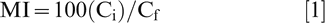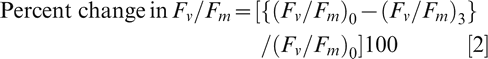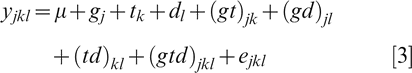Introduction
The Virginia-Carolina (VC) region is the most important peanut production region for the large seeded, virginia-type peanut (Arachis hypogaea L.) in the United States. Although historically the region has had adequate climatic conditions for high peanut yields, recent occurrence of high temperatures has been frequently observed in particular during early growth stages of peanut. For example, frequent temperatures above 30 C have been reported during May and June in the VC region (NCDC, 2012) and predictions are for an additional 2.4 to 6.4 C increase by the end of 21st century (Meehl et al., 2007). Optimum temperature for peanut growth is between 25 and 30 C but pod yields can be substantially reduced if temperatures exceed 33 C (Cox, 1979; Prasad et al., 2003). During early growth stages, soil temperatures above 32 C reduced the early vegetative growth of peanut, with negative effects on pod yield and total plant biomass at maturity (Golombek and Johansen, 1997).
Under elevated abiotic stress, compatible solutes proline, glycine betaine, and mannitol and antioxidants ascorbate, and glutathione are produced to protect the cellular and thylakoid membranes (Alscher et al., 2002; Apel and Hirt, 2004; Asada, 2006; Dat et al., 2000; Foyer and Noctor, 2005; Kovtun et al., 2000; McKersie et al., 1990; Mittler et al., 2004; Noctor and Foyer, 1998; Pei et al., 2000; Xu et al., 2006). Specifically, heat stress lead to increased pinitol levels in soybean (Guo and Oosterhuis, 1995), and galactinol and raffinose in Arabidopsis (Kaplan et al., 2004). Heat tolerant cultivars of turfgrass accumulated higher levels of certain sugars, sugar alcohols, and organic acids in response to heat exposure and had reduced MI and higher Fv /Fm ratio (Du et al., 2011).
To maintain high yields under an increasingly hotter climate, peanut cultivars adapted to high temperatures would have to be developed. Genetic variation for heat tolerance at post-flower stages has been reported worldwide (Awal et al., 2003; Craufurd et al., 1999; Prasad et al., 2003; Vu, 2005), but little is known about pre-flower stage heat tolerance in peanut in general and in virginia-type peanut in particular (Selvaraj et al., 2011). To our knowledge the data reported in this paper is the first attempt to understanding the physiological and metabolic mechanisms of the virginia-type peanut seedlings in response to high temperature stress. Our objectives were to identify the physiological and metabolic mechanisms developed by peanut at early growth stages in response to heat stress, evaluate the relationship between the physiological characteristics and metabolite levels, and assess the genetic variability for these mechanisms among eight virginia-type cultivars and breeding lines.
Materials and Methods
Plant Materials, Growth Conditions, and Temperature Treatments
Virginia-type peanut cultivars (CHAMPS, Bailey, and Phillips) and advanced breeding lines (N04074FCT, N05006, N05008, N05024J, and SPT 06-07) were selected for this experiment. They were selected based on their differential response to reduced soil moisture in the field, therefore potentially different response to heat (Singh et al., 2014). Seeds were surface sterilized with 70% ethanol for 1 min and rinsed twice with sterile water. Seeds were wrapped in moist germination paper and allowed to germinate in the dark for 6 d in a growth chamber at 30 C. The seedlings were then transferred to plastic boxes (29.2 cm × 18.7 cm × 15.2 cm) containing half strength Hoagland’s nutrient solution (Hoagland and Arnon, 1950). Before imposing the heat treatment, seedlings were allowed to grow in a growth chamber for 7 d at 30/25 C (day/night) temperature and 16/8 hr (day/night) photoperiod, and light intensity of 300 to 400 µmol/m2/s1 at the plant level. Thirteen-day-old plants were then exposed to two temperature regimes 40/35 C (heat treatment) and 30/25 C (optimum temperature regime), sequentially using the same growth chamber. Plants were watered well to avoid drought stress during the heat treatment and the nutrient solution was replaced every 4 d to allow proper root aeration and to minimize microbial contamination. Each genotype within a temperature regime was replicated four times (single plant per replication) and two identical experiments were performed. Two leaflets from youngest, fully mature leaves per single plant replication were harvested at 1, 2, 4, and 7 d after beginning of the heat treatment for the physiological characteristics (membrane injury and the Fv /Fm ratio); three individual leaves from four single plants per each replication, genotype, and temperature regime were harvested at the same time-points for metabolite measurements.
Membrane Injury
We used a modified Membrane injury (MI) assay of Blum and Ebercon (1981) on two fully expanded youngest leaflets per replication. Leaf discs of 11.1 mm in diameter were cut from the two leaflets with a leaf disk sampler, rinsed twice with distilled water, and placed in 20 ml plastic vials containing 15 ml distilled water. After shaking the vials for 24 hr, initial conductivity (Ci) of the bathing solution was measured with a conductivity meter (Seven-multi conductivity module and InLab® 741 electrode, Mettler-Toledo Inc., Columbus, OH). Leaf discs were later autoclaved at 120 C for 45 min, and placed on a shaker for 24 hr before recording the final conductivity (Cf) of the bathing solution. The percentage MI was calculated using the formula:
Chlorophyll Fluorescence
Two leaflets from the youngest fully expanded leaves were harvested for the chlorophyll fluorescence measurements with a modulated chlorophyll fluorometer (OS1p, OptiSciences Inc., NH, USA). Chlorophyll florescence was measured as the Fv /Fm ratio, the ratio of variable (Fv ) to maximum (Fm ) chlorophyll fluorescence as described (Burke, 2007) with incubation temperature modified from 39 to 45 C. Leaf discs were cut using an 11.1 mm disc sampler and subsequently arranged on a moist tissue paper in a Pyrex® glass dish, covered with GLAD® cling wrap transparent film (GLAD Products Company), and carefully pressed flat to remove air bubbles, and to ensure good contact between the leaf discs and film. Initial Fv /Fm values were recorded prior to incubation, in the dark at 45 C for 3 hr. The Fv /Fm ratios were recorded at hourly intervals. The percent change in Fv /Fm ratio was further calculated using the formula:
where (Fv /Fm )0 and (Fv /Fm )3 are the Fv /Fm readings after 0 and 3 hr of incubation at 45 C, respectively.
Metabolite Sampling, Extraction, and Analysis
Three individual leaves from four single plants per each replication, genotype, and temperature regime were harvested at four different time-points, snap-frozen in liquid nitrogen, and stored at −80 C until further GC-MS-FID based metabolite and lipid-derived fatty acid analyses (Duran et al., 2003; Collakova et al., 2008; 2013). Briefly, after lyophilizing, metabolites from 4.00 ± 0.05 mg of dry powderized tissue were extracted with equal volumes of chloroform and water (400 µl each) containing ribitol and heptadecanoic acid as internal standards. For untargeted polar metabolite profiling, 50 µl of the aqueous phase was dried under a stream of nitrogen gas and derivatized in two steps with methoxyamine.HCl and N-methyl-N-(trimetylsilyl) trifluoroacetamide containing 1% (v/v) trimethylchlorosilane (Thermo Fisher Scientific, Waltham, MA). Trimethylsilyl derivatives of metabolites were analyzed by GC-MS as described (Duran et al., 2003; Collakova et al., 2008; 2013). For lipid-derived fatty acid analysis, 200 µl of the organic phase was dried as for polar metabolites and fatty acid methylesters were prepared and analyzed as described (Lu et al., 2011; Collakova et al., 2013).
The GC-MS-FID analyses were performed on an Agilent 7890A series GC-FID and 5975C series single quadrupole MS (Agilent Technologies, Santa Clara, CA) equipped with a DB-5MS-DG capillary column (30 m × 0.25 mm × 0.25 µm; Agilent Technologies) for untargeted polar metabolites and a 30-m DB-23 column (0.25 mm × 0.25 μm, Agilent Technologies) for fatty acid methylester analysis. Peak identification and data analysis were performed as described by Collakova et al. (2013) and by using the Agilent Enhanced Mass Selective Detector ChemStation software and three different libraries: FiehnLib spectral and retention time library [Kind et al., 2009], our own custom-built spectral and retention time library, and the spectral NIST library (National Institute of Standards and Technology, Gaithersburg, MD). Final relative levels of polar metabolites and absolute levels of fatty acids were standardized for dry weight and internal standard recoveries.
Statistical Analysis
The two experiments were combined for statistical analysis because trends in seedling responses were similar. Data sets containing a total of eight replications per genotype and temperature regime were analyzed using JMP 9.0 software program (SAS Institute Inc., Version 9.0). Analysis of variance (ANOVA) for individual metabolites and physiological characteristics was carried out and the statistical model was:
where μ is the overall mean effect, g j the main effect of the j th genotype (j = 1 to 8), t k the main effect of the k th temperature treatment (k = 1, 2), d l the day of the l th sampling effect (l = 1 to 4), (gt)jk the interaction of the j th genotype and k th treatment, (gd)jl the interaction of the j th genotype and l th day of sampling, (td)kl the interaction of the k th treatment and l th day of sampling, (gtd)jkl the interaction of the j th genotype, k th temperature treatment, and l th day of sampling, and ejkl the random error associated with the experimental unit.
Correlations between the physiological characteristics and changes in metabolite levels were calculated. Step-wise regression analysis was carried out to identify the most discriminating metabolites that explained the largest proportion of variation in the physiological data. These response variables were then used to construct a dendrogram of genotype clusters based on Ward’s distance matrix.
Principal component analysis (PCA) was performed on the metabolite data. Loading and score plots were used to reveal the correlations and degree of variation present in the metabolite data. The first two principal components explained the highest percentage of variation between samples; hence further analyses were based on these two principal components.
Results
Membrane Injury
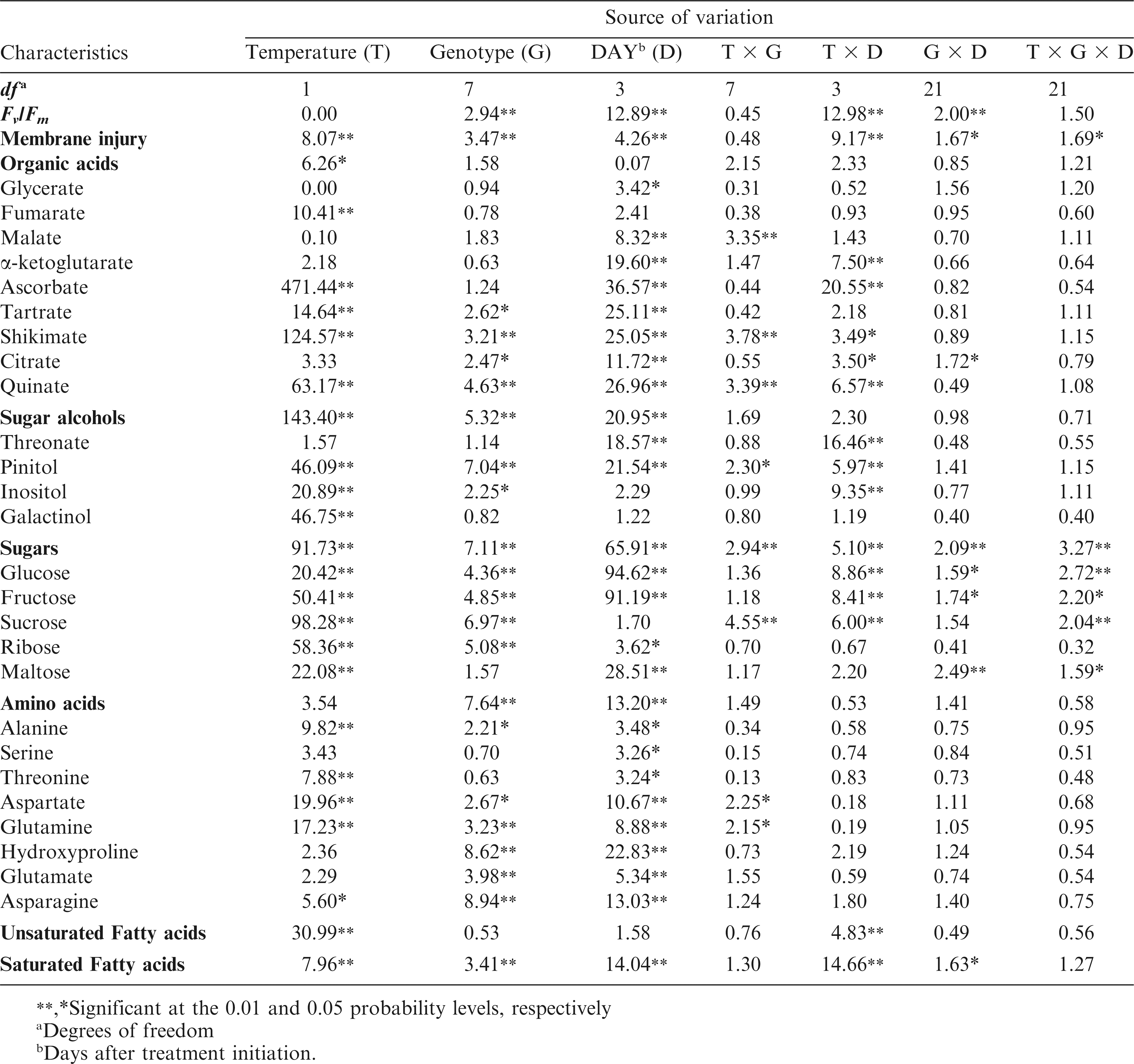
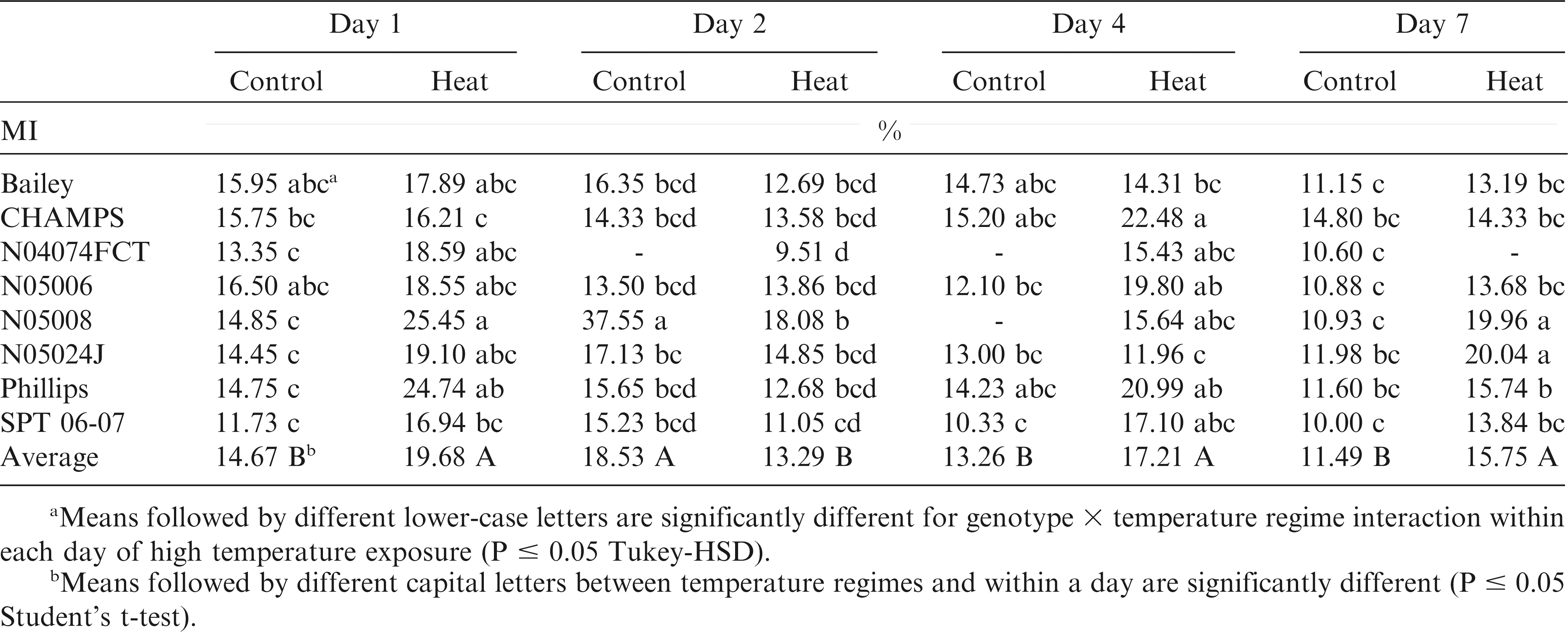
The ANOVA for MI showed temperature-by-genotype-by-day of measurement effect (p < 0.05) indicating that genotypes performed differently at each time-point and temperature treatment (Table 1). However, the three way interaction for MI could have been caused by a strong effect of line N05008 on Day 2 unusually showing twice more MI in control than in heat stressed leaves and twice more than for other genotypes; for the other genotypes on Day 2, MI was not significantly different between control vs. heat treated seedlings (Table 2), possibly due to initiation of heat acclimation processes (Alscher et al., 2002; Apel and Hirt, 2004; Asada, 2006). Seedlings under heat stress had on average 14% more MI than control plants (Table 2). For the heat stressed plants, MI at Day 1 was 33%, at Day 4 30% and at Day 7 37% higher than for control. N05008 at Days 1 and 7, Phillips at Day 1, and N05024J at Day 7 showed higher MI in heat stressed than control plants. When averaged across the time-points, genotypes N05008 and Phillips showed the highest MI (20 and 18%), and Bailey and SPT 06-07 the lowest (14.5% each) MI under heat stress.
Chlorophyll Fluorescence
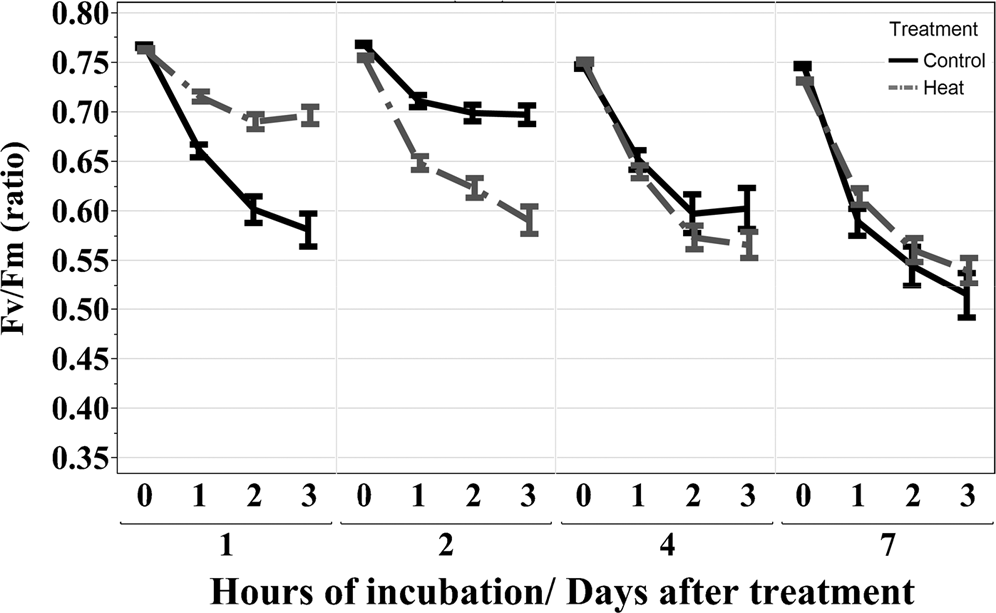
The Fv /Fm ratio decreased gradually over the 3 h of dark incubation period at 45 C for both control and heat stressed plants (Fig. 1). On Day 1, the Fv /Fm ratio decreased more for the control than for heat stressed plants (p < 0.05), but this trend was reversed on Day 2. After 4 d of differential temperature regime, the Fv /Fm ratio decreased similarly in control and heat stressed plants. Percent change in the Fv /Fm ratio from 0 to 3 hr of incubation 45 C was calculated for individual genotypes and temperature regimes. Because the temperature-by-genotype and temperature-by-genotype-by-day of measurement interactions were not statistically significant for the Fv /Fm (% change) (Table 1), the days of measurement or time-points were combined for each genotype. Overall, Bailey and Phillips exhibited the smallest (14 and 17%, respectively) and N05024J the largest (28%) decline in the Fv /Fm (% change) among the eight genotypes.
The Fv /Fm chlorophyll fluorescence ratio of eight virginia-type peanut cultivars and breeding lines grown under two temperature treatments (heat, 40/35 C; control, 30/25 C), at four time-points after the temperature treatment initiation (day 1, 2, 4, and 7 combined), and after 3 hours of dark incubation at 45 C.
Metabolite Profiling
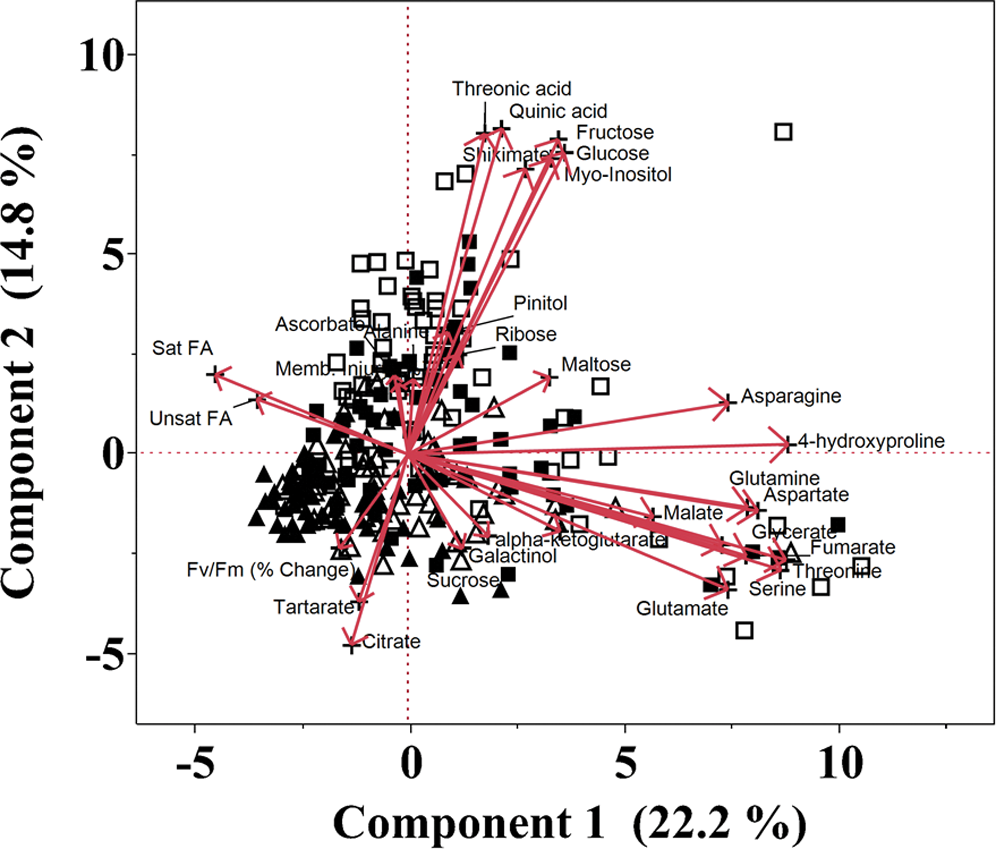
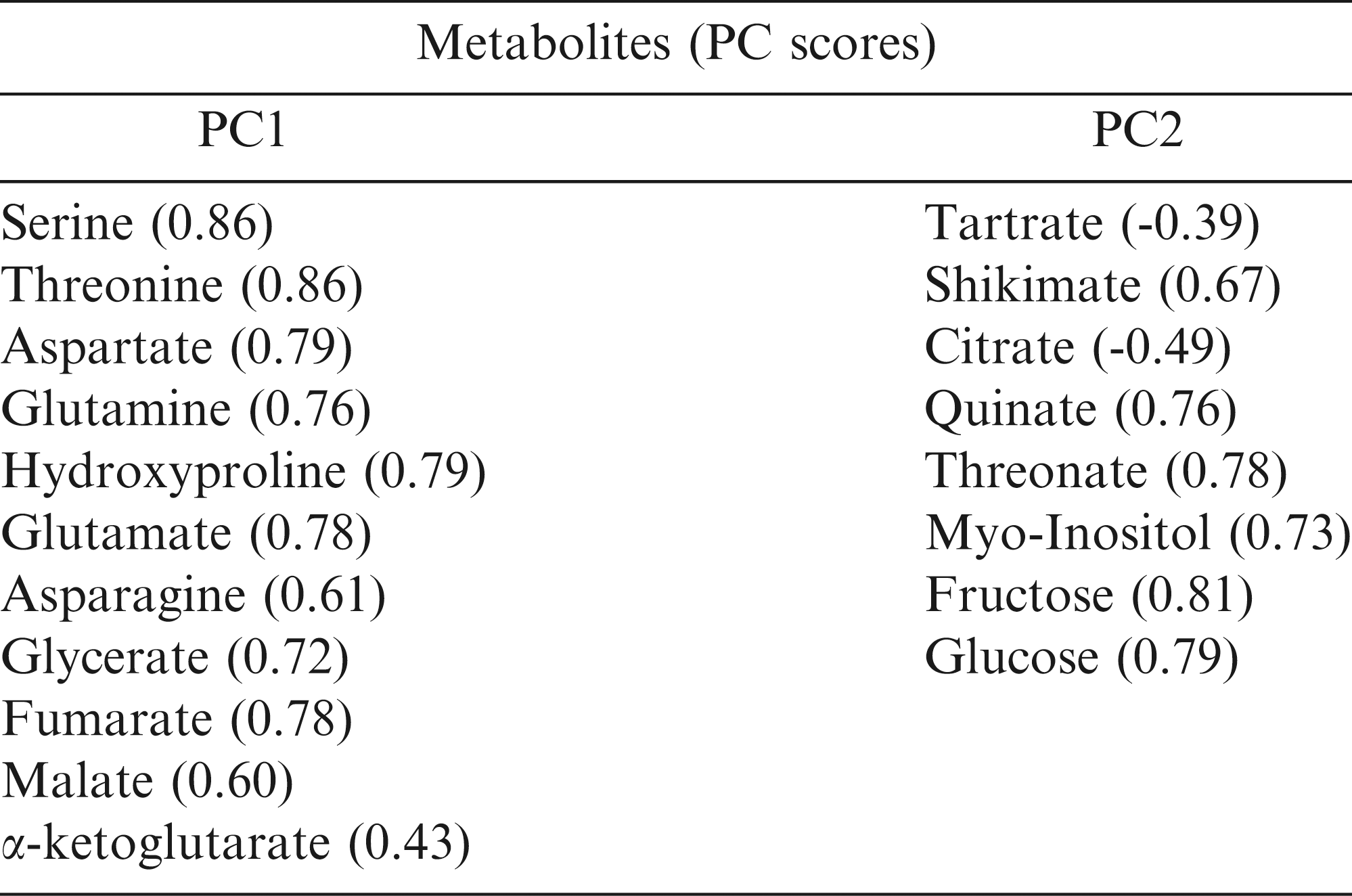
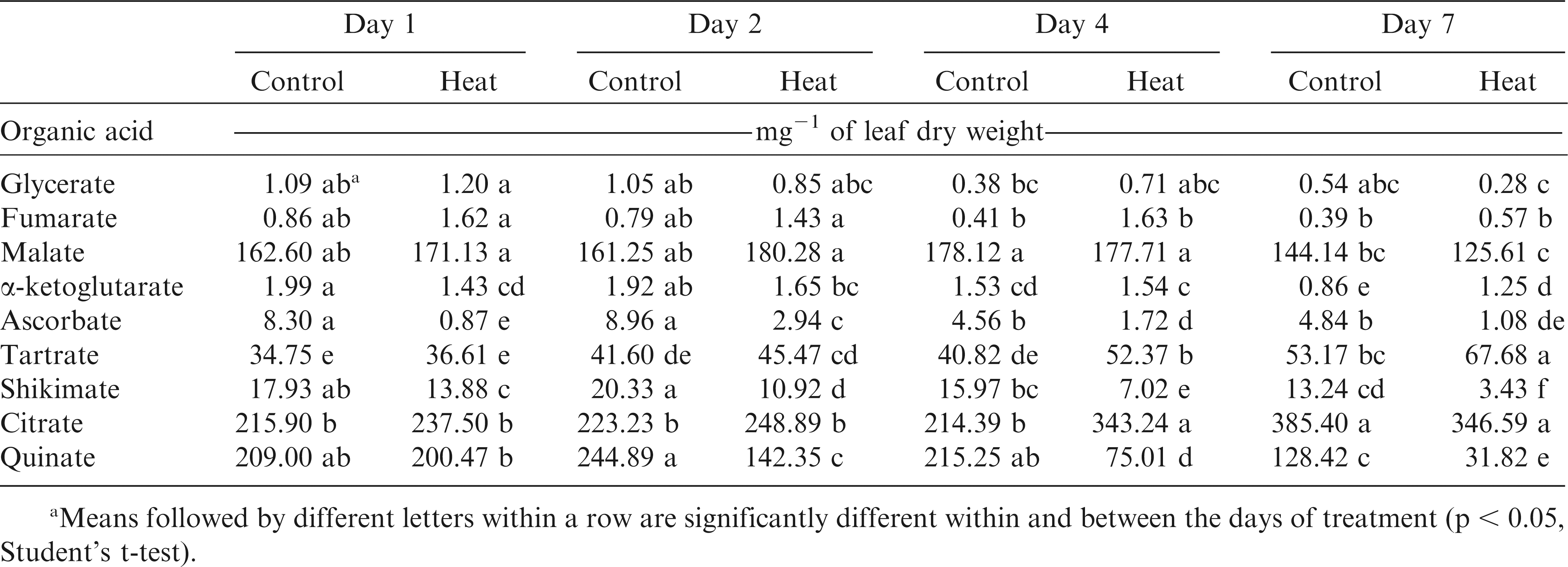
Out of the total peaks identified during the metabolite profiling analysis, the 26 most abundant metabolites were chosen for further analysis (Table 1). These metabolites belong to four major groups. There were eight amino acids, nine organic acids, five sugars, and four sugar alcohols and cyclic polyols. Significant within-treatment and between-time-point variations were observed with the first two principal components (PCs) explaining 37% of the total variance in the metabolite and fatty acid data (Fig. 2). The data cluster from Day 1 was separated from Day 7 by PC2, which was associated with tartrate, shikimate, citrate, quinate, threonate, myo-inositol, fructose, and glucose relative levels. The first two PCs along with their associated variables and component scores are presented in Table 3.
A total of nine organic acids were identified: glycerate, fumarate, citrate, malate, α-ketoglutarate, ascorbate, quinate, shikimate, threonate, and tartrate. For glycerate and fumarate, there were no or small changes in response to temperature regime or the number of days of heat exposure (Table 4). However, changes with the day of heat exposure were significant for the relative levels of malate, and citrate. Significant changes due to temperature regime were recorded for α-ketoglutarate and ascorbate relative levels (p < 0.05). For shikimate, tartrate, quinate, and threonate, temperature regime and the number of days of heat significantly changed their relative levels in the peanut seedlings (Table 4). For example, significant decline with time under heat treatment were recorded for relative levels of shikimate (4.1 fold) and quinate (6.3 fold). Relative shikimate levels decreased in heat-stressed seedlings of all genotypes, except for N04074FCT (Table 5). Among genotypes, N05006 had the lowest and N05024J, Bailey, and N04074FCT the highest relative levels of α-ketoglutarate in heat-stressed seedlings. Relative quinate levels decreased (p < 0.05) in heat-stressed Bailey, N05006, N05008, and SPT06-07 and remained unchanged for all other genotypes.
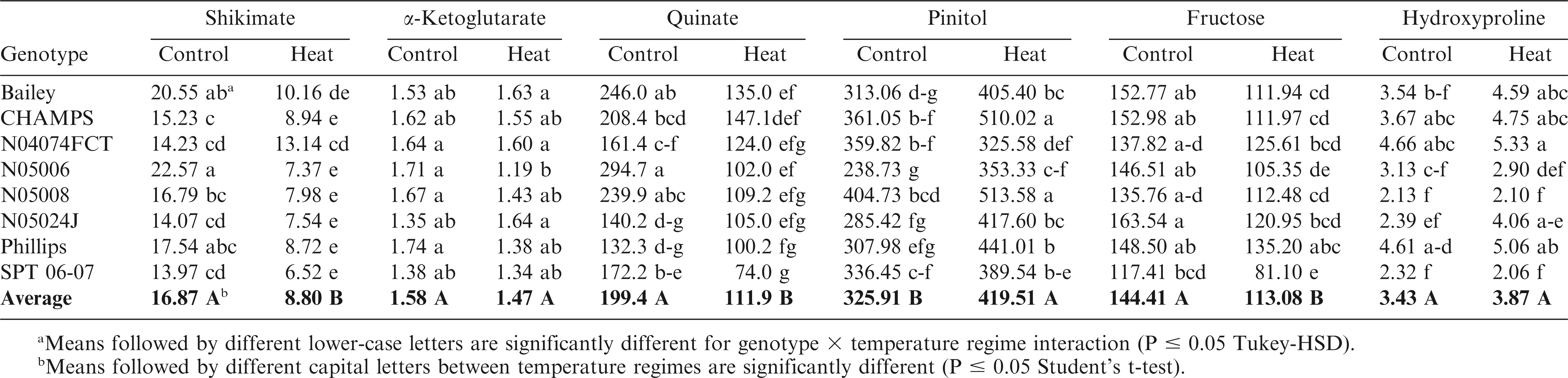
Relative levels of shikimate, α-ketoglutarate, quinate, pinitol, fructose, and hidroxyproline (mg-1 of leaf dry weight) in the leaves of eight virginia-type peanut cultivars and breeding lines under optimum (control) (30/25 C) and high temperature (40/35 C) regimes. Data from days 1, 2, 4, and 7 of exposure to temperature regimes were combined.
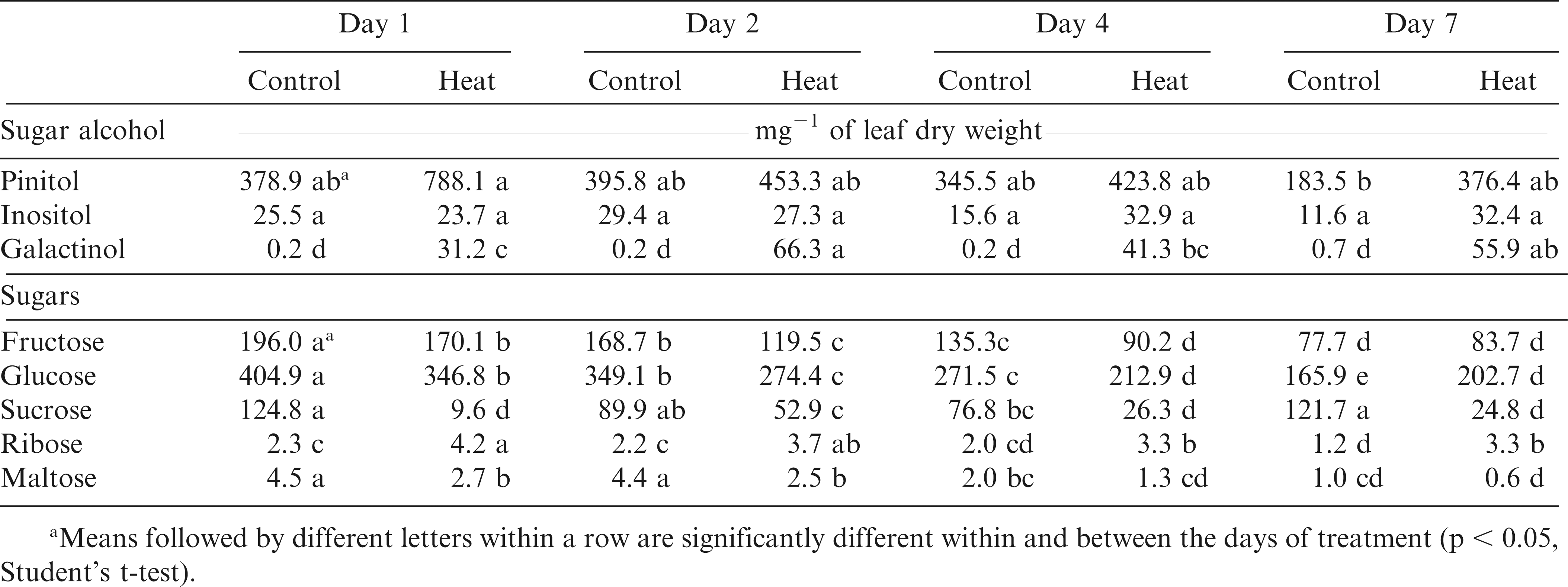
Pinitol, inositol, and galactinol were the major cyclic polyols and sugar alcohols identified in peanut samples, and their relative levels increased in response to heat stress (Table 6). For example, galactinol was over 100 fold more in heat-stressed than in control plants at all time-points. No significant differences in relative galactinol levels were observed among genotypes in control plants. At high temperatures, Bailey, CHAMPS, N05024J, Phillips, and SPT 06-07 had approximately 66% more galactinol accumulation than N05006, N05008, and N04074FCT (data not shown). With the exception of N04074FCT and SPT 06-07, all other genotypes showed higher accumulation of pinitol in heat-stressed than in control plants. For example, N05008 and CHAMPS had almost 70% more pinitol compared to N04074FCT and N05006 under heat treatment (Table 5).
Glucose, fructose, and maltose also showed significant (p < 0.05) genotype-by-day of measurement and temperature-by-genotype-by-day interactions (Table 1). On average, barring ribose, relative levels of all other sugars decreased in response to heat stress (Table 6). Relative levels of sucrose and ribose changed only in response to temperature, whereas fructose, glucose and maltose levels changed in response to both temperature and number of days of exposure to high temperatures (Table 6). Relative fructose levels decreased at Days 1, 2, and 4 but no change was observed at Day 7 of the heat treatment. Relative fructose levels decreased for Bailey, CHAMPS, N05006, and SPT 06-07 in the heat-stressed compared to their respective controls (Table 5). N05006 and SPT 06-07 had the lowest and Phillips and N04074FCT the highest levels of fructose among the eight genotypes under heat stress.
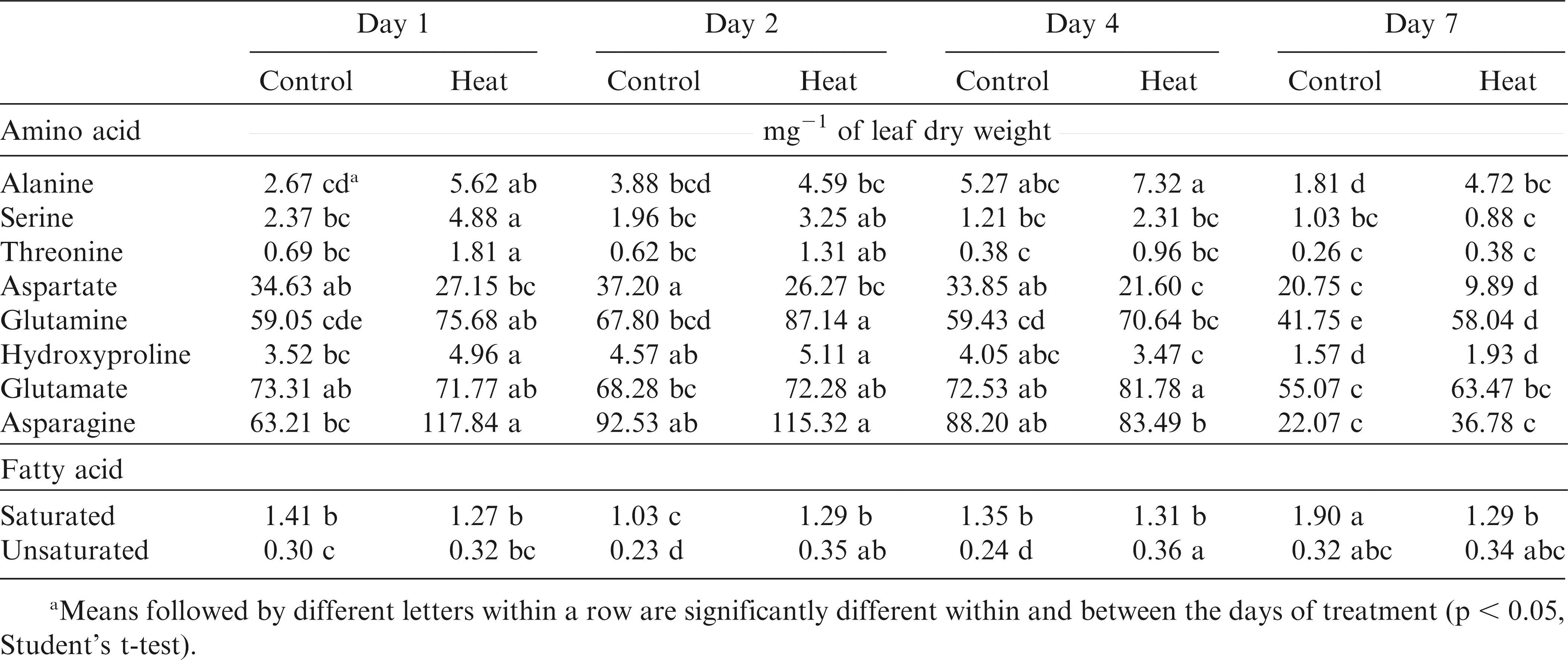
The genotype-by-day of measurement and temperature-by-genotype-by-day of measurement interactions were absent for all amino acids (Table 1). In general, accumulation of amino acids increased in heat-stressed compared to the control plants (Table 7). For example, concentrations of alanine, serine, threonine, glutamine, hydroxyproline, and asparagine were higher in heat-stressed plants after one day of exposure to 40/35 C temperature regime. With the exception of alanine, aspartate, and glutamine, the other amino acids remained unchanged after Day 1 of the heat stress treatment initiation. Aspartate levels decreased gradually after 2, 4, and 7 d of heat stress whereas glutamate levels remained unchanged throughout the heat treatment time-course (Table 7). Total amino acid levels remained unchanged during Days 1, 2, and 4 and decreased significantly on Day 7 in both control and heat stressed seedlings. Among the genotypes, N04074FCT and Phillips had the highest and SPT 06-07 and N05008 the lowest levels of hydroxyproline in heat stressed plants (Table 5). Similarly, threonine levels were 65% higher for heat stressed seedlings of N04074FCT and Phillips than for SPT 06-07 (data not shown).
Mean relative amino acid levels (mg-1 of leaf dry weight) and fatty acid content (µg mg-1 leaf dry weight) in the leaves of eight virginia-type peanut cultivars and breeding lines after 1, 2, 4, and 7 days of optimum (control) (30/25 C) and high temperature (40/35 C) under controlled conditions.
Fatty Acids
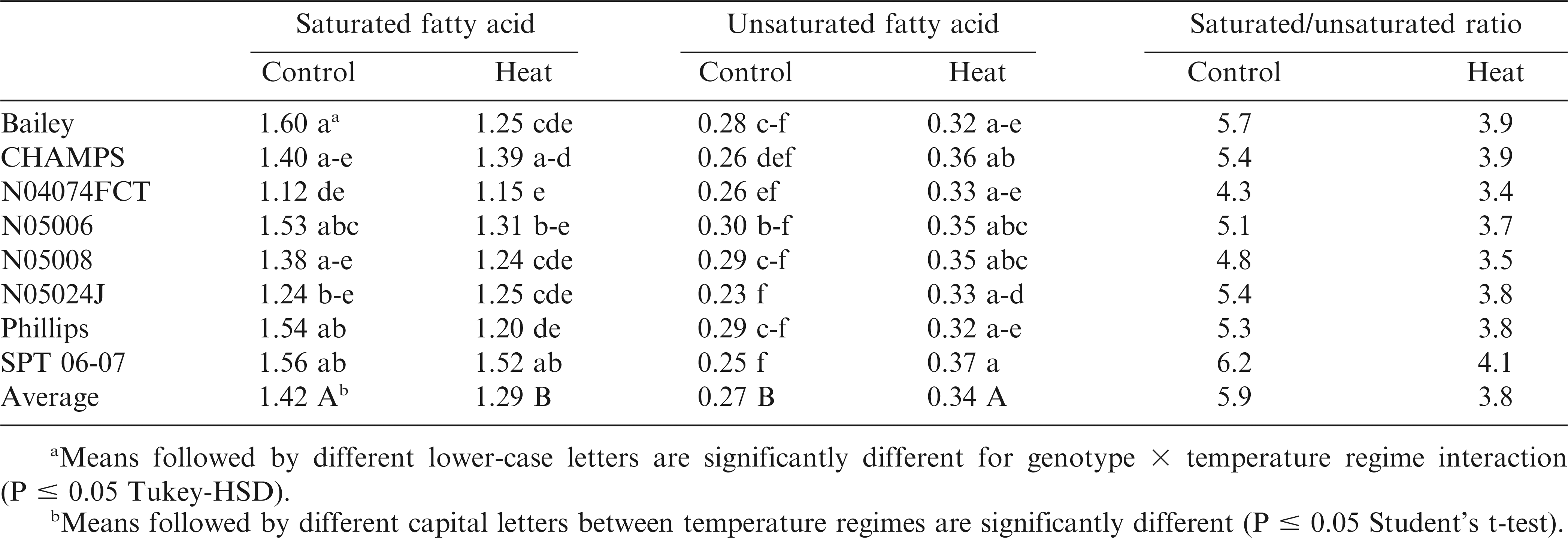
Saturated FA level was five times higher than unsaturated FA in control plants (1.42 vs. 0.27 µg mg−1) and four times in heat stressed seedlings (1.29 vs. 0.34 µg mg−1) (Table 7) so that the saturated/unsaturated FA ratio was on average 5.2 for control and 3.8 for heat-stressed plants, constantly throughout the temperature treatment duration. A smaller ratio for stressed than for control seedlings resulted from higher unsaturated and lower saturated FA content in stressed compared to control seedlings. SPT 06-07 had the highest saturated/unsaturated FA ratio in control (6.24) and heat-stressed treatments (4.11) among the genotypes. N04074FCT had the smallest saturated/unsaturated FA ratios, 4.31 in control and 3.48 in stressed plants (Table 8).
Correlations
The Fv /Fm decline was negatively correlated with the overall organic acids (r = − 0.24, p < 0.05) and saturated fatty acid (r = − 0.35, p < 0.01) levels at Day 1 of the heat treatment, but no other relationships were identified afterwards. Membrane injury was not correlated with any individual metabolite under heat stress.
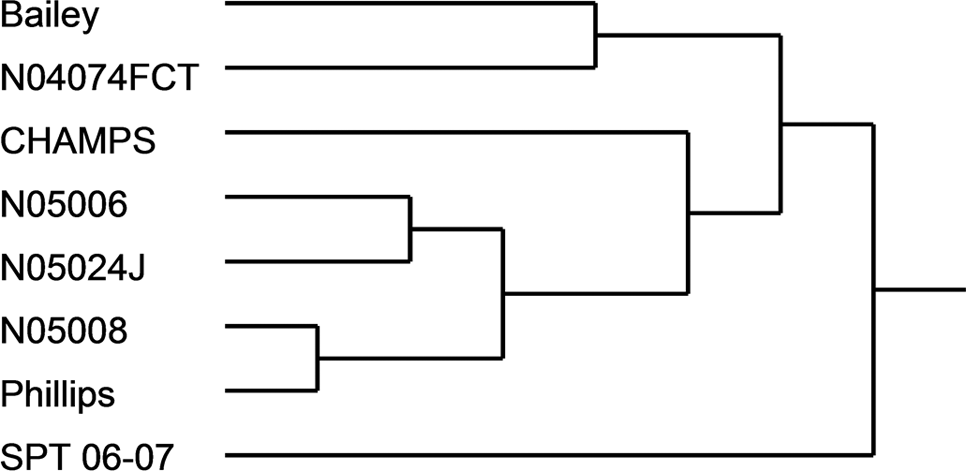
A dendrogram was constructed based on the physiological characteristics and the most significant metabolites through step-wise variable selection (Fig. 3). Based on the metabolic and physiological similarities, the eight genotypes were classified into two distinct groups. Group I consisted of seven genotypes and was different from Group II with SPT 06-07 as the sole member. Within Group I, N05008 and Phillips were closely associated to each other, N05006 was similar phenotypically to N05024J, and Bailey was closely associated with N04074FCT.
Discussion
Membrane Injury
During abiotic stress, lipid peroxidation can cause severe membrane injury (MI) and as such, it can be measured to assess the degree of stress in crops including peanuts (Bajji et al., 2002; Blum and Ebercon, 1981; Srinivasan et al., 1996). MI and the Fv /Fm ratio have previously been used in screening peanut genotypes for salt, heat, and drought stress tolerance (Lauriano et al., 2000; Qin et al., 2011; Srinivasan et al., 1996). Peanut seedlings exposed to a 40/35 C temperature treatment in this study showed on average 18% increased MI compared to control plants (Table 2). Genotypes Bailey, N04074FCT, and SPT 06-07 exhibited the least MI under heat stress in comparison with the other genotypes for which MI changes were inconsistent throughout the heat stress treatment. High levels of several amino acids, sugars, and sugar alcohols have been frequently associated with improved membrane stability during stress (Gounaris, 1984; Liu and Huang, 2000; Dhindsa, 1981). In this study we could not identify individual metabolites associated with reduced MI. However, when multiple regression analysis was used, the combination of fructose, glucose, maltose, inositol, pinitol and aspartate were positively associated with MI. In contrast, ribose, hydroxyproline and, surprisingly, saturated fatty acids were negatively correlated with MI. Combinations of these metabolites explained from 41 to 61% of the variation in the MI during the heat stress treatment.
Chlorophyll Fluorescence
Temperature effect was not significant for individual genotypes but overall Bailey and Phillips had the least (14 and 17%), and N05024J the greatest (28%) decline in the Fv /Fm % change among the eight genotypes after heat exposure (data not shown). Less Fv /Fm ratio decline under drought stress compared to well-watered plants has been reported in peanut (Burke, 2007; Kottapalli et al., 2009). Burke (2007) explained this to be the result of stress-induced sucrose accumulation, which provided better respiratory metabolic substrate under high temperature stressed compared with non-stressed plants. In our experiment, a negative correlation between the Fv /Fm % change and total sugar alcohols was observed under heat stress, suggesting that for our genotypes accumulation of sugar alcohols was rather associated with decline in the Fv /Fm ratio in heat stressed peanut seedlings. Although seedlings exhibited symptoms of heat stress in terms of increased MI and change in the levels of the majority of metabolites, the Fv /Fm % change due to heat stress exposure was small for Bailey and absent for the other genotypes. The lack of genotypic differences may be due to the short duration (7 d) of the heat stress period which may have been insufficient to cause significant damage to the photosystem II complex, often reflected by decreases in Fv /Fm ratio in stressed plants (Balota and Lichtenthaler, 1999; Lichtenthaler et al., 1998; Maxwell, 2000).
Polar Metabolites
Principal component analysis showed low variance among time points, indicating a weak differentiation of time-points on the basis of physiological characteristics and metabolites. In general, organic acids decreased under heat stress (Table 4). For example, major organic acids including, ascorbate, shikimate, and quinate showed significant decreases in their relative steady-state levels in heat-stressed seedlings, especially during the last two time-points (Days 4 and 7). Similar results were reported in drought-stressed wheat (Bowne et al., 2012) and field grown mature peanut plants (Singh, 2013). However, fumarate, malate, tartrate, citrate, and threonate increased either throughout entire heat stress exposure or in the first days of heat stress. Increased levels of organic acids were often positively related to cell growth and respiration (Glassop et al., 2007; Vasquez-Robinet et al., 2008). The relationship between organic acid levels and maintenance of high Fv /Fm ratio (low Fv /Fm % change) was positive (p < 0.05) at Day 1 during the heat treatment, suggesting that higher levels of organic acids during early heat stress could be an indicator of increased photochemical efficiency of peanut seedlings during stress as shown in other crops (Widodo et al., 2009; Liu and Huang, 2000). Among the sugar alcohols identified during this study, galactinol levels increased over 100 fold as a result of heat stress (Table 6). The osmoprotective and anti-oxidation roles of galactinol during abiotic stresses have been documented before (Nishizawa et al., 2008; Kaplan et al., 2004; Peters et al., 2007). In the present study, accumulation of galactinol in peanut plants is in agreement with this literature and highlights galactinol’s role in heat stress tolerance of peanut seedlings (Kaplan et al., 2004). Increased levels of pinitol are also known to confer tolerance to various abiotic stresses in other crop plants (Matos et al., 2010; McManus et al., 2000), but they were unchanged in our study. Indeed, in our research, a combination of several sugars, sugar alcohols, amino acids, and fatty acids better explained the variation in MI, 41 to 61% of the total.
A steady decline in sugar content was observed following prolonged heat stress with the lowest levels at Day 7 of the heat treatment (Table 6). Sugars that contributed to this trend were glucose, fructose, and the disaccharide maltose. Abiotic stresses affect the process of photosynthesis through various means, i.e., ROS-mediated membrane damage and reduced chlorophyll production (Chu et al., 1974; Dhindsa et al., 1981; Nishizawa et al., 2008). Lower sugar levels can be a direct consequence of reduced photosynthesis (Shah and Paulsen, 2003) and altered carbon metabolism under stress (Mangelsen et al., 2011). Decline in sugar levels was seen in wheat seedlings exposed to drought (Bowne et al., 2012). Heat-induced accumulation of soluble sugars such as glucose, fructose, and sucrose was also reported in Arabidopsis and other crops (Du et al., 2011; Kaplan et al., 2004; Rizhsky et al., 2004). In the present study, a significant decrease in sugar levels during heat stress (7 d) was observed.
Several studies reported an increase in amino acid levels in response to various stresses in sensitive cultivars (Du et al., 2011; Vasquez-Robinet et al., 2008; Zuther et al., 2007). The relative levels of hydroxyproline and asparagine were higher at Day 1, glutamate at Day 7, and glutamine throughout the time course in the heat-stressed than in control plants (Table 7). Their increase is sometimes attributed to stress-induced protein degradation due to tissue damage and senescence (Diaz et al., 2005; Widodo et al., 2009). For example, increased hydroxyproline and glutamine levels for N04074FCT could be indicative of sensitivity of this genotype to heat stress.
Fatty acids
As part of the cellular membrane complex, fatty acids play an important role in keeping the cellular membrane integrity during periods of environmental stress (McKersie et al., 1990; Simon, 1974). In this study the unsaturated fatty acid content increased and saturated fatty acid content decreased during heat stress relative to the controls (Table 7). However, the ratio of saturated vs. unsaturated fatty acids was variable among genotypes. The highest saturated to unsaturated fatty acid ratio was observed in SPT 06-07; this possibly coincided with lower MI for this genotype compared to other genotypes.
Conclusions
This study investigated the effect of high temperature stress on seedlings of eight virginia-type peanut cultivars and breeding lines in a controlled environment. From the physiological perspective, MI increased and chlorophyll fluorescence (measured as the Fv /Fm ratio) decreased in response to high temperature. Comparative metabolomics revealed changes in the levels of specific metabolites in heat-treated relative to control peanut seedlings. Even though we were able to highlight some metabolites, e.g., hydroxyproline, galactinol, and pinitol, that could explain specific differential physiological (Fv /Fm ratio and MI) responses in peanut plants, overall our data suggested general stress responses by seedlings rather than adaptive mechanisms under heat stress. Among the cultivars, Bailey seedlings seemed to have better tolerance to early season heat stress; MI was low (Table 2) and the Fv /Fm change due to the heat exposure was significantly less than for other genotypes (Singh, 2013). At least in part, this may explain the good crop stand and high yields observed in farmer fields for this cultivar, grown on over 85% peanut acreage in the VC region. Among the lines, SPT 06-07 exhibited lower membrane injury, increased galactinol, reduced hydroxyproline, and higher saturated vs. unsaturated fatty acids accumulation under heat stress compared to other genotypes. The uniqueness of SPT 06-07 in comparison with the other genotypes was also shown by the step-wise variable classification analysis, where SPT 06-07 was selected as the sole genotype of Group II, which was significantly different from Group I, that included the remaining genotypes. SPT 06-07 has exhibited improved drought tolerance of mature plants in field studies (Singh, 2013; Singh et al., 2014). Further, this shows that SPT 06-07 could be an important source for improvement of peanut tolerance to heat and drought stress at multiple growth stages.
Acknowledgements
The Virginia Peanut Growers Association, National Peanut Board, Virginia Crop Improvement Association, and Virginia Agricultural Council funded this work. We thank the staff at the Tidewater Agricultural Research and Extension Center and Virginia Tech Blacksburg campus for technical assistance.
Literature Cited
Alscher R.G. Erturk N. and Heath L.S. 2002 Role of superoxide dismutases (SODs) in controlling oxidative stress in plants J. Exp. Bot. 53 : 1331 - 1341 .
Apel K. and Hirt H. 2004 Reactive oxygen species: metabolism, oxidative stress, and signal transduction Ann. Rev. Plant Biol. 55 : 373 - 99 .
Asada K. 2006 Production and scavenging of reactive oxygen species in chloroplasts and their functions Plant Physiol 141 : 391 - 396 .
Awal M.A. Ikeda T. and Itoh R. 2003 The effect of soil temperature on source–sink economy in peanut (Arachis hypogaea) Environ. Exp. Bot. 50 ( 1 ): 41 - 50 .
Bajji M. Kinet J.-M. and Lutts S. 2002 The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat Plant Growth Regul. 36 : 61 - 70 .
Balota M. and Lichtenthaler H.K. 1999 Red chlorophyll fluorescence as an ecophysiological method to assess the behavior of wheat genotypes under drought and heat Cereal Res. Commun. 27 : 179 - 187 .
Blum A. and Ebercon A. 1981 Cell membrane stability as a measure of drought and heat tolerance in wheat Crop Sci. 21 : 43 - 47 .
Bowne J.B. Erwin T.A. Juttner J. Schnurbusch T. Langridge P. Bacic A. and Roessner U. 2012 Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level Mol. Plant 5 : 418 - 429 .
Burke J.J. 2007 Evaluation of source leaf responses to water-deficit stresses in cotton using a novel stress bioassay Plant Physiol. 143 : 108 - 121 .
Chu T. Aspinall D. and Paleg L. 1974 Stress metabolism VI. Temperature stress and the accumulation of proline in barley and radish. Funct. Plant Biol. 1 : 87 - 97 .
Collakova E. Aghamirzaie D. Fang Y. Klumas C. Tabataba F. Kakumanu A. Myers E. Heath L.S. and Grene R. 2013 Metabolic and transcriptional reprogramming in developing soybean (Glycine max) embryos Metabolites 3 : 347 - 372 .
Collakova E. Goyer A. Naponelli V. Krassovskaya I. Gregory J.F. Hanson A.D. and Shachar-Hill Y. 2008 Arabidopsis 10-formyl tetrahydrofolate deformylases are essential for photorespiration Plant Cell 20 : 1818 - 1832 .
Cox F.R. 1979 Effect of temperature treatment on peanut vegetative and fruit growth 1 Peanut Sci. 6 : 14 - 17 .
Craufurd P.Q. Wheeler T.R. Ellis R.H. Summerfield R.J. and Williams J.H. 1999 Effect of temperature and water deficit on water-use efficiency, carbon isotope discrimination, and specific leaf area in peanut Crop Sci. 39 : 136 - 142 .
Dat J. Vandenabeele S. Vranová E. Van Montagu M. Inzé D. and Van Breusegem F. 2000 Dual action of the active oxygen species during plant stress responses Cell. Mol. Life Sci. 57 : 779 - 795 .
Dhindsa R.S. Plumb-Dhindsa P. and Thorpe T.A. 1981 Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase J. Exp. Bot. 32 : 93 - 101 .
Diaz C. Purdy S. Christ A. Morot-Gaudry J.-F. Wingler A. and Masclaux-Daubresse C. 2005 Characterization of markers to determine the extent and variability of leaf senescence in Arabidopsis A metabolic profiling approach. Plant Physiol. 138 : 898 - 908 .
Du H. Wang Z. Yu W. Liu Y. and Huang B. 2011 Differential metabolic responses of perennial grass Cynodon transvaalensis× Cynodon dactylon (C4) and Poa pratensis (C3) to heat stress Physiol. Plant. 141 : 251 - 264 .
Duran A.L. Yang J. Wang L.J. and Sumner L.W. 2003 Metabolomics spectral formatting, alignment and conversion tools (MSFACTs) Bioinformatics. 19 : 2283 - 2293 .
Foyer C.H. and Noctor G. 2005 Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context Plant Cell Environ. 28 : 1056 - 1071 .
Glassop D. Roessner U. Bacic A. and Bonnett G.D. 2007 Changes in the sugarcane metabolome with stem development Are they related to sucrose accumulation? Plant Cell Physiol. 48 : 573 - 584 .
Golombek S.D. and Johansen C. 1997 Effect of soil temperature on vegetative and reproductive growth and development in three spanish genotypes of peanut (arachis hypogaea l.) Peanut Science 24 : 67 - 72 .
Gounaris K. Brain A.R.R. Quinn P.J. and Williams W.P. 1984 Structural reorganisation of chloroplast thylakoid membranes in response to heat-stress BBA - Bioenergetics 766 : 198 - 208 .
Guo C. and Oosterhuis D.M. 1995 Pinitol occurrence in soybean plants as affected by temperature and plant growth regulators J. Exp. Bot. 46 : 249 - 253 .
Hoagland D.R. and Arnon D.I. 1950 The water-culture method for growing plants without soil Calif. Agric. Exp. Stn. Circ 347:32 pp .
Kaplan F. Kopka J. Haskell D.W. Zhao W. Schiller K.C. Gatzke N. Sung D.Y. and Guy C.L. 2004 Exploring the temperature-stress metabolome of Arabidopsis Plant Physiol. 136 : 4159 - 4168 .
Kottapalli K.R. Rakwal R. Shibato J. Burow G. Tissue D. Burke J. Puppala N. Burow M. and Payton P. 2009 Physiology and proteomics of the water-deficit stress response in three contrasting peanut genotypes Plant Cell Environ. 32 : 380 - 407 .
Kovtun Y. Chiu W.-L. Tena G. and Sheen J. 2000 Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants Proc. Natl. Acad Sci. U.S.A. 97 : 2940 - 2945 .
Lauriano J.A. Lidon F.C. Carvalho C.A. Campos P.S. and do Céu Matos M. 2000 Drought effects on membrane lipids and photosynthetic activity in different peanut cultivars Photosynthetica 38 : 7 - 12 .
Lichtenthaler H.K. Wenzel O. Buschmann C. and Gitelson A. 1998 Plant stress detection by reflectance and fluorescence Ann. N.Y. Acad. Sci. 851 : 271 - 285 .
Liu X. and Huang B. 2000 Heat Stress Injury in Relation to Membrane Lipid Peroxidation in Creeping Bentgrass Crop Sci. 40 : 503 - 510 .
Lu Y. Savage L.J. Larson M.D. Wilkerson C.G. and Last R.L. 2011 Chloroplast 2010: A database for large-scale phenotypic screening of Arabidopsis mutants Plant Physiol. 155 : 1589 - 1600 .
Mangelsen E. Kilian J. Harter K. Jansson C. Wanke D. and Sundberg E. 2011 Transcriptome analysis of high-temperature stress in developing barley caryopses: Early stress responses and effects on storage compound biosynthesis Mol. Plant 4 : 97 - 115 .
Matos M. Campos P. Passarinho J. Semedo J. Marques N. Ramalho J. and Ricardo C. 2010 Drought effect on photosynthetic activity, osmolyte accumulation and membrane integrity of two Cicer arietinum genotypes Photosynthetica 48 : 303 - 312 .
Maxwell K. 2000 Chlorophyll fluorescence--a practical guide J. Exp. Bot. 51 : 659 - 668 .
McKersie B.D. Hoekstra F.A. and Krieg L.C. 1990 Differences in the susceptibility of plant membrane lipids to peroxidation BBA - Biomembranes 1030 : 119 - 126 .
McManus M.T. Bieleski R.L. Caradus J.R. and Barker D.J. 2000 Pinitol accumulation in mature leaves of white clover in response to a water deficit Environ. Exp. Bot. 43 : 11 - 18 .
Meehl G.A. Stocker T.F. Collins W.D. Friedlingstein P. Gaye A.T. Gregory J.M. Kitoh A. Knutti R. Murphy J.M. A. Raper S.C.B. Watterson I.G. Weaver A.J. , and Zhao Z.-C. 2007 : Global Climate Projections. In: Climate Change 2007: The Physical Science Basis Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change [Solomon et al. (eds.)] Cambridge University Press, Cambridge , United Kingdom and New York, NY, USA .
Mittler R. Vanderauwera S. Gollery M. and Van Breusegem F. 2004 Reactive oxygen gene network of plants Trends Plant Sci. 9 : 490 - 498 .
National Climate Data Center (NCDC) 2012 https://www.ncdc.noaa.gov (Accessed 01-15-2012) .
Nishizawa A. Yabuta Y. and Shigeoka S. 2008 Galactinol and raffinose constitute a novel function to protect plants from oxidative damage Plant Physiol. 147 : 1251 - 1263 .
Noctor G. and Foyer C.H. 1998 Ascorbate and glutathione: Keeping active oxygen under control Ann. Rev. Plant Phys. 49 : 249 - 279 .
Pei Z.-M. Murata Y. Benning G. Thomine S. Klusener B. Allen G.J. Grill E. and Schroeder J.I. 2000 Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells Nature 406 : 731 - 734 .
Peters S. Mundree S.G. Thomson J.A. Farrant J.M. and Keller F. 2007 Protection mechanisms in the resurrection plant xerophyta viscosa (baker): Both sucrose and raffinose family oligosaccharides (rfos) accumulate in leaves in response to water deficit J. Exp. Bot. 58 : 1947 - 1956 .
Prasad P.V.V. Boote K.J. Hartwell A. L. and Thomas J.M.G. 2003 Super-optimal temperatures are detrimental to peanut (Arachis hypogaea L.) reproductive processes and yield at both ambient and elevated carbon dioxide Glob. Change Biol. 9 : 1775 - 1787 .
Qin L. Li L. Bi C. Zhang Y. Wan S. Meng J. Meng Q. and Li X. 2011 Damaging mechanisms of chilling- and salt stress to Arachis hypogaea L. leaves Photosynthetica 49 : 37 - 42 .
Rizhsky L. Liang H. Shuman J. Shulaev V. Davletova S. and Mittler R. 2004 When defense pathways collide The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 134 : 1683 - 1696 .
SAS Institute Inc 2012 SAS Inst Cary, NC .
Selvaraj M. Burow G. Burke J. Belamkar V. Puppala N. and Burow M. 2011 Heat stress screening of peanut (Arachis hypogaea L.) seedlings for acquired thermotolerance Plant Growth Regul. 65 : 83 - 91 .
Shah N.H. and Paulsen G.M. 2003 Interaction of drought and high temperature on photosynthesis and grain-filling of wheat Plant Soil 257 : 219 - 226 .
Simon E.W. 1974 Phospholipids and plant membrane permeability New Phytol. 73 : 377 - 420 .
Singh D Collakova E. Isleib T.G. Welbaum G.E. Tallury S.P. and Balota M. 2013 Physiological and metabolic responses to water-deficit and heat stress of virginia-type peanut cultivars and breeding lines M.S. Thesis. Virginia Polytechnic Institute and State University .
Singh D. Collakova E. Isleib T.G. Welbaum G.E. Tallury S. and Balota M. 2014 Differential physiological and metabolic responses to drought stress of peanut cultivars and breeding lines Crop Sci. 54 : 2262 - 2274 .
Srinivasan A. Takeda H. and Senboku T. 1996 Heat tolerance in food legumes as evaluated by cell membrane thermostability and chlorophyll fluorescence techniques Euphytica 88 : 35 - 45 .
Vasquez-Robinet C. Mane S.P. Ulanov A.V. Watkinson J.I. Stromberg V.K. De Koeyer D. Schafleitner R. Willmot D.B. Bonierbale M. Bohnert H.J. and Grene R. 2008 Physiological and molecular adaptations to drought in Andean potato genotypes J. Exp. Bot. 59 : 2109 - 2123 .
Vu J.C.V. 2005 Acclimation of peanut (Arachis hypogaea L.) leaf photosynthesis to elevated growth CO2 and temperature Environ. Exp. Bot. 53 : 85 - 95 .
Widodo Patterson J.H. Newbigin E. Tester M. Bacic A. and Roessner U. 2009 Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance J. Exp. Bot. 60 : 4089 - 4103 .
Xu S. Li J. Zhang X. Wei H. and Cui L. 2006 Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress Environ. Exp. Bot. 56 : 274 - 285 .
Zuther E. Koehl K. and Kopka J. 2007 Comparative metabolome analysis of the salt response in breeding cultivars of rice, p. 285-315 . In Jenks M. et al. (Ed.) Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops Springer , Netherlands .
Notes
- Genetics Department, Kansas State University, Manhattan, KS 66506
- Tidewater Agricultural Research and Extension Center, Virginia Polytechnic Institute and State University, Suffolk, VA 23437
- Plant Pathology, Physiology, and Weed Science, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061
- Department of Crop Science, North Carolina State University, Raleigh, NC 27695
- Horticulture Department, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061
- Plant Genetic Resources Conservation Unit, Griffin, GA, 30223 *Corresponding author email: mbalota@vt.edu
Author Affiliations