magnifying the effects of soil conditions on plant growth. Furthermore, no differences in weed biomass were detected among the genotypes (Table 7) and there was no interaction between genotype and management (weedy and weed-free conditions) effects on peanut biomass (Table 4). The only indication of variation among genotypes in tolerance to weed interference was in the difference between weedy and weed-free peanut biomass in 2008 (Table 7). The genotypes N01013T, N02020J, NC 10C, and Phillips showed a reduction in peanut biomass due to weed presence, while the genotypes N99027L, NC 12C, NC-V 11, and VA 98R did not have a detectable reduction in peanut biomass due to weed interference. These findings could be interpreted as a possible variability among genotypes in tolerance to weed interference, but these differences were not consistently detected.
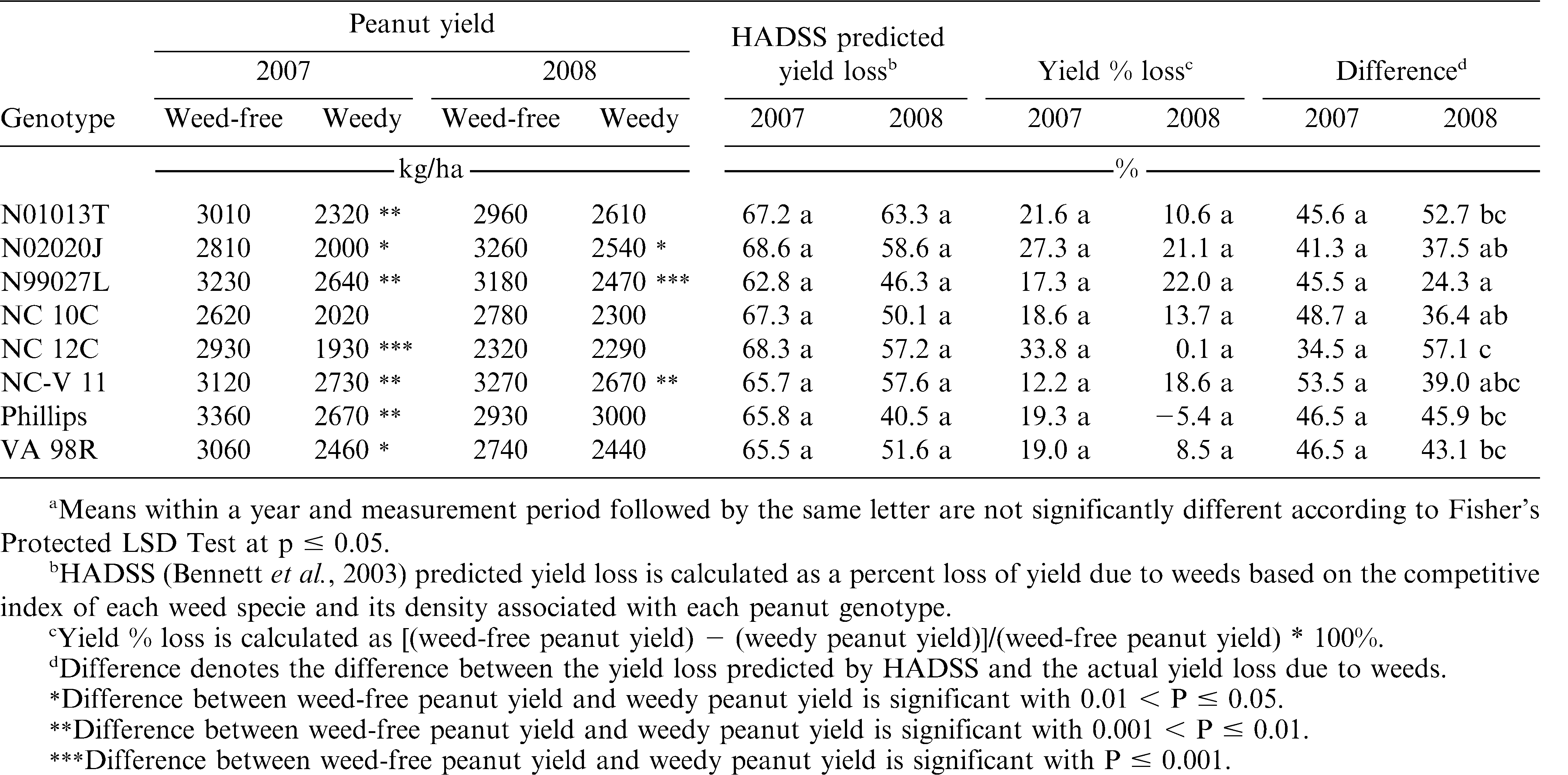
No differences among genotypes were detected for the densities of the dominant weeds associated with the genotypes (data not shown). The presence of some minor weeds was variable across each location (data not shown), thus HADSS was utilized to estimate a yield percent loss due to overall weed interference associated with each genotype. No interaction was detected between genotype and management (weedy and weed-free conditions) for peanut pod yield (Table 4), suggesting little to no differences between the genotypes in yield response to weed interference. However, the differences between weed-free and weedy peanut pod yield were variable between genotypes. NC 10C was the only genotype that showed no difference in yield due to weeds in both years (Table 8). N01013T, NC 12C, Phillips, and VA 98R showed no difference in yield due to weeds in 2008 only. Genotypes did not differ in the presence of weeds as shown by no differences in the HADSS predicted yield loss due to weeds associated with the genotypes (Table 8). Similarly, when comparing the yield loss percentage due to weeds among genotypes, no differences were detected (Table 8). The difference between the HADSS predicted yield loss percentage and the actual percent yield loss was compared with the hypothesis that a larger difference between the predicted and actual yield loss might indicate a genotype more tolerant of weed interference. No such weed tolerant genotypes were detected in 2007 (Table 8). However, in 2008 several genotypes resulted in less yield loss than anticipated by HADSS (Table 8) with genotypes N01013T, NC 12C, NC-V 11, Phillips, and VA 98R resulting in the least yield loss as estimated from the weed species and densities growing with those genotypes.
In any discussion of competitive genotypes, the term competition itself must be addressed. Competition can be examined from two perspectives: suppressiveness and tolerance. Suppressiveness is the ability of a plant to reduce the biomass and/or reproductive production of plants in close proximity to a greater than expected extent. Tolerance is the ability of a plant to endure the close proximity of other plants and have a less than expected decrease in biomass and/or reproductive production. In this series of experiments, it was hypothesized that an increased canopy percent ground cover would indicate an advantage in competition for solar radiation and result in a reduction in weed biomass. While some differences existed between cultivars and breeding lines in the ground canopy cover, there were no differences in weed biomass. It was also hypothesized that less than expected peanut yield loss due to weeds would indicate a peanut genotype tolerant to weed interference. The HADSS predicted percent yield loss due to weed species competitive indices and densities measured was consistently higher than the actual percent yield loss in weed-free and weedy comparisons. Yield loss prediction models have been demonstrated to overestimate yield loss due to weeds in some cases (Willis et al., 2006). This discrepancy was the same for all peanut genotypes tested, indicating no differences in weed tolerance.
Results from our research suggest few consistent differences among virginia market type genotypes in weed competitive ability. Virginia market type peanut cultivars seldom reach a canopy height more than 50 cm due to a morphological growth that is generally prostrate in habit (Mozingo et al., 1987). Crop height is an advantageous characteristic for weed competition (Jannink et al., 2000). Weeds that are not controlled early in the season can often grow taller than peanut and can quickly overwhelm peanut even if differences in some morphological characteristics would be advantageous. Spanish and valencia market type peanut cultivar groups tend to have a more erect growth habit than virginia market types (Gregory et al., 1951) and may compete more effectively with weeds. Hiremath et al. (1997) reported differences in cultivar tolerance to weed competition when comparing a virginia market type cultivar with a spanish and valencia market type cultivar. In their study, the highest yielding peanut was the virginia market type in weed-free conditions. However, in the presence of weeds, the spanish and valencia market type cultivars out yielded the virginia market type. Agostinho et al., (2006) showed weed tolerance differences among peanut cultivars in relation to the reduction of seed weight due to weeds but the influence of erect or prostrate growth was not consistent.
Other peanut characteristics have also been theorized to affect competitive ability. In another study, a runner type peanut cultivar was better able to avoid reductions of dry matter caused by common cocklebur (Xanthium strumarium L.) (Fiebig et al., 1991). The researchers concluded that late maturity seemed to increase the weed tolerance ability of the peanut genotype but that such a characteristic was not preferable for many peanut producers.
Summary
While differences may exist among peanut cultivar groups in weed competitive ability, our results indicate that peanut cultivar selection within virginia market type cultivars does not seem to be a promising weed management tactic by itself.
Sources of Materials
1Temik 15G®. Bayer CropScience LP, Research Triangle Park, NC 27709.
2Gramoxone INTEON herbicide®. Syngenta Crop Protection, Greensboro, NC 27419.
3Strongarm Herbicide®. Dow AgroSciences, Indianapolis, IN 46268.
4Dual Magnum Herbicide®. Syngenta Crop Protection, Greensboro, NC 27419.
5Induce® nonionic surfactant. Proprietary blend of alkyl aryl polyoxyalkane ethers, free fatty acids, and dimothyl polysiloxane, 90%. Helena Chemical Company, Collierville, TN 38107.
6Select 2EC herbicide®. Valent USA Corporation, Walnut Creek, CA 94596.
7Agri-Dex® spray adjuvant. Proprietary blend of alkyl aryl polyoxyalkane ethers, free fatty acids, and dimothyl polysiloxane, 90%. Helena Chemical Company, Collierville, TN 38107.
88002 Spray nozzles. Spraying Systems Company, Wheaton, IL 60189-7900.
9Canon PowerShot A360 Digital Camera (8.0 mega pixels), Canon 30-2, Shimomaruko 3-chome, Ohta-ku, Tokyo, 146-8501, Japan.
10Adobe Photoshop 5.0, Adobe Systems Incorporated, 345 Park Avenue, San Jose, CA 95110.
11PixelCounter 1.0, a Javascript software developed at North Carolina State University, Raleigh, NC 27695.
12HADSS™, “Herbicide Application Decision Support System,” is a trademark of North Carolina State University and is distributed by AgRenaissance Software LLC, P.O. Box 68007, Raleigh, NC 27613, www.AgRenaissance.com.
13SAS software for Windows, Version 9.1.3. SAS Institute Inc., 100 SAS Campus Dr., Cary, NC 27513.
Acknowledgements
Appreciation is expressed to staff at the Upper Coastal Plain Research Station, Dewayne Johnson, Carl Murphey, Adam Smith, Carrie Brinton, and William Blount for assistance with these experiments. This research was supported financially by the North Carolina Peanut Growers Association, Inc., the National Peanut Board, the Golden Leaf Foundation of North Carolina, and the USAID Peanut CRSP LAG-G-00-96-0013-00.
Literature Cited
Agostinho F.H Gravena R Alves P.L.C.A Salgado T.P and Mattos E.D 2006 The effect of cultivar on critical periods of weed control in peanuts Peanut Sci. 33 : 29 – 35 .
Bennett A.C Price A.J Sturgill M.C Buol G.S and Wilkerson G.G 2003 HADSS™, Pocket HERB™, and WebHADSS™: Decision Aids for Field Crops Weed Technology. 17 : 412 – 420 .
Branch W.D and Culbreath A.K 2008 Disease and insect assessment of candidate cultivars for potential use in organic peanut production Peanut Sci. 35 : 61 – 66 .
Brandenburg R.L 2009 Peanut insect management . Pages 77 – 94 In 2009 Peanut Information North Carolina Coop. Ext. Serv. Ser. AG-331 132 pp.
Callaway M.B 1992 A compendium of crop varietal tolerance to weeds American J. of Alternative Agric. 7 : 169 – 180 .
Culbreath L 2005 Are they nuts? Southern researchers and farmers tackle organic peanuts, The New Farm on line at: http://newfarm.rodaleinstitute.org/features/2005/1105/peanuts/culbreath.shtml. Verified April 24, 2009 .
Fiebig W.W Shilling D.G and Knauft D.A 1991 Peanut genotype response to interference from common cocklebur Crop Sci. 31 : 1289 – 1292 .
Gibson K.D Fischer A.J Foin T.C and Hill J.E 2003 Crop traits related to weed suppression in water-seeded rice (Oryza sativa L.) Weed Sci. 51 : 87 – 93 .
Gregory W.C Smith B.W and Yarbrough J.A 1951 Morphology, genetics, and breeding, . pp. 62 – 70 In The Peanut - the Unpredictable Legume Nat. Fert. Assoc. , Washington, D.C .
Guerena M and Adam K 2008 Peanuts: organic production ATTRA. On line at http://attra.ncat.org/new_pubs/attra-pub/PDF/peanuts.pdf?id=DC Verified April 24, 2009.
Haefele S.M Johnson D.E Bodj D.M Wopereis M.C.S and Miezan K.M 2004 Field screening of diverse rice genotypes for weed competitiveness in irrigated lowland ecosystems Field Crops Res. 88 : 39 – 56 .
Hiremath S.M Shiv Raj A Sajjan A.S Kamatar M.Y and Chetti M.B 1997 Effect of herbicides on weed control efficiency in diverse groundnut genotypes World Weeds. 4 : 163 – 168 .
Isleib T.G Rice P.W Bailey J.E Mozingo R.W and Pattee H.E 1997 Registration of ‘NC 12C’ peanut Crop Sci. 37 : 1976 .
Isleib T.G Rice P.W Mozingo R.W Copeland S.C Graeber J.B Pattee H.E Sanders T.H Mozingo R.W and Coker D.L 2006 Registration of ‘Phillips’ peanut Crop Sci. 46 : 2308 – 2309 .
Jannink J.L Orf J.H Jordan N.R and Shaw R.G 2000 Index selection for weed suppressive ability in soybean Crop Science. 40 : 1087 – 1094 .
Jordan D.L 2009 Peanut production practices . Pages 27 – 49 In 2009 Peanut Information North Carolina Coop. Ext. Ser. AG-331 132 pp.
Lamb M.C 2007 The economics of organic versus conventional peanuts 2006 Proc. Am Peanut Res. Educ. Soc. 38 : 92 .
Liebman M and Gallandt E 1997 . Many little hammers: ecological management of crop-weed interactions . pp. 291 – 343 In: Jackson L.E (ed.) Ecology in Agricultural Academic , San Diego, CA .
Mozingo R.W Coffelt T.A and Wynne J.C 1987 Characteristics of virginia-type peanuts released from 1944–1985 Southern Coop. Ser. Bull. No. 326 .
Mozingo R.W Coffelt T.A and Isleib T.G 2000 Registration of ‘VA 98R’ peanut Crop Sci. 40 : 1202 – 1203 .
Organic Farming Research Foundation 2001 Final results of the third biennial national organic farmers survey, On line at http://ofrf.org/publications/pubs/3rdsurvey_results.pdf Verified April 24, 2009 .
Parker B 2007 Organic peanut production in the US: the sheller's perspective 2006 Proc. Am. Peanut Res. Educ. Soc. 38 : 92 .
Ramsel R.E and Wicks G.A 1988 Use of winter wheat (Triticum aestivum) cultivars and herbicides in aiding weed control in an ecofallow corn (Zea mays) rotation Weed Sci. 36 : 394 – 398 .
Remison S.U 1978 The performance of cowpea (Vigna unguiculata (L.) Walp.) as influenced by weed competition J. of Agricultural Sci., Cambridge 90 : 523 – 530 .
Sharma H.C Pampapathy G Dwivedi S.L and Reddy L.J 2003 Mechanisms and diversity of resistance to insect pests in wild relative of groundnut J. of Economic Entomol. 96 : 1886 – 1897 .
Shew B.B 2009 Peanut disease management: Pages 95–120 in 2009 Peanut Information North Carolina Coop. Ext. Ser. AG-331 132 pp.
Stewart A.M Edminsten K.L Wells R and Collins G.D 2007 Measuring canopy coverage with digital imaging Communications in Soil Science and Plant Analysis. 38 : 895 – 902 .
Williams E.J and Drexler J.S 1981 A non-destructive method for determining peanut pod maturity, pericarp, mesocarp, color, morphology, and classification Peanut Sci. 8 : 134 – 141 .
Willis J.B Murray D.S and Murdock S.W 2006 Validation of weed competitive indices for predicting peanut yield losses in Oklahoma Weed Tech. 20 : 688 – 694 .
Wooley J.N and Smith M.E 1986 Maize zea-mays plant types suitable for present and possible bean phaseolus-vulgaris relay systems in Central America Field Crops Res. 15 : 3 – 16 .
Wynne J.C Beute M.K and Nigam S.N 1991a Breeding for disease resistance in peanut (Arachis hypogaea L.) Annual Review of Phytopathology. 29 : 279 – 303 .
Wynne J.C Beute M.K Bailey J and Mozingo R.W 1991b Registration of ‘NC 10C’ peanut Crop Sci. 31 : 484 .
Wynne J.C Coffelt T.A Mozingo R.W and Anderson W.F 1991c Registration of ‘NC-V11’ peanut Crop Sci. 31 : 484 – 485 .
Notes
- Research Associate, Crop Science Department, North Carolina State University, Raleigh, NC
- Associate Professor, Crop Science Department, North Carolina State University, Raleigh, NC
- Professor, Crop Science Department, North Carolina State University, Raleigh, NC *Corresponding author's E-mail: david_jordan@ncsu.edu
Author Affiliations