Introduction
Peanut ( Arachis hypogaea L.) is the second most cultivated crop in Zambia. In 2013, groundnut was cultivated on 207,249 Ha, mostly by subsistence farmers, and the average yield was 515 Kg/Ha (FAOSTAT, 2013). Within Zambia, peanut production is highest in the Eastern Province where the crop is grown on 80,000 Ha in different agroecological zones, from the cooler and wetter plateau, i.e., Chipata District, to the hotter and drier Luangwa Valley, i.e., Mambwe District. Like in most countries in southern Africa, the cropping season starts at the onset of the rains in December, and peanut is grown in rotation with other staples and cash crops such as maize, cotton, sunflower, tobacco, etc. Farmers, however, prioritize sowing corn, cotton, tobacco, and sunflower over peanut, which are planted up to 3 weeks after the rains commence.
Aflatoxins are chemical compounds produced by the opportunistic pathogens Aspergillus flavus Link ex Fries, (Teleomorph Petromyces flavus) A. parasiticus Speare (Teleomorph P. parasiticus), and A. nomius (Teleomorph P. nomius) (Abbas et al., 2004; Amaike and Keller, 2011; Horn et al., 2009a; Horn et al., 2009b; Horn et al., 2011; Paterson et al., 1997), however, only A. flavus and A. parasiticus have an impact on agriculture (Horn, 2003; Klich, 2007). Oil seed crops such as corn, peanut, cotton, and sunflower are susceptible to aflatoxin contamination. Aflatoxins are a common contaminant of foods, especially the staple diets of many in developing countries (Williams et al., 2004) and the US Food and Drug Administration considers aflatoxins to be unavoidable contaminant of foods (Williams et al., 2004). Dietary exposure to aflatoxin can lead to aflatoxicosis, defined by Williams et al. (2004), as poisoning from ingesting aflatoxins. Aflatoxicosis, can either be acute, which is severe intoxication that leads to direct liver damage followed by sickness or death (Azziz-Baumgartner et al., 2005), or chronic, which is subsymptomatic exposure that may lead to nutritional and immunological consequences (Williams et al., 2004). However, all doses i.e., chronic and acute, have an accumulative effect on the risk for cancer (Williams et al., 2004). In developing countries, exposure to aflatoxins may begin early, when infants are exposed tog weaning and adult foods (Gong et al., 2002).
Aflatoxin contamination is a serious problem in Africa (Atehnkeng et al., 2008; Kaaya and Warren, 2005; Matumba et al., 2015; Monyo et al., 2012; Mutegi et al., 2009; Setamou et al., 1997; Wagacha and Muthomi, 2008) and also worldwide (Amaike and Keller, 2011; Munkvold, 2003). Despite the magnitude of the aflatoxin problem, only a few studies have attempted large scale comparisons of soil populations of aflatoxigenic fungi over large geographic areas which represent differences in climatic, soil type, and cropping systems (Horn, 2003). Reports on characterization of aflatoxigenic fungi have been published mostly from the southeastern US (Abbas et al., 2004; Boyd and Cotty, 2001; Horn, 2003; Horn, 2005; Horn et al., 1995; Sweany et al., 2011; Jamie-Garcia and Cotty, 2006). In Africa, there have been reports from Kenya (Okoth et al., 2012) where the authors tested isolates from two different agroecologies – the hotter and drier Makueni, where the acute outbreak of aflatoxicosis occurred (Lewis et al., 2005), and the cooler and wetter Nandi, a corn growing region in the Rift Valley. Okoth et al. (2012) reported that there were no differences in the occurrence of A. flavus between the two tested regions, however, S-strain A. flavus was more prevalent in Makueni compared to Nandi, where L-stains were more prevalent. In Nigeria, Atehnkeng et al. (2008) collected maize samples from three agroecological zones and determined the identity of isolated species and also the incidence of toxigenic versus atoxigenic strains. However, most reports from Africa document the prevalence of aflatoxin contamination in different commodities and researchers often do not differentiate or characterize Aspergillus species (Monyo et al., 2012; Matumba et al., 2015).
It is important to study and document factors that influence aflatoxin contamination, such as understanding the activities and population structure of aflatoxigenic fungi in the soil, because this is a pre-requisite for developing effective measures to control aflatoxin contamination (Horn, 2003). Other important factors also should be considered when developing preharvest aflatoxin mitigation measures, i.e., the crops grown and their resistances to aflatoxin contamination, soil characteristics, agronomic and cultural practices, weather, and the occurrence of drought. Taken together, these would be used to develop a holistic approach to aflatoxin mitigation.
To our knowledge, there are no published reports from Zambia on the population density of A. flavus , and characterization of aflatoxigenic fungi. Therefore, the objective of this study was to identify aflatoxigenic fungi from soils cropped to peanut, estimate the population densities of A. flavus , and to characterize isolates for aflatoxin production.
Materials and Methods
Population density of A. flavus .
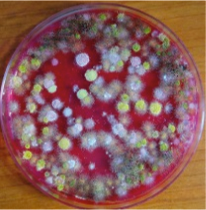
Geo-referenced soil samples were collected from fields cropped to groundnuts in Nyimba, Petauke, Mambwe, and Chipata Districts during May to June 2012. All these districts are important in groundnut production, with Nyimba and Mambwe being located in the hotter Luangwa Valley, and Petauke and Chipata being in the cooler Plateau. From each field, soils were sampled from at least 5 distant spots, 0 to 10 cm deep along the ridge, and aggregated into a single composite sample. From Nyimba, Petauke, Mambwe, and Chipata, a total of 100, 100, 95, and 104, soil samples were collected, respectively. For processing, samples were taken to ICRISAT laboratories in Lilongwe, Malawi. Estimation of the population densities of A. flavus was done using methods from Horn et al. (1995). Briefly, soil samples were air dried for 1 week and then a subsample of 3.3 g was measured into test-tubes containing 10 ml of 0.2% water agar, and vortexed. 2 ml of the mixture was then added into another test-tube containing 8 ml of 0.2% water agar and vortexed. Four replicates, 0.2 ml each, of the mixture were then plated on Modified Dichloran Rose Bengal (MDRB) media. Petri dishes were then incubated for 3 days at 37 °C and colonies (Fig. 1) were counted under the dissecting microscope (Jenco International Inc, Portland, USA).
Identification of Aspergillus spp.
The following reference isolates were obtained from the Centraalbureau voor Schimmelcultures (CBS) Fungal Biodiversity Centre: i) A. parasiticus CBS 100939 98.0708 EX-B12-1045; ii) A. terreus var terreus CBS 601.65 10.1474 EX-B12-1045; iii) A. nomius CBS 260.88 07.1480 EX-B12-1045; iv) A. caelatus CBS 763.97 05.0604 EX-B12-1045; v) A. fumigatus CBS 127.803 10.1913 EX-B12-1045; and vi) A. flavus var flavus CBS 573.65 07.1168 EX-B12-1045. Working cultures of these isolates were grown on Czapek's agar (CzA), Potato Dextrose Agar (PDA), and Malt Extract Agar (MEA). Test tubes with slants of CzA were used for long-term storage at both room temperature and at 4 °C. A total of 91 isolates were randomly selected from cultures growing on MDRB soil dilution plates and transferred onto plates with CzA. Two weeks later, hyphal-tip transfers were taken from the edge of these cultures and transferred onto plates with CzA, PDA, and MEA. Plates were then held at room temperature and the morphological characteristics of the colonies were recorded two weeks later. To observe microscopic characteristics, slides from each of the cultures were mounted and observed at ×200, ×400, and ×1000, using a Jenco brightfield compound microscope (Jenco International Inc, Portland, USA). Aspergillus spp. were then identified by comparing with the isolates from CBS and also by using the Aspergillus spp. taxonomic key from Domsch et al. (2007).
Toxigenicity of Aspergillus spp.
Hyphal-tip isolates were grown on CzA for two weeks. Single spore isolate transfers were aseptically taken from the edge of the colony using a sterile needle, into Yeast Extract Sucrose (YES) media in 10-ml vials. The vials were then sealed and incubated at room temperature for 1 week. The negative and positive controls were, non-inoculated and inoculated YES media with a toxigenic strain from CBS collection, respectively. 1 week later, 10-g of the YES media was weighed into sterile polypropylene Falcon® tubes. 20 ml of 70% (v/v) methanol was then added into the tubes and vortexed for 1 minute. The mixture was then filtered through a Whatman # 1 filter paper into a conical flask. 50 μl of the filtrate was then pipetted into micro-wells and total aflatoxin (B1+B2+G1+G2) production was determined using strip tests Agra Strip ® (Romer Labs, Austria) at 4 and 20 ppb.
Results and Discussion
In this study, we documented the population densities of A. flavus across two agroecologies in eastern Zambia. Population densities of A. flavus varied amoung districts. The mean population density of A. flavus was 2.6, 1.8, 2.0, and 2.4 log CFU/g of dry soil in Chipata, Mambwe, Nyimba, and Petauke Districts, respectively. Mambwe and Nyimba Districts, which fall in the comparatively warmer Luangwa Valley, had lower populations of A. flavus than Chipata and Petauke Districts, located in the cooler and wetter plateau.
Several studies have documented population densities at different scales—from single fields to larger geographical zones, at different sampling frequencies, and also from fields with different cropping histories. Horn (2005) sampled two cultivated fields in Georgia at five different times and showed that for populations of A. flavus L strain, field A had lower mean densities at 2.7 log CFU/g compared to field B at 3.1 log CFU/g. A similar observation was also reported on population densities of A. flavus S strains from these fields (Horn, 2005). From an earlier study, also conducted in Georgia, Horn (1995) found that populations of A. flavus and A. parasiticus did not differ significantly at the beginning of the season and remained stable during the season in fields cropped to corn and peanuts. However, populations of A. flavus relative to A. parasiticus increased significantly in one drought stressed corn field after harvest and debris dispersal. In Virginia, which has relatively cooler weather, populations of A. flavus did not exceed 2.0 log CFU/g soil in peanut cultivated soil (Griffin and Garren, 1974).
Variations in population densities of A. flavus , both between and within districts were therefore expected. However, we expected higher populations in the hotter and drier Luangwa Valley compared to the cooler and wetter plateau. Several factors, such as flooding, could explain for the documented differences in population densities of A. flavus . Parts of the Luangwa Valley are prone to floods. Farmers interviewed during sample collection noted that some fields cropped to groundnuts had been exposed to recurrent flooding events, with water standing in these fields for a few days. For example, in the sampled Chikowa region of Mambwe District, the Kasengwa, Lwuo, and Kabilubilu rivers flooded during the 2011/2012 growing season, whereas in the Masumba region of Mambwe District, the Msandile river flooded affecting fields cropped to groundnuts and other crops. In such areas, it is common practice to plant along the flood plains of the rivers, because farmers try to take advantage of the fertile alluvial soils deposited whenever these rivers break their banks.
Population densities of A. flavus , an aerobic fungus found in the upper profiles of the soil (Horn, 2003), could therefore have been reduced because of these flooding events. Colony forming units estimated through soil dilution plating mostly arise from conidia and hyphae (Horn, 2003). Conidia are asexual spores that are hydrophobic and are mostly found in the upper 6 cm of the soil (Horn, 2003). However, in agricultural soils, Aspergillus spp. can be found up to 30 cm deep, possibly due to ploughing activities that result in mixing of soil at such depths (Horn, 2003). Flooding events could reduce the densities of both conidia and hyphae in the upper soil profiles by either depositing soils from the flooding river, or by washing down these propagules further down the soil profile.
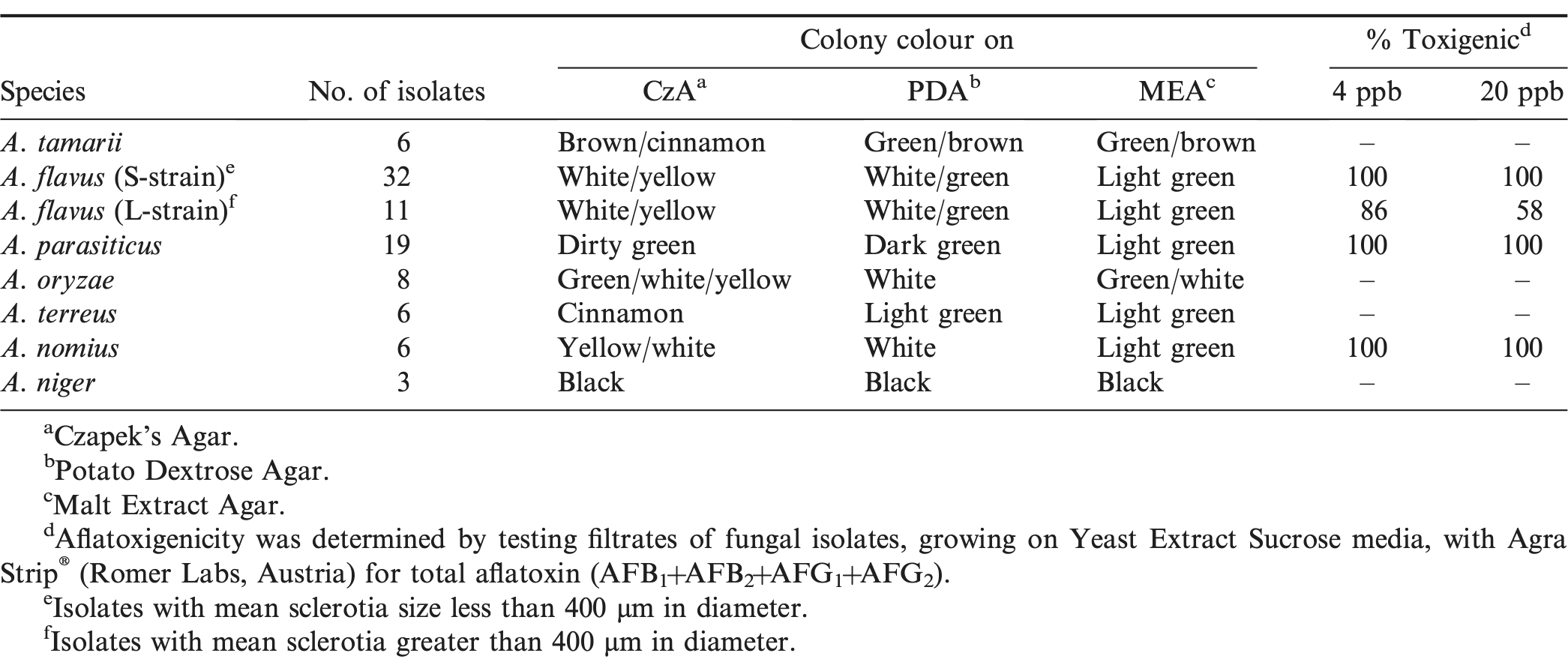
Many papers on characterizing Aspergillus in both agricultural and non-agricultural fields have been published (Boyd and Cotty, 2001; Karthikeyan et al., 2009; Okoth et al., 2012; Solozarno et al., 2014). Boyd and Cotty (2001) reported that A. flavus and A. tamarii were the only species isolated from plant debris and pod samples collected from the dessert in Arizona. In contrast, Okoth et al. (2014), reported that A. flavus was the most abundant species from section Flavi isolated from corn in two districts in different agroecologies in Kenya. A. tamarii and A. parasiticus were also isolated. Interestingly, Okoth et al. (2014) reported that A. parasiticus and A. tamarii were isolated more frequently in the cooler district of Nandi compared to Makueni which is also receives significantly lower rainfall. Of the 91 Aspergillus spp. isolates characterized in our study, the most abundant species was A. flavus (47%), followed by A. parasiticus (21%). Other species identified were A. tamarii (7%), A. oryzae (9%), A. terreus (7%), A. nomius (7%) and A. niger (3%) (Table 1).
A. flavus populations are genetically diverse (Chang et al., 2005; Karthikeyan et al., 2009) and is evident in the different capabilities of isolates producing toxin (Karthikeyan et al., 2009; Okoth et al., 2014). There are many methods, both cultural and analytical that can be used to determine toxigenicity. Abbas et al. (2004) reported that cultural methods were equally as effective in determining toxigenicity and were cheaper than testing for toxin using HPLC or ELISA.
In our study 100% of the S-strain A. flavus isolates produced AFB1 at 4 and 20 ppb, whereas 86% and 56% of the L-strain isolates produced AFB1 at 4 and 20 ppb, respectively. Non-toxigenic isolates have sequence breakpoints in the aflatoxin gene cluster, and Chang et al. (2005) showed that such deletions are not a rare occurrence in natural populations. Recently, Solozarno et al. (2014) reported that 60% of A. flavus isolates from a corn field in Mississippi were toxigenic however they did not differentiate isolates based on S- and L- morphotypes. Interestingly, S morphotypes have been identified in Asia, United States, Africa, South America, but not in Europe (Boyd and Cotty, 2001; Okoth et al., 2014; Perrone et al., 2014). Karthikeyan et al. (2009) also tested isolates of A. flavus recovered from corn for toxin production and reported that 63% (32 out of 52 isolates) produced AFB1 and the rest did not.
Our results also showed that 79% and 64% of tested A. parasiticus isolates produced aflatoxins at 4 and 20 ppb, respectively. A. nomius isolates were also tested for toxin production and all isolates produced aflatoxins at 4 and 20 ppb (Table 1). However, by adopting for this study, a cut-off lower detection limit of 4 ppb, we could be under-reporting on the percentage of toxigenic isolates present. Interestingly, A. flavus isolates may lose the ability to produce aflatoxin in-vitro following successive culture transfers (Sweany et al., 2011) and this too, could have affected our results. Therefore, to better determine toxigenicity, a combination of assays may be used. Abbas et al. (2004), reported that out of 517 isolates tested, combining cultural assays reduced false positives for aflatoxigencity to 0% and false negatives to 7%.
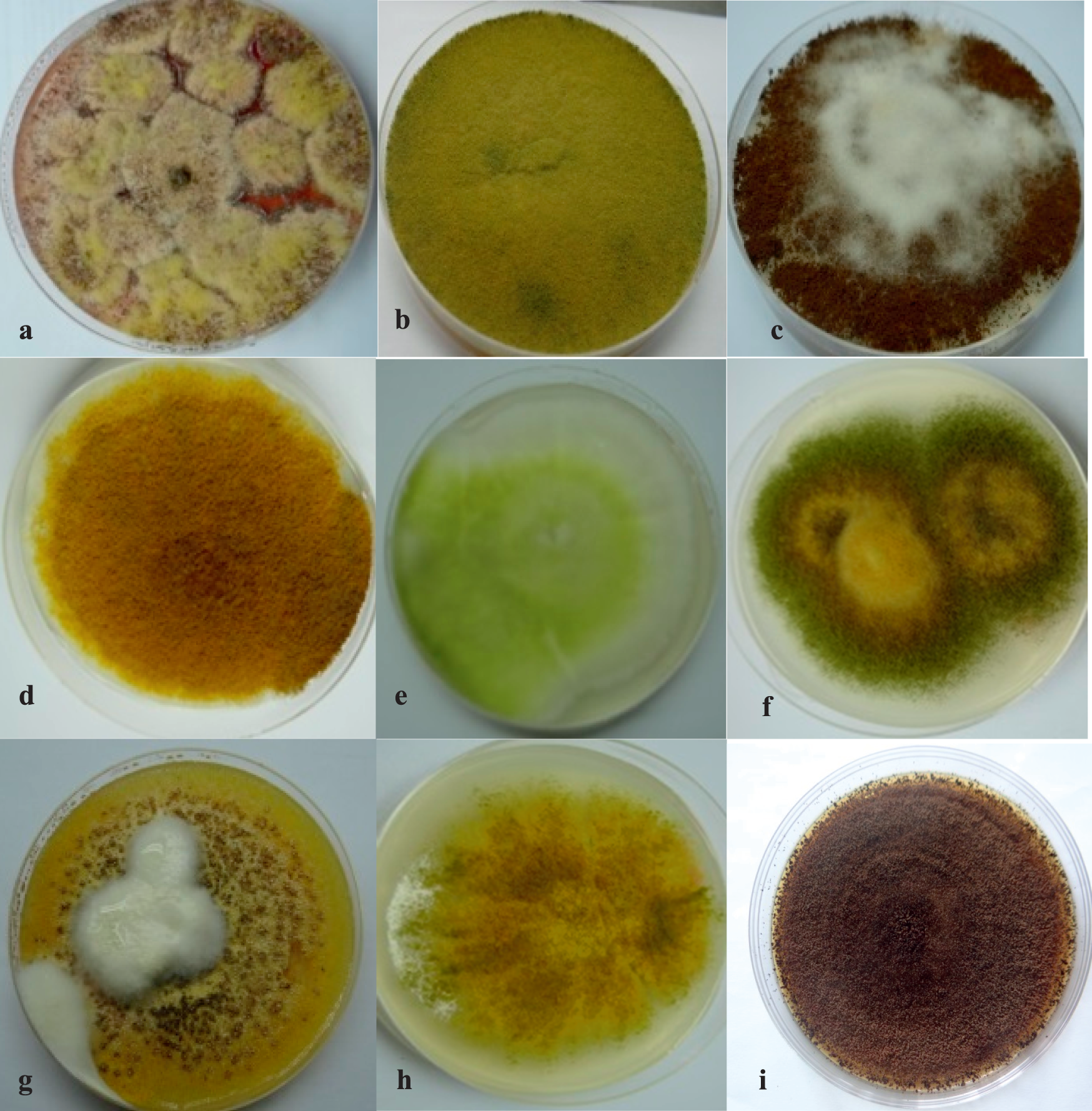
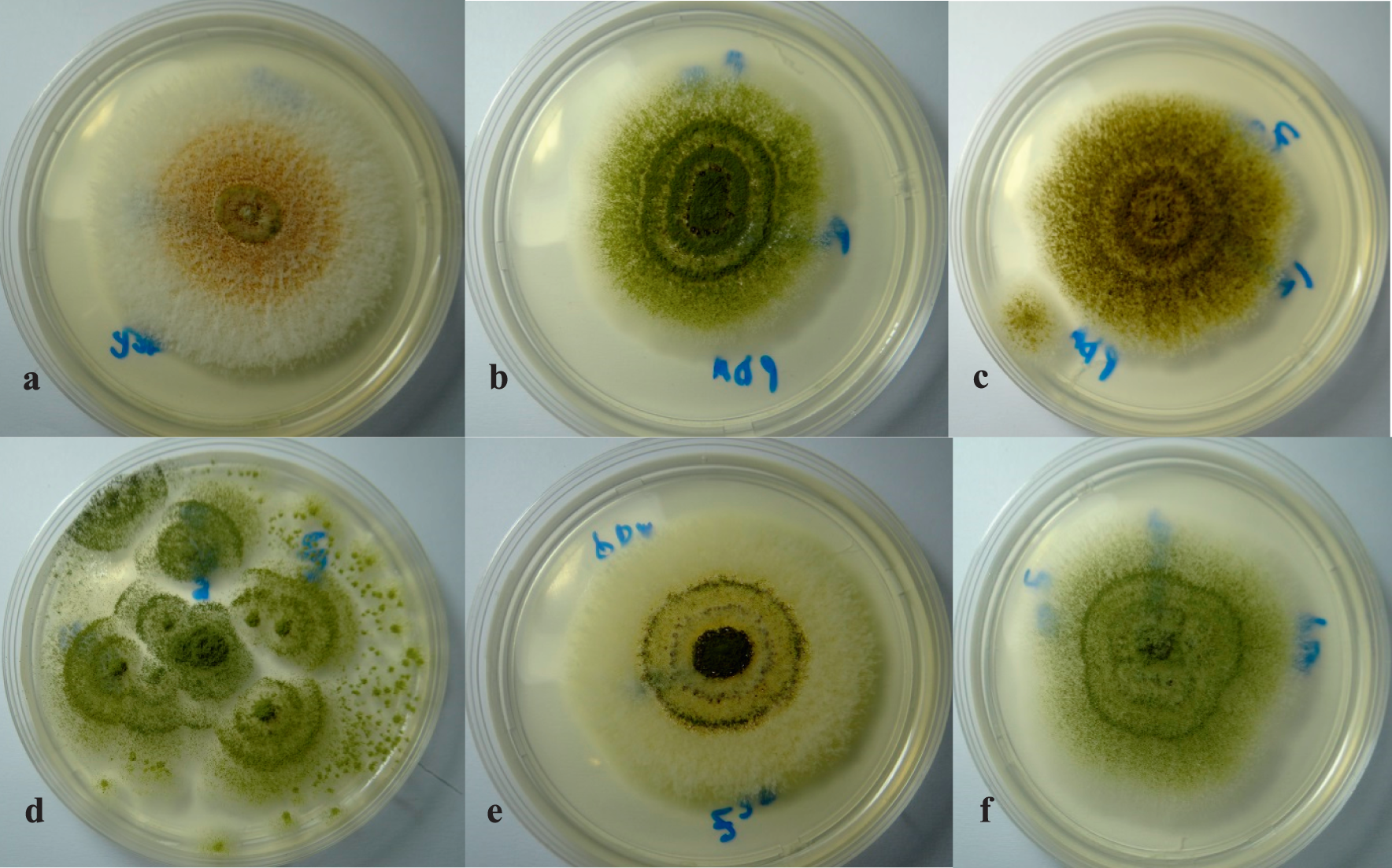
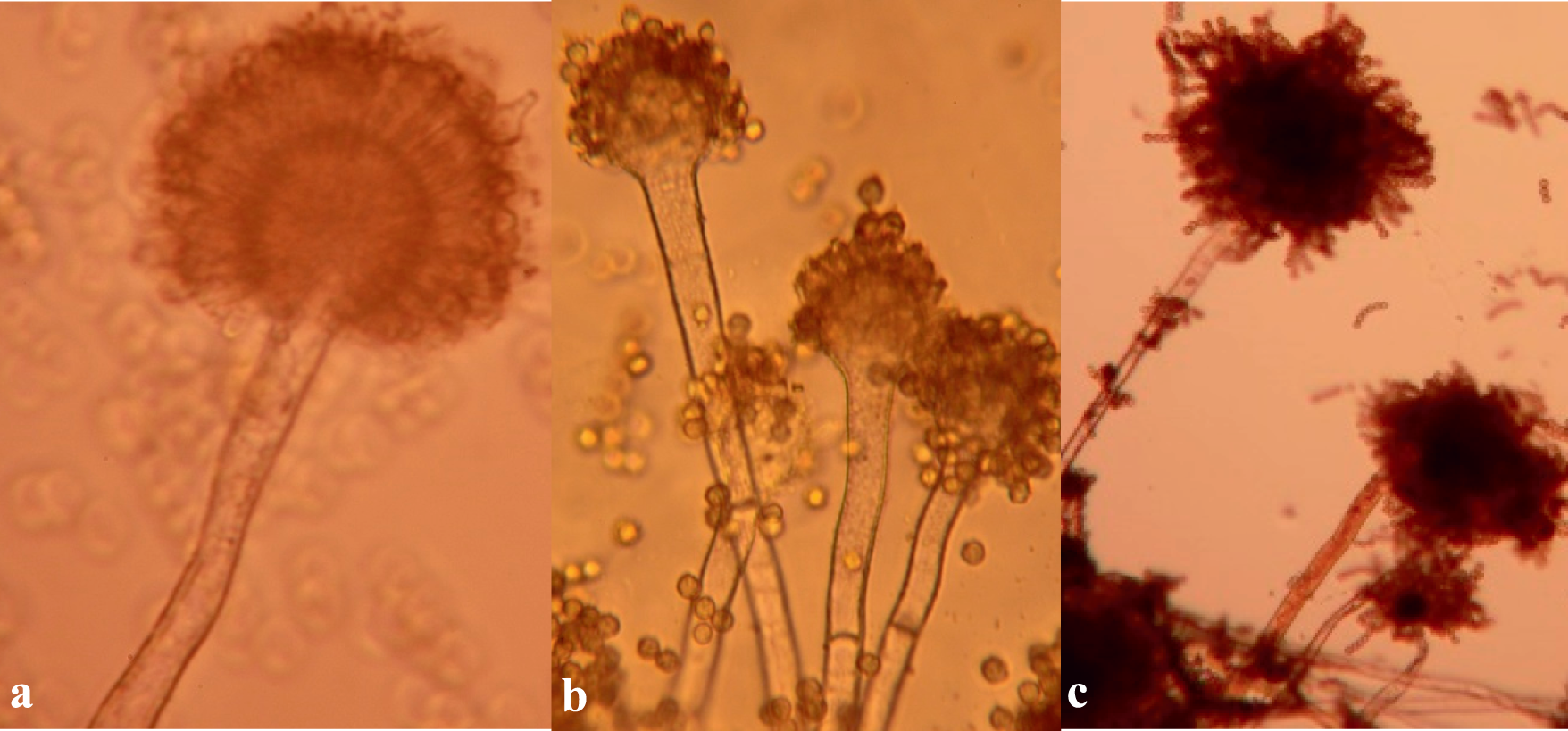
In this study, we also sought to identify and characterize for the first time in eastern Zambia, fungi from Aspergillus spp. isolated from soils cropped to groundnut. 91 Aspergillus spp. isolates, randomly selected from soil dilution plates (Fig. 1), were identified and characterized for aflatoxin production. We identified A. flavus (small (S) and large (L) sclerotial strains), A. parasiticus, A. niger, A. nomius, A. oryzae , A. tamarii, and A. terreus . A. flavus colonies were identified as greenish-yellow to yellow-white colonies on CzA (Fig. 2A), but were mostly green on MEA (Fig. 3A), and white-green colonies on PDA (Fig. 4A). Some A. flavus colonies were sclerotial with sparse hyphal growth on nutrient agar. A. flavus S- and L-strain isolates had sclerotia ≤400 μm or >400 μm in diameter, respectively. Under microscopic examination, conidia of A. flavus colonies were mostly smooth-walled and were borne on phialades, which were attached to metulae on globose vesicles (Fig. 5A). A. flavus is a common fungus in the soil and has been isolated in arid, temperate, and humid agroecologies worldwide (Abbas et al., 2004; Boyd and Cotty, 2001; Griffin and Garren, 1974; Horn, 2005; Horn 2003; Okoth 2014; Sweany et al., 2011)
In contrast, A. parasiticus colonies were pale green on both CzA (Fig. 2B) and MEA (Fig. 3B), and dark green on PDA (Fig. 4B). Under microscopic examination, conidial walls of A. parasiticus were echinulate and the stalks of conidiophores were rough-walled. Conidia were borne on phialades which lack metulae (Fig. 5B). A. parasiticus is also a common soil inhabitant which is mostly isolated in cooler temperate areas but is distributed worldwide (Domsch et al. 2007).
A. tamarii were comparatively easier to identify. Colonies of A. tamarii were chocolate-brown to cinnamon-brown on CzA (Fig. 2C and D), whereas on MEA, colonies were greenish with tints of brown (Fig. 3C), and green with more pronounced brown on PDA (Fig. 4C). Under microscopic observation, conidia of A. tamarii were echinulate and characteristically attached in chains, radiating from phialades lacking metulae (Fig. 5C). A. tamarii does not produce aflatoxin but produces another neurotoxin—cyclopiazonic acid (CPA) (Boyd and Cotty, 2001). The impact of CPA contamination in food is however an area not well researched into, especially in Africa. In the US, A. tamarii is mostly isolated from soil but occasionally isolated from plants (Horn, 2007). However, Horn (2007) discusses that the distribution of A. tamarii is not well defined in the US, but in contrast, Okoth et al. (2011) reported that A. tamarii was mostly found in the cooler areas of Nandi in Kenya, compared to the hotter and drier regions of Makueni.
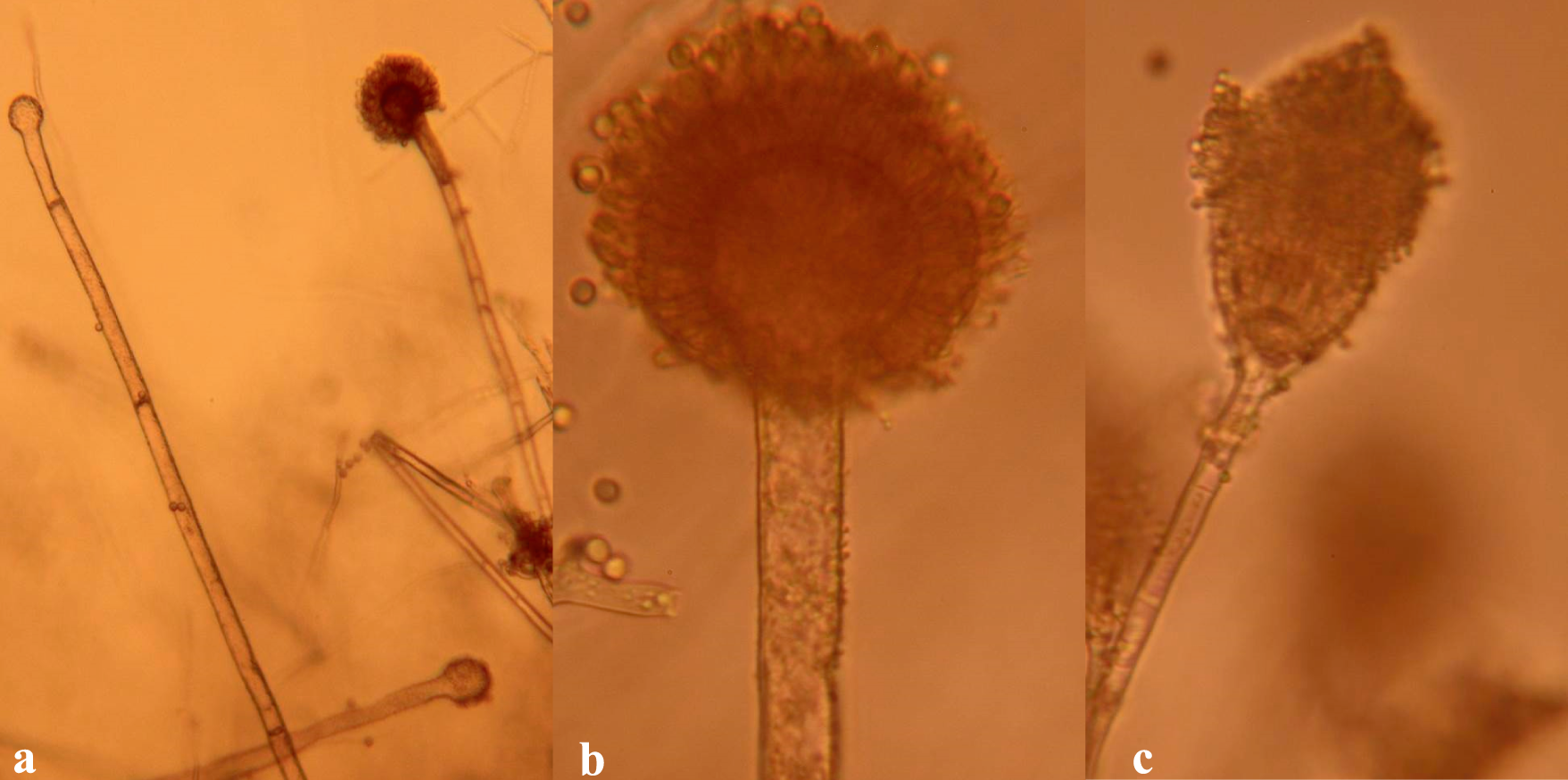
For A. oryzae , colonies on CzA varied from yellow-white (Fig. 2E) to green-brown (Fig. 2F), whereas on MEA colonies were white (Fig. 3D) or green (Fig. 3E). Compared to A. flavus , conidia of A. oryzae were echinulate, and were borne on conidiophores with characteristically long stalks (Fig. 6A). In addition, unlike for A. flavus , A. oryzae colonies did not form sclerotia. A. oryzae is important in food processing in Asia where it is used to ferment products and in the making of soy sauce, sake, etc (Domsch et al., 2007). The fungus has also been isolated worldwide in soils from cultivated fields and also in agricultural products.
For A. nomius, colonies were green to yellow with white sectors on CzA (Fig. 2G), light green on MEA (Fig. 3F) and white fluffy colonies on PDA (Fig. 4E). A. nomius colonies formed sclerotia (Figs. 2G and 3F), mostly larger than 400 μm in diameter. Under the microscope, conidia were slightly echinulate with globose vesicles and had roughened conidiophores (Fig. 6B). There are few reports on the isolation and identification of A. nomius, a comparatively newer aflatoxin producing species. In the US, A. nomius is most reliably found in agricultural soils of the Mississippi Delta (Horn, 2007).
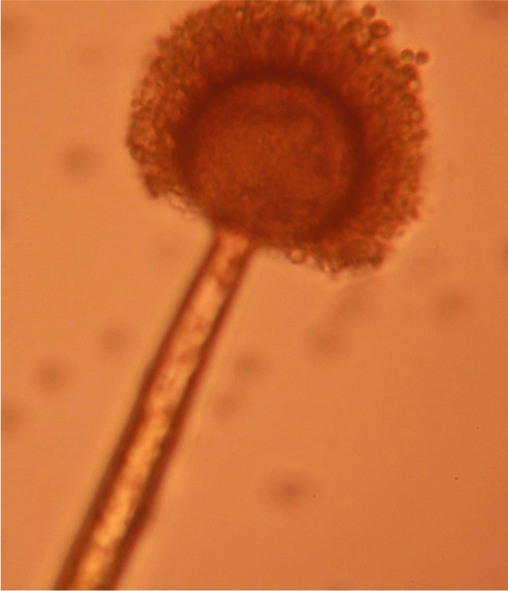
For A. terreus , colonies were cinnamon to light brown on CzA (Fig. 2H) and light green on both MEA (Fig. 3H) and on PDA (Fig. 4F). Under microscopic observation, vesicles of A. terreus were semi-spherical and had conidia arising from phialades attached to metulae (Fig. 6C). Conidia heads of A. terreus were strongly columnar arising from the top sides of the vesicles (Fig. 6C). Lastly, colonies of A. niger were characteristically black, both on CzA (Fig 2I) and on PDA. A. niger (Fig. 7) is an important spoilage fungus that can also attack young peanut seedling in the field causing crown rot (Melouk and Damicone, 1997), and produces ochratoxins (Perrone et al., 2007). Its importance in causing crown rot and reducing plant stand has not been documented in Zambia. A niger is ubiquitous in soil (Melouk and Damicone, 1997).
Summary and Conclusions
Despite the importance of aflatoxin contamination and increased focus on mitigation efforts, information on the identification of aflatoxigenic fungi in Africa is still limited. To our knowledge, this is the first peer reviewed report from Zambia documenting the population densities of A. flavus across different agroecologies. In addition, it is also the first report on the identification of different Aspergillus spp., such as A. nomius, A. terreus , A. oryzae , and A. tamarii from Zambia. However, our findings on the presence of these different Aspergillus spp. is not surprising since these fungi have been isolated in many different ecologies worldwide (Abbas et al., 2004; Boyd and Cotty, 2001; Domsch et al., 2007; Horn, 2003; Horn, 2005; Horn et al., 1995; Jamie-Garcia and Cotty, 2006; Monyo et al., 2012; Okoth et al., 2012). The significance of our research is the documentation of the presence of different toxigenic species, such as the S-strain A. flavus and also A. nomius, but also L- strain A. flavus and A. parasiticus.
Acknowledgements
We thank Joseph Maruwo (ICRISAT-Malawi), Dickson Mbughi (ICRISAT-Malawi), Willard Sinkala (Zambia Agriculture Research Institute-Msekera Station), and Griven Phiri (Zambia Agriculture Research Institute-Msekera Station) for technical support. This research was funded by USAID Feed the Future project on Aflatoxin Mitigation in Zambia, grant number EEM-G-00-04-0003-00 modification # 11/12. The study was carried out as part of CGIAR Research Program on Grain Legumes and also as part of CGIAR Research Program on Agriculture for Nutrition and Health.
Literature Cited
K. H., Abbas, W. T Shier, B. W Horn and M. A Weaver (2004). Cultural methods for aflatoxin detection. Toxins Reviews 23 : 295 – 315 .
K.H., Abbas, R.M Zablotowicz, M.A Weaver, B.W Horn, W Xie and W.T Shier (2004). Comparison of cultural and analytical methods for determination of aflatoxin production by Mississippi Delta Aspergillus isolates. Can. J. Microbiol 50 : 193 – 199 .
S Amaike, and N.P Keller (2011). Aspergillus flavus . Ann. Rev. Phytopathol 49 : 107 – 133 .
J., Atehnkeng, P.S Ojiambo, M Donner, T Ikotun, R.A Sikora, P.J Cotty and R Bandyopadhyay (2008). Distribution and toxigenicity of Aspergillus species isolated from maize kernels from three agroecological zones in Nigeria. International Journal of food Microbiology 122 : 74 – 84 .
E., Azziz-Baumgartner, K Lindblade, K Gieseker, H.S Rogers, (2005). Kieszak., , S H Njapau, R Schleicher, L.F McCoy, A Misore, K DeCock, C Rubin and L Slutsker Case-control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environmental Health Perspectives 113 : 1779 – 1783 .
M.L Boyd, and P.J Cotty (2001). Aspergillus flavus and aflatoxin contamination in leguminous trees of the Sanoran Desert in Arizona. Phytopathology 91 : 913 – 919 .
P. K., Chang, B. W Horn and J. W Dorner (2005). Sequence breakpoints in the aflatoxin biosynthesis gene cluster and flanking regions in non-aflatoxigenic Aspergillus flavus isolates. Fungal Genetics and Biology 42 : 914 – 923 .
Domsch, K.H., W Gams and T Anderson Eds. 2007 In Aspergillus. Compendium of soil fungi.672 pp IHW-Verlag, Eching Bei Müˇnchen .
FAOSTAT 2013 . Food and Agriculture Organization Statistical Database.: Groundnut production information for Zambia http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor (Accessed 12 May 2015) .
Y. Y., Gong, K Cardwell, A Hounsa, S Egal, P. C Turner and C. P Wild (2002). Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: cross sectional study. BMJ 325 : 20 – 21 .
G. J., Griffin, K. H Garren (1974). Population levels of Aspergillus flavus and their Aspergillus niger group in Virginia peanut field soils. Phytopathology 64 : 322 – 325 .
B. W Horn, (1995). Effect of corn and peanut cultivation on soil populations of Aspergillus flavus and A. parasiticus in Southwestern Georgia. Applied and Environmental Microbiology 61 ((7)) : 2472 – 2475 .
B.W Horn, (2003). Ecology and population biology of aflatoxigenic fungi in soil. Journal of Toxicology 22 : 351 – 379 .
B.W Horn, (2005). Colonization of wounded peanut seeds by soil fungi: selectivity for species from Aspergillus section Flavi . Mycologia 97 ((1)) : 202 – 217 .
B.W., Horn, R.L Greene and J.W Dorner (1995). Effect of corn and peanut cultivation on soil populations of Aspergillus flavus and A. parasiticus in Southwestern Georgia. Applied and Environmental Microbiology 61 : 2472 – 2475 .
Horn, B.W., G.G Moore and I Carbone 2009 a The sexual state of Aspergillus parasiticus Mycologia 101 (2) : 275 – 280 .
Horn, B.W., G.G Moore, and I Carbone 2009 b Sexual reproduction in Aspergillus flavus Mycologia 101 (3) : 423 – 429 .
B.W., Horn, G.G Moore and I Carbone (2011). Sexual reproduction in aflatoxin producing Aspergillus nomius . Mycologia 103 ((1)) : 174 – 183 .
R Jamie-Garcia, and P.J Cotty (2006). Spatial relationships of soil texture and crop rotation to Aspergillus flavus community structure in South Texas. Phytopathology 96 : 599 – 607 .
N.A Kaaya, and H.L Warren (2005). A review of past and present research on aflatoxin in Uganda. African Journal of Food Agriculture and Nutritional Development 5 : 1 – 18 .
M., Karthikeyan, R Sandosskumar, S Mathiyazhagan, M Mohankumar, V Valluvaparidasan, S Kumar and R Velazhahan (2009). Genetic variability and atoxigenic potential of Aspergillus isolates from maize. Archives of Phytopathology and Plant Protection 42 : 83 – 91 .
M.A Klich, (2007). Environmental and developmental factors influencing aflatoxin production by Aspergillus flavus and Aspergillus parasiticus . Mycoscience 48 : 71 – 80 .
L., Lewis, M Onsongo, H Njapau, H Schurz-Rogers, G Luber, S Kieszak, J Nyamongo, L Backer, A.M Dahiye, A Misore, K DeCock and C Rubin (2005). Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in Eastern and Central Kenya. Environmental Health Perspectives 113 ((12)) : 1763 – 1767 .
L., Matumba, M Sulyok, S.M.C Njoroge, E.N Ediage, C van Poucke, S De Saeger and R Krska (2015). Uncommon occurrence ratios of aflatoxin B1, B2, G1, and G2 in maize and groundnuts in Malawi. Mycotoxin Res 31 : 57 – 62 .
Melouk, H. A., and J. P Damicone 1997 Aspergillus crown rot . Pages 7-8 in: Compendium of Peanut Diseases 2nd Edition, N Kokalis-Burelle, D. M Porter, R Rodriguez-Kabana, D. H Smith, and P Subrahmanyam, eds. APS Press, St Paul, Minnesotta
E.S., Monyo, S.M.C Njoroge, R Coe, M Osiru, F Madinda, F Waliyar, R.P Thakur, T Chilunjika and S Anitha (2012). Occurrence and distribution of aflatoxin contamination in groundnuts ( Arachis hypogaea L) and population density of Aflatoxigenic Aspergilli in Malawi. Crop Prot 42 : 149 – 155 .
G.P Munkvold, (2003). Cultural and genetic approaches to managing mycotoxins in maize. Ann. Rev. Phytopathol 41 : 99 – 116 .
C.K., Mutegi, H.K Ngugi, S.L Hendriks and R.B Jones (2009). Prevalence and factors associated with aflatoxin contamination of peanuts from Western Kenya. International Journal of Food Microbiology 130 : 27 – 34 .
Okoth, S., B Nyongesa, V Ayugi, E Kang'ethe, H Korhonen and Joutsjoki. 2012 Toxigenic potential of Aspergillus species occurring on maize kernels from two agroecological zones in Kenya Toxins 4 : 991 – 1007 Doi:10.3390/toxins4110991 .
R.R.M., Paterson, J Kelley and M Gailagher (1997). Natural occurrence of aflatoxins and Aspergillus flavus (Link) in water. Letters in Applied Microbiology 25 : 435 – 436 .
M., Setamou, K.F Cardwell, F Schulthess and H.K Hell (1997). Aspergillus flavus infection and aflatoxin contamination of preharvest maize in Benin. Plant Dis 81 : 1323 – 1327 .
Solorzano, C. D., H. K Abbas, R. M Zablotowicz, P. K Chang and W. A Jones 2014 Genetic variability of Aspergillus flavus isolates froma Mississippi corn field The Scientific World Journal . Volume 20014. 8 pages. http://dx.doi.org/10.1155/2014/356059
Wagacha, J.M and J.W Muthomi 2008 Mycotoxin problem in Africa: current status, implications to food safety and health and possible management strategies Int. J. Food Microbiol.124:1-12 .
J.H., Williams, T.D Phillips, P.E Jolly, J.K Stiles, C.M Jolly and D Aggarwal (2004). Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr 80 : 1106 – 1122 .
Notes
- First author: International Crops Research institute for the Semi-Arid Tropics (ICRISAT), P.O. Box 1096, Lilongwe, Malawi; Second author: Zambia Agriculture Research Institute, Chipata, Zambia; Third and fifth authors: ICRISAT, P. O. Box 39063, Nairobi, Kenya; Fourth author: ICRISAT, Patancheru 502 324, Telagana, India. [^] *Corresponding author's E-mail: s.njoroge@cgiar.org
Author Affiliations