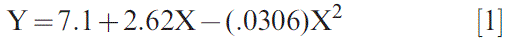Introduction
The majority component of peanut (Arachis hypogaea L.) is oil, typically accounting for 45-52% of the shelled kernel weight (Ahmed and Young 1982). Accordingly, oil composition is critical to final product quality of peanut based products, including nutritional profile, flavor and shelf life (Braddock et al. 1995). Fatty acids are basic constituents of fats and oils, and in the case of peanut, oleic acid and linoleic acid are the major fatty acids followed by palmitic acid and these three typically account for about 90% of the fatty acid composition, with smaller quantities of various longer chain fatty acids such as arachidic, behenic and lignoceric acid (Dean et al. 2011). Oleic and linoleic acid are both 18 carbon atoms long but differ in level of unsaturation, with oleic acid having one double bond and linoleic acid having two double bonds. Oleic acid commonly ranges from 43 to 83% of total peanut oil fatty acid content and linoleic from 1 to 37% (Andersen et al. 1998; Davis et al. 2008; Shin et al. 2010). Furthermore, the sum of these 2 fatty acids equates to approximately 80% of the oil fatty acid composition in peanut, and the two concentrations are highly inversely correlated, that is, as oleic acid increases, linoleic acid concentration decreases and vice versa (Andersen et al. 1998). These observed concentrations and correlations result from the underlying genetics and biochemical pathways responsible for producing these two fatty acids which have been well elucidated over the past approximate 20 years (Chu et al. 2009; Isleib et al. 2006; Moore and Knauft 1989). As these 2 fatty acids are so prevalent and so highly correlated in peanut, the typical convention for reporting their concentrations is the oleic/linoleic acid (O/L) ratio. For conventional U.S. peanut varieties, the O/L ratio is around 1.5 to 2.5. Varieties are considered to be high oleic when this ratio is greater than 9.0. Comparative studies have established that high O/L peanuts have exceptional shelf life compared to conventional peanuts, as the overall unsaturation of the oil is decreased enhancing resistance to oxidative rancidity, a primary factor dictating shelf life of peanut based products. The first high O/L peanut was documented in the University of Florida breeding program in the late 1980’s as a naturally occurring mutant (Norden et al. 1987). Since the discovery of the first high oleic peanut, numerous commercial high oleic cultivars have been released and most major breeding programs in the U.S. are now actively releasing, or planning to release, high oleic varieties (Barkley et al. 2011).
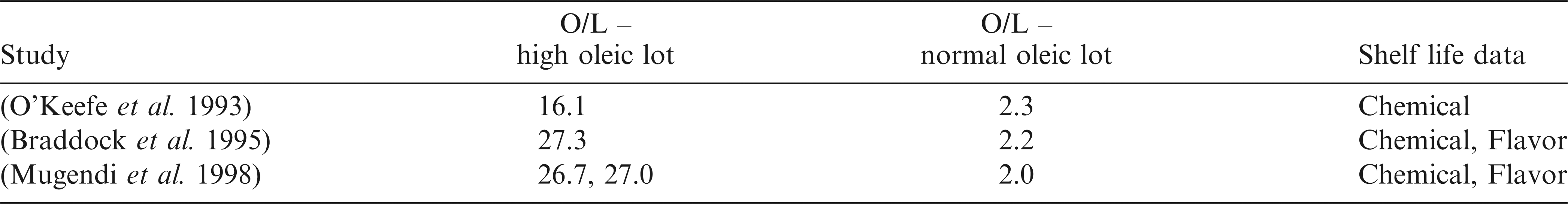
Chemical measurements, of oil derived from peanuts, such as peroxide value (PV), or the active oxygen method (AOM), suggest an approximate 10 fold improvement in shelf life when comparing high oleic and normal oleic lots (Braddock et al. 1995; O’Keefe et al. 1993). For example, roasted high oleic peanuts (average O/L = 27.3) stored at 25 C and a 40% relative humidity were estimated to reach a PV of 10 meq/kg at approximately 360 d versus 32 d for normal oleic peanuts (average O/L = 2.2) (Braddock et al. 1995). Related, AOM induction times for oil extracted from high oleic peanuts (average O/L = 16.1) was reported as 9.5 times longer than that of oil extracted from normal oleic peanuts (average O/L = 2.3) (O’Keefe et al. 1993). Furthermore, after roasting, high oleic peanut lots compared to normal oleic peanut lots have shown an approximate two fold improvement in off note generation as determined by descriptive sensory flavor analysis (Braddock et al. 1995; Mugendi et al. 1998; O’Keefe et al. 1993). Specifically, high oleic peanuts have an enhanced resistance to flavor fade, i.e. loss of the characteristic roasted peanut flavor descriptor during storage (Braddock et al. 1995; Mugendi et al. 1998; O’Keefe et al. 1993). However, while critical in establishing baseline shelf life data and the benefits of high oleic peanuts, a drawback of the previously mentioned studies is that the range of O/L ratios compared across these samples is limited as outlined in Table 1.
Among normal and high oleic varieties, the O/L ratio can vary significantly as a function of environment and maturity within a given harvested lot (Pattee et al. 1974; Singkham et al. 2010; Worthington at al. 1972). The commonly accepted threshold for high oleic seed is an O/L greater than 9.0 (Knauft et al. 2000). However, in practice O/L ratios can readily range from ~1.3 to 30+ (Chamberlin et al. 2011; Davis et al. 2013), and detailed shelf life data has not been collected across this industrially relevant range. Accordingly, for the current study, model oil blends were systematically prepared across a range of O/L values and a variety of chemical, physical, shelf life and sensory data were subsequently collected on those samples. Information derived from this study will improve the understanding of peanut shelf life as a function of O/L chemistry.
Materials and Methods
Peanut Oils & Blending
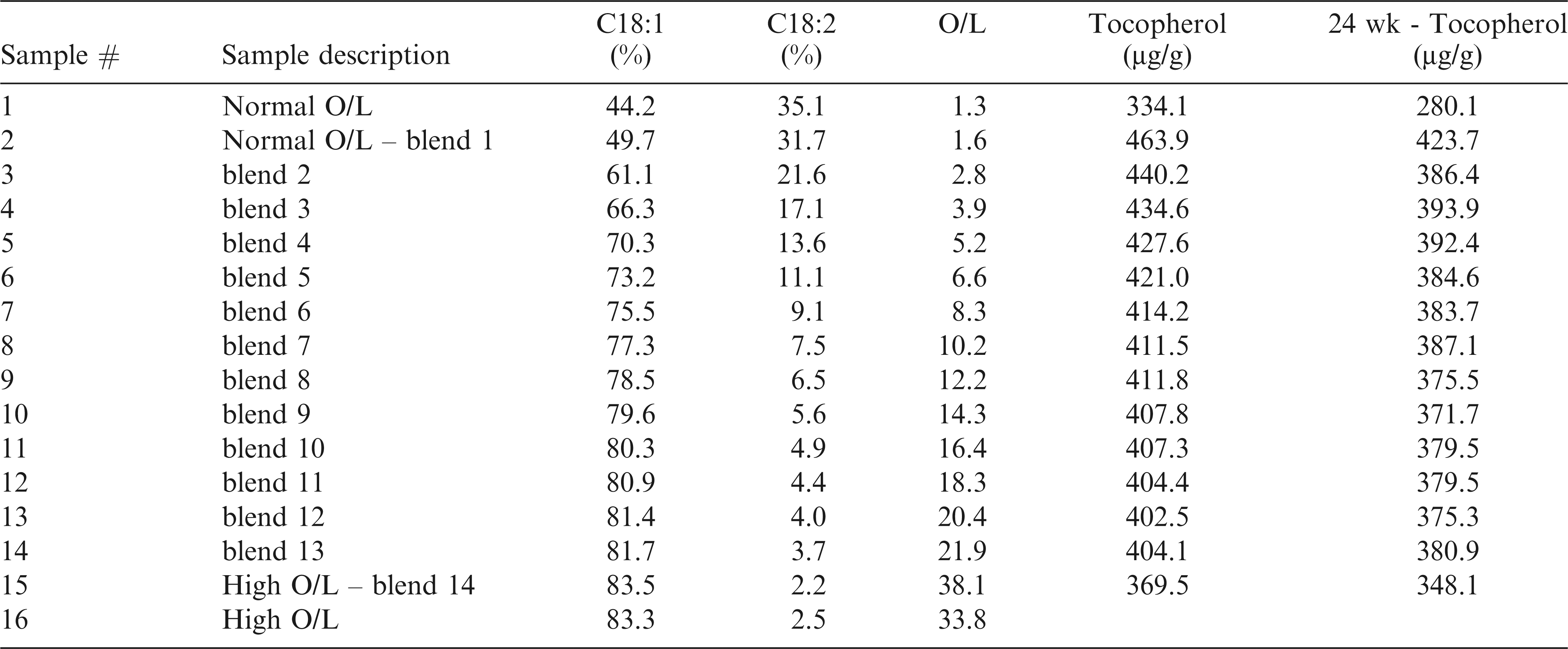
Crude peanut oil, i.e. unrefined, was mechanically expressed using a lab scale Carver Press or received from a commercial supplier. Peanuts, including two commercial lots of normal oleic peanuts, and several high oleic accessions from the 2011 Uniform Peanut Performance Tests (UPPT) program, were utilized to prepare the 16 test samples (Table 2). Sample 2, which was normal O/L oil provided by Golden Peanut and Treenuts (Alpharetta, GA) and Sample 15, which was a mixture of oil from three high O/L accessions from the UPPT program, were blended to create samples 3-14. Based on initial calculations, blends were created to cover a range of industrially relevant O/L ratios.
Fatty acid profile analyses
Fatty acids profiles of the oils were determined by gas chromatography (GC) as previously described (Davis et al. 2008). All chemicals and reagents were purchased from Thermo Fisher Scientific (Fair Lawn, NJ) unless otherwise noted. In brief, 20 to 30 mg of oil were saponified with 1 mL of 0.5 N NaOH in methanol. One mL of 14 % boron trifluoride in methanol (Sigma Chemical Corp., St. Louis, MO) was added to the samples to serve to catalyze the formation of the methyl esters from the fatty acids. The methyl esters were then extracted into 1 mL of hexane. The recovered hexane was dried over sodium sulfate and then injected into a Perkin Elmer Autosystem XL gas chromatograph (Perkin Elmer, Shelton, CT). The instrument was fitted with a 70% cyanopropyl polysilphenylene-siloxane capillary column (BPX-70, 30m length, 0.25mm interior diameter, 0.25 micron dry film, SGE Analytical Science, Austin, TX). The temperature program was an initial temperature of 60 C with a hold time of 2 min, then an increase of 10 C/min to 180 C with no hold time, followed by an increase at 4 C/min to 235 C. The carrier gas was helium with a flow rate of 1.85 mL/min. The injector was held at 220 C with a split flow rate of 76.9 mL/min. The detector was by flame ionization (FID) set to 235 C. A standard mix of fatty acids methyl esters (Kel Fir Fame 5, Matreya LLC, Pleasant Gap, PA) was injected with the samples to establish retention times for identification. The fatty acids determined were quantified as the percent of the total fatty acids present based on peak area according to AOCS Official Method Cd1-62 (Firestone 2004).
Density determination
Oil density was measured using an Anton-Paar (Graz, Austria) DMA 5000 oscillating tube density meter (Davis et al. 2008). A minimum of two replications were collected for each sample and the average value was used in subsequent analyses. Temperature control was internal to the instrument and automatically controlled at 20 C for this study. The specified accuracy of the instrument is ±5 x 10-6 g/ml and ± 0.01 C. The specified reproducibility of the instrument is ±1 x 10-6 g/ml and ± 0.001 C. The instrument was gently rinsed with hexane and then acetone between samples to clean, and then dried with an air pump supplied with the instrument.
Refractive index
Refractive index of seed oil was measured using an Anton-Paar (Graz, Austria) Abbemat 550 refractometer (Davis et al. 2013). Oil droplets were added to the measuring prism using a disposable pipette. The measurement prism was cleaned between measurements using a nonabrasive wipe. A minimum of two replications were collected for each sample and the average value was used in subsequent analyses. The specified accuracy of the instrument is ± 0.00002 nD at standard refractometric conditions. Temperature control was internal to the instrument and automatically controlled. Measurement temperatures for this study included 20, 40, 60, 80 and 100 C. The specified temperature accuracy is ± 0.03 C with a stability of ± 0.002 C.
Viscosity measurements
Dynamic and kinematic viscosities were determined using an Anton-Paar (Graz, Austria) SVM3000 Stabinger-type dual viscometer/density meter based on ASTM D7042–04 (Davis et al. 2008; Davis et al. 2009). Temperature control was internal to the instrument, automatically controlled and specified to a reproducibility of 0.02 C. Measurement temperatures for this study included 20, 40, 60, 80 and 100 C. The instrument was gently rinsed with hexane and then acetone between samples to clean, and then dried with an air pump supplied with the instrument.
Tocopherol analyses
Tocopherols were analyzed by HPLC as previously described (Davis et al. 2010; Hashim et al.1993). In brief, 200 mg of oil was diluted to 1 mL with mobile phase (1% isopropanol in hexane). The solutions were injected onto a Agilent 1100 high pressure liquid chromatograph (Agilent Technologies, Santa Clara, CA). The column was a Luna silica type (Phenomenex, Torrance, CA). The column dimensions were 250 mm in length and 0.45 mm inner diameter with 10 Å spherical packing. The flow rate was 1.2 mL/min. Detection was by UV set to 294 nm. Quantification was by peak area comparision to standard solutions of tocopherols diluted in hexane. The standards were alpha, beta, gamma and delta tocopherols purchased from Sigma Chemical Corp (St. Louis, MO). All other chemicals and reagents were purchased from Thermo Fisher Scientific (Fairlawn, NJ). A minimum of 3 replications were collected for each sample and averaged.
Metal analysis
Samples were ashed in a graphite furnace. After ashing and digestion in acid treatment, the copper (Cu) and Iron (Fe) contents were determined using a Perkin Elmer 8000Inductively Coupled Plasma-Optical Emission Spectrometer (Perkin Elmer, Shelton, CT). Standard solutions of Cu and Fe ions (Thermo Fisher Scientific, Fairlawn, NJ) were used to construct standard curves. A minimum of two replications were collected for each sample and averaged.
Oxidative stability index (OSI)
OSI was determined at 110 C as previously described (Bolton and Sanders 2002; Grimm et al.1996). In brief, 5 mL of oil was added to glass tubes and fitted into an Omnion OSI instrument (Ultra Scientific, North Kingstown, RI). The inflection point in hours was determined by the instrument in accordance with AOCS Official Method Cd 12B-92 (Firestone 2004). A minimum of 3 replications were collected for each sample and averaged.
Storage conditions
Each sample was evenly sub-sampled into 10 (2 reps, 5 time points) open test tubes and incubated at 24 C at 50% relative humidity. Samples were subsequently removed for peroxide value, tocopherol and sensory analysis at 2, 6, 12, 18 and 24 wks.
Peroxide value (PV) determination
Peroxide values of oil samples were determined potentiometrically using standardized solutions of sodium thiosulfate and a Mettler DL40GP MemoTitrator (Toledo, OH) based on AOAC Official Method 965.33. A minimum of 2 replications were collected for each sample and averaged. Method reproducibility is 0.33 meq/kg.
Sensory analysis
A trained descriptive panel (n = 7) of individuals highly experienced in peanut flavor evaluated samples during the storage study. Descriptive sensory terms relevant to lipid oxidation, including cardboardy and painty, were documented (Johnsen et al. 1988) using a truncated, semi-quantitative scale similar to Warner and Nelsen (1996). Samples were categorized into one of three categories: 1) bland, free of off-notes thru trace cardboardy or painty, i.e. just perceptible off-notes, 2) moderate cardboardy and/or painty intensities and 3) excessive cardboardy and/or painty off-notes rendering the sample unacceptable.
Results and Discussion
Oil Composition
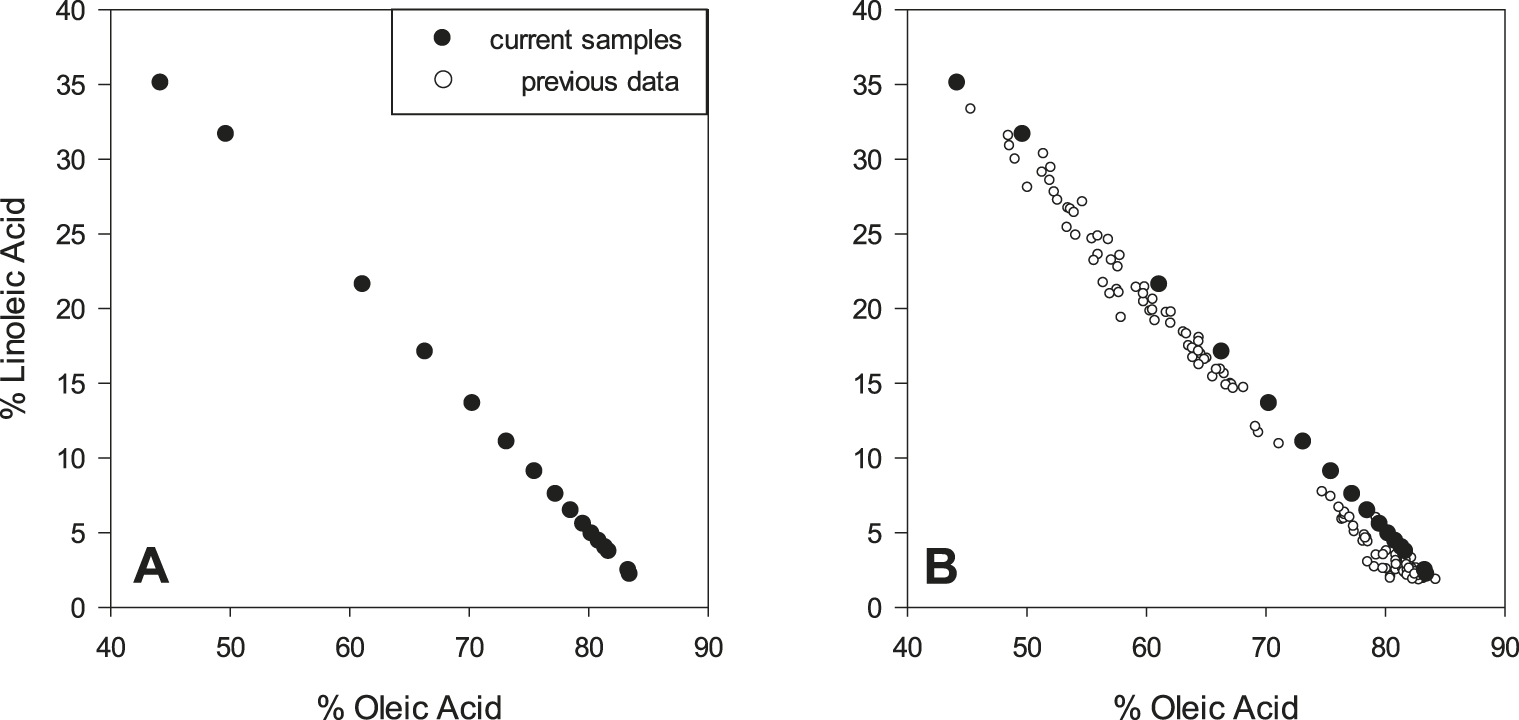
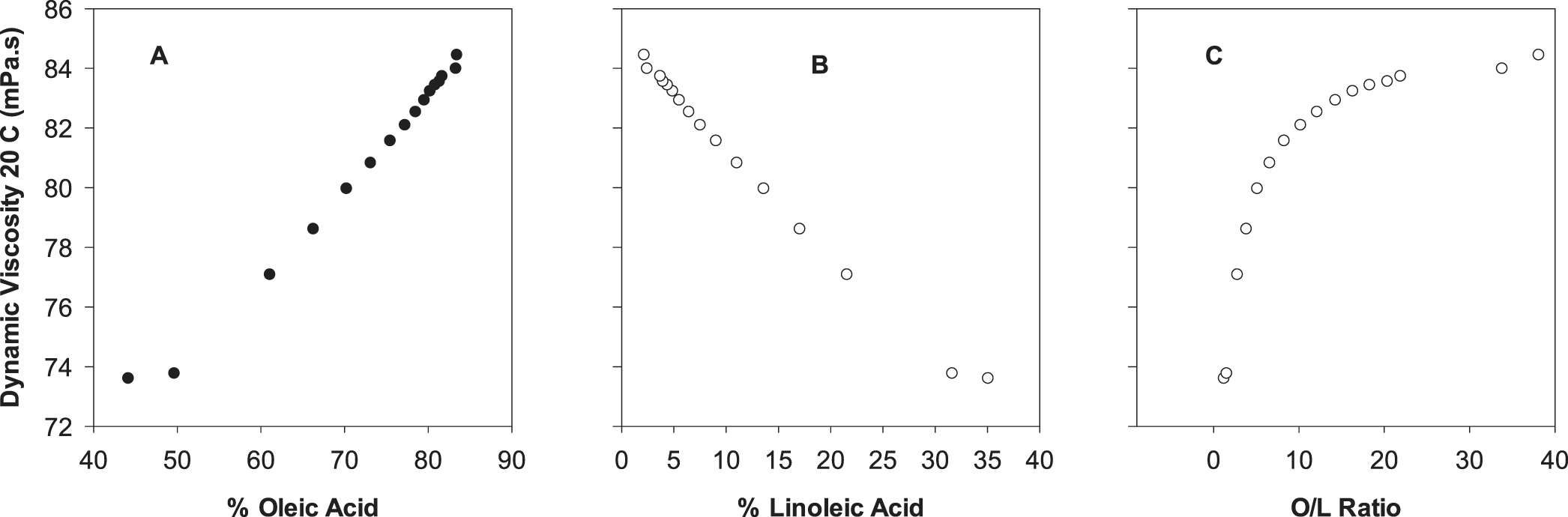
Complete fatty acid profiles were collected for all samples using GC. Relative (%) concentrations of oleic acid (C18:1), linoleic acid (C18:2) and the calculated O/L ratio are provided in Table 2. Samples 2-15 (blends) were specifically prepared to bracket a range of industrially relevant O/L ratios. Increased coverage was desired around the O/L ratio of 9.0, because this value is a commonly accepted threshold for classifying peanut as high O/L. The exponential response of the O/L ratio is emphasized: for example, at values < 3, an approximate one point change in the ratio corresponds to an approximate 10% change in oleic and linoleic acid contents, whereas at O/L values > 20, a one point change in the ratio corresponds to less than 1% changes in oleic and linoleic acid content (Table 2). A plot of % oleic acid versus % linoleic acid for current samples is highly linear and inversely correlated (Figure 1A). This response is well documented in peanuts (Andersen et al. 1998; Chu et al. 2009), and for further verification, current data was overlaid with equivalent data collected from previous samples (n = 130) analyzed in our lab, including both individual and bulk seed oil derived from a variety of normal and high O/L varieties (Figure 1B).
A primary goal of this study was to gain a more detailed understanding of the influence of O/L chemistry on shelf life and physical property measurements of model oil blends; however, tocopherols (antioxidants), and trace metals (prooxidants), such as copper and iron, are naturally present in peanut oil and could also impact these measurements. When preparing model oil blends, tocopherol content and trace metals were also (unavoidably) being systemically changed with blending. As such, tocopherol, copper and iron contents of the fresh (prior to oxidation experiments) samples were measured to estimate their effect, if any, on subsequent oxidation results. Tocopherols (Vitamin E) are a primary class of lipid soluble antioxidants naturally present in various seed oils, including peanut, with importance to oil stability and human health. Values for total tocopherols in the fresh oil ranged from 334 to 464 µg/g (Table 2), in agreement with data previously reported for peanut (Shin et al. 2009). The increase in tocopherol content with decreasing O/L ratio for samples 2-15 results from blending of the parent oils (samples 2 and 15). Tocopherol content of fresh oils from runner peanuts has been shown to be minimally affected by O/L ratio (Shin et al. 2009). Copper (Cu) and Iron (Fe) are pro-oxidants present at low levels in seed oils, including peanut. Variation in copper and iron among samples was minimal, ranging from 0.02 to 0.4 ppm for Cu and 0.09 to 0.56 ppm for Fe. Previous work has shown the effect of Cu to be more pronounced than Fe, but overall, the effects of these metals on oxidative stability compared to fatty acid unsaturation is minimal (Knothe and Dunn 2003). In current experiments, while minor effects cannot be ruled out, the effects of tocopherols, Cu, and Fe on measurements of oxidative stability appeared to be minor compared to the pronounced effect of O/L chemistry as discussed next.
Oil shelf life
Two methods were used to estimate oil shelf life. The first was the oxidative stability index (OSI), sometimes referred to as the oil stability index, a semi-quantitative predictor of the resistance of oxidation for an oil or other lipid (Knothe and Dunn 2003). In a highly controlled fashion using specific instrumentation, dry air is bubbled through the oil at a controlled temperature, usually elevated (110 C for current experiments), and the resulting volatile acids generated under the oxidizing conditions are collected in separate container of deionized water. These compounds increase the conductivity of the water, which is measured over time until a critical ‘induction time’ is reached. This induction time is the OSI, and when comparing multiple samples, increasing OSI correlates to increased resistance to oxidation. As such, this method is useful for quickly (results typically available by 1 to 3 d depending on conditions) comparing various samples to predict relative resistance to oxidation compared to traditional shelf life studies which may take months; however, specific quantitative extrapolation from OSI data to a true shelf life in a given food systems is generally not possible.
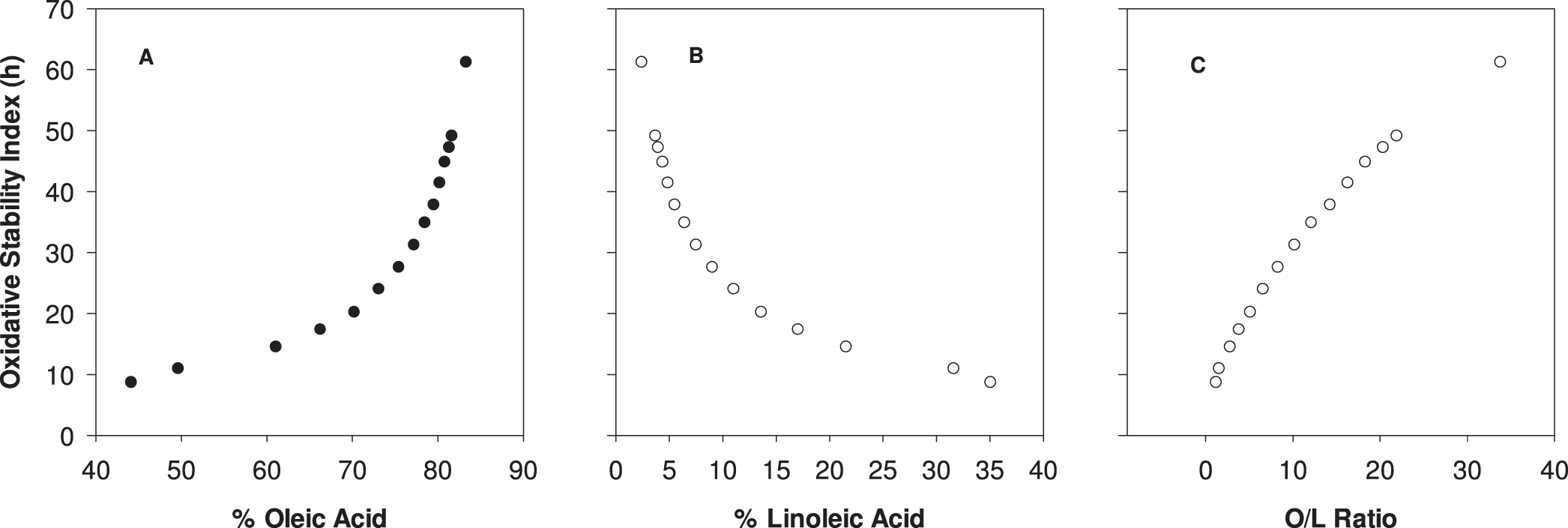
OSI response to % oleic acid (Figure 2A) and % linoleic acid (Figure 2B) were positively and negatively steeply curvilinear, respectively, whereas OSI response to the ratio of these FA displayed more of a positive linear response, albeit still slightly curvilinear (Figure 2C). While the slope in OSI vs O/L ratio was steepest across lower values of the ratio, there was still appreciable improvement in OSI with increasing O/L across the entire range tested, i.e. thru an O/L of 33.8. Studies have shown that linoleic acid is approximately 40-50X more oxidatively labile than oleic acid (Choe et al. 2005), and seemingly even relatively minor changes in concentrations of these two FA for very high O/L samples, i.e. those greater than 20, increased the OSI. Data in Figure 2C was well described (R2 = 0.99) by the equation:
where X = O/L ratio and Y = OSI. This suggests the excellent potential to readily predict OSI for any given O/L ratio. An important factor when considering OSI, or any determination of lipid shelf life, is temperature. Oxidation rates typically increase exponentially to increasing temperature (Wang 2002); however, excessively high temperatures can promote reaction pathways that might not be completely relevant to actual storage conditions. Current OSI data was collected at 110 C which is typical in the scientific literature for this method, as it allows for rapid data collection that can be utilized to predict relative shelf life (Coppin and Pike 2001); however, the most realistic shelf life data is collected at temperatures and overall conditions that most closely mimic actual product storage.
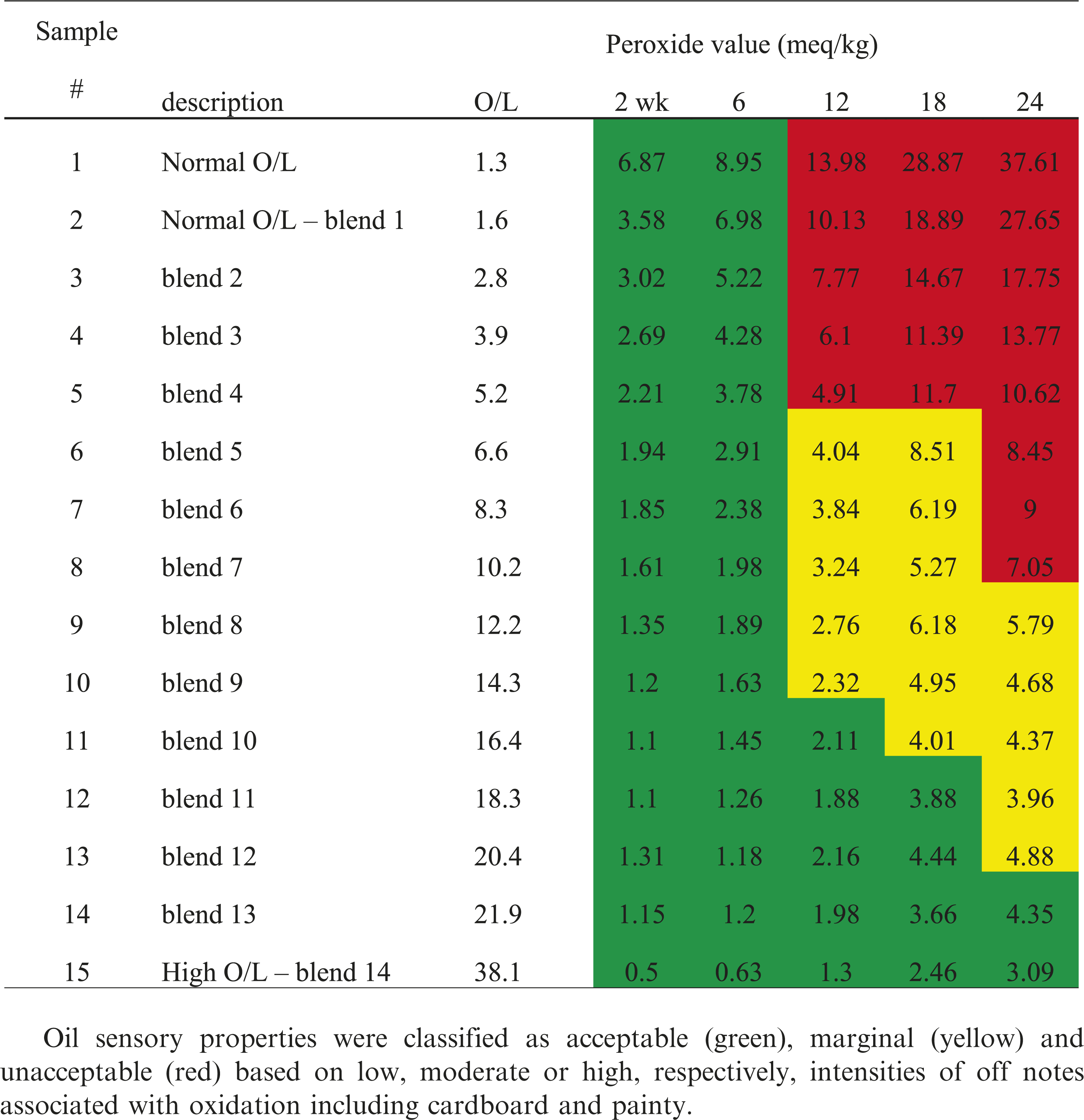
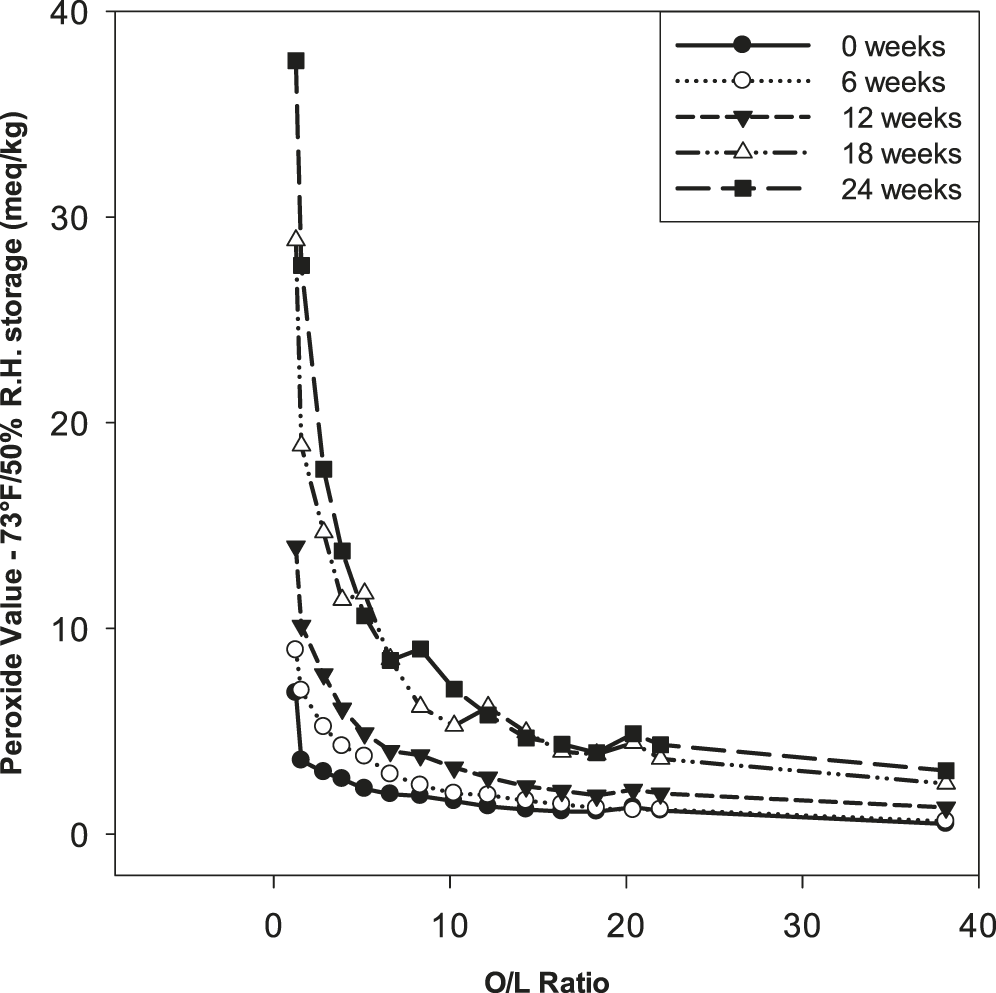
To gain further insights into oil oxidative stability under typical storage conditions, oils were stored in open vessels at 24 C/50% relative humidity and periodically sampled for peroxide value, flavor, and tocopherol content over 24 wk. Peroxides are primary products of lipid oxidation and the peroxide value (PV) is indicative of the extent to which an oil, or other lipid, is oxidized. Determination of PV is widely used in research settings and industrially to document lipid oxidation as the highly reactive peroxides promote formation of aldehydes and other small molecular weight compounds that negatively affect flavor (O’Brien 2002). Peroxides do degrade with time such that during storage oils commonly show a maximum in PV followed be a subsequent decrease, making it important to document storage history when comparing PV’s among samples. PV increased substantially from 0 to 24 wk for all oils, with the greatest increases occurring for low O/L oils (Table 3, Figure 3). As reference, for soybean oil, PV’s of 1 to 5, 5 to 10, and > 10 correspond to low, medium and high oxidation according to AOCS Method Cg 3-91 (Firestone 2004). For any given time point, PV increased curvilinearly with decreasing O/L ratio (Figure 3), and there was a notable inflection at an O/L ratio of approximately 9.0 at 18 and 24 wk of storage, demonstrating substantial improvement for samples with O/L values greater than 9.0. This inflection also demonstrates that relatively small increases in O/L ratio for values less than 9.0 result in substantial improvements in PV generation. These curvilinear responses are attributed to the O/L ratio and the substantially increased lability to oxidation of linoleic acid versus oleic acid as previously discussed. Depression of PV with increasing O/L was observed across the entire range of samples, and the PV never exceeded 5.0 for any sample with an O/L ≥ 14.3, even after 24 wk (Table 3). Slight decreases in PV from 18 to 24 wk for samples 5, 6, 9 and 10 (Table 3) are attributable to inherent experimental error.
Good flavor, free of off-notes, is critical to the quality of all foods including peanut. Oxidation leads to ‘flavor fade’ in roasted peanuts during storage, which is the steady decline of the characteristic ‘roasted peanutty’ flavor (Williams et al. 2006). Excessive oxidation during storage also leads to off-notes including cardboardy and painty in peanuts, which are classic flavor descriptors produced from lipid oxidation. While chemical measurements can help predict flavor impact of oxidation, no measurement can fully predict/describe flavor aside from sensory analyses. Accordingly, documenting oil flavor as a function of O/L chemistry in the storage study was ultimately the most important measure of product quality as flavor is the primary driver in the consumption of most peanut-based products (Neta et al. 2010). A descriptive sensory flavor lexicon was used to describe oil flavor, and samples were subsequently categorized as acceptable (green), marginal (yellow) and unacceptable (red) (Table 3), with these categories based on low, moderate or high, respectively, collective intensities of off notes associated with oxidation including cardboard and painty. Flavor acceptability decreased with storage time, and increasing O/L ratio was clearly associated with the potential for acceptable flavor during storage. For example, all samples with an O/L ≤ 5.2 had unacceptable flavor at 12 wk, whereas samples 6-8 with O/L ranging from 6.6-10.2 maintained marginal flavor thru 18 wk. At 24 wk, a transition from unacceptable to marginal was observed at an O/L between 10 and 12, and only the 2 samples with O/L values greater than 21 had acceptable flavor.
Comparison of flavor data with corresponding PV’s revealed that while increasing PV was associated with less acceptable flavor, definite thresholds were not observed (Table 3). For example, all samples at 6 wk had acceptable flavor despite several of the low O/L samples having PV’s greater than 5; however, at 12 wk, several samples with PV’s ranging from ~2-4 had moderately oxidized flavors. By 24 wk, all samples with a PV > 7 had unacceptable flavor, defined by excessive intensities of cardboardy and/or painty off-notes (Table 3). Previously, a multi-lab study specifically compared none (PV: 0), low (PV: 3.1-4.6), moderate (PV: 10.4-11.9) and highly (PV: 17.2-17.9) oxidized soy, canola and sunflower oils, and found that 85-90% of the 14 panels, for a given oil, correctly ranked samples using sensory flavor analyses (Warner and Nelsen 1996). While current flavor and PV trends generally agree with this earlier study, there are discrepancies which are attributed to the more complex set of samples being evaluated in the current study, that is a continuum of 15 samples over multiple time points, as opposed to 4 distinctly oxidized samples (for a given oil) in the previous study. While PV measurements are important as a general indicator of oil quality, the current data emphasizes the importance of documenting flavor.
After 24 wk of storage, total tocopherol contents were relatively constant, with no more than 17% degradation in any sample, and there was a slight trend of increased tocopherol degradation for low O/L samples (Table 2). Considering many of the samples clearly had unacceptable flavor after 24 weeks and elevated peroxide values (Table 3), data indicated that substantial tocopherol degradation was not a prerequisite for unacceptable quality.
As our primary goal was to better understand the shelf life of whole roasted peanuts, an important limitation of the current experimental design was the use of model oil blends, as opposed to whole, roasted seed for shelf life determinations. Clearly, the natural matrix of the roasted seed impacts shelf life and must be considered; however, to source roasted whole seed across the range of O/L values covered in this study would likely not be possible. Furthermore, numerous confounding variables would have to be considered which would be challenging to isolate, including cultivar, environment, age of seed before roasting, maturity and roasting conditions, to name a few. However, oil extraction and analysis is a common first step in most peanut shelf life testing protocols. Accordingly, the current study provides some detailed foundational data, which could complement additional, more targeted shelf life studies in the future.
Oil physical properties
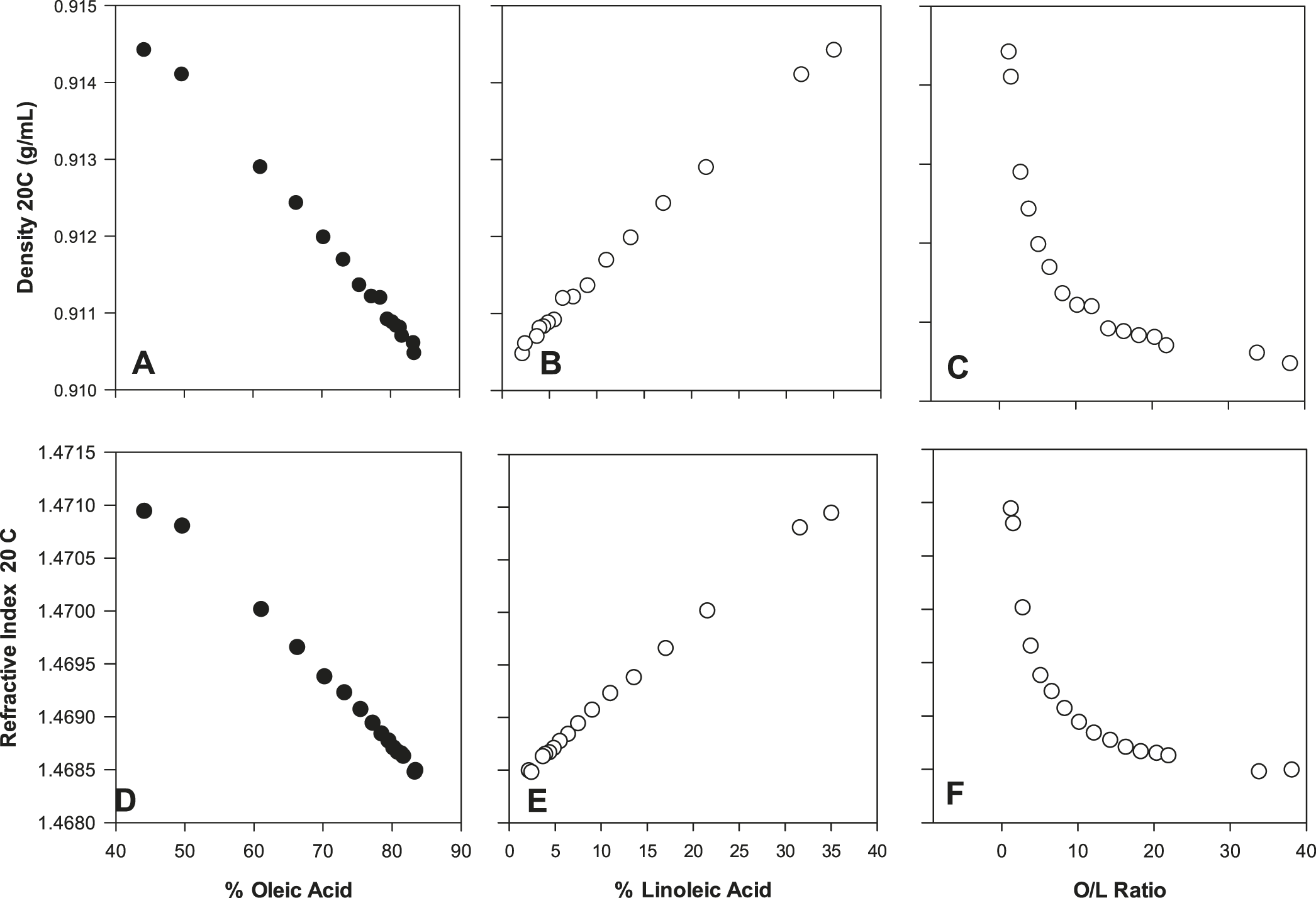
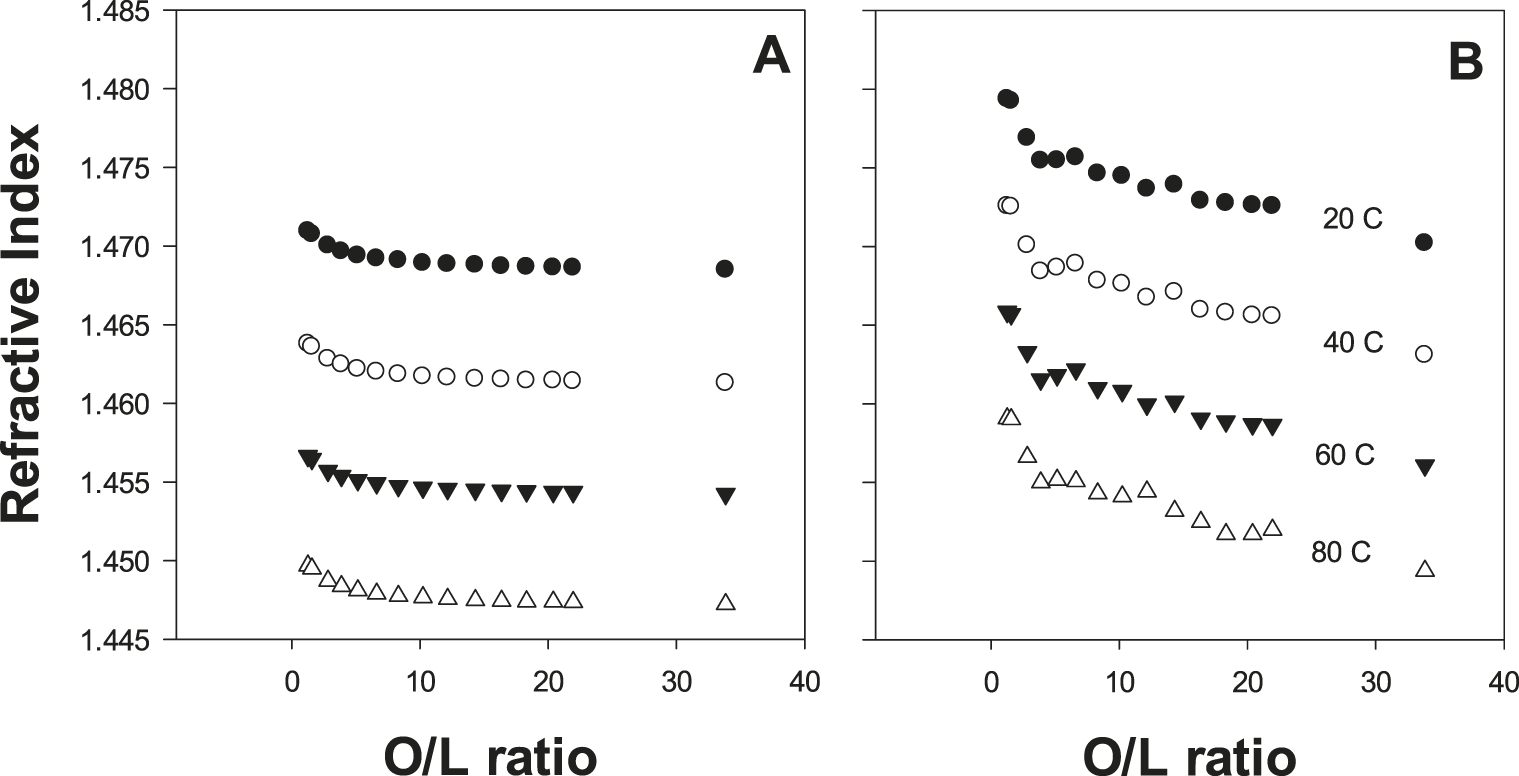
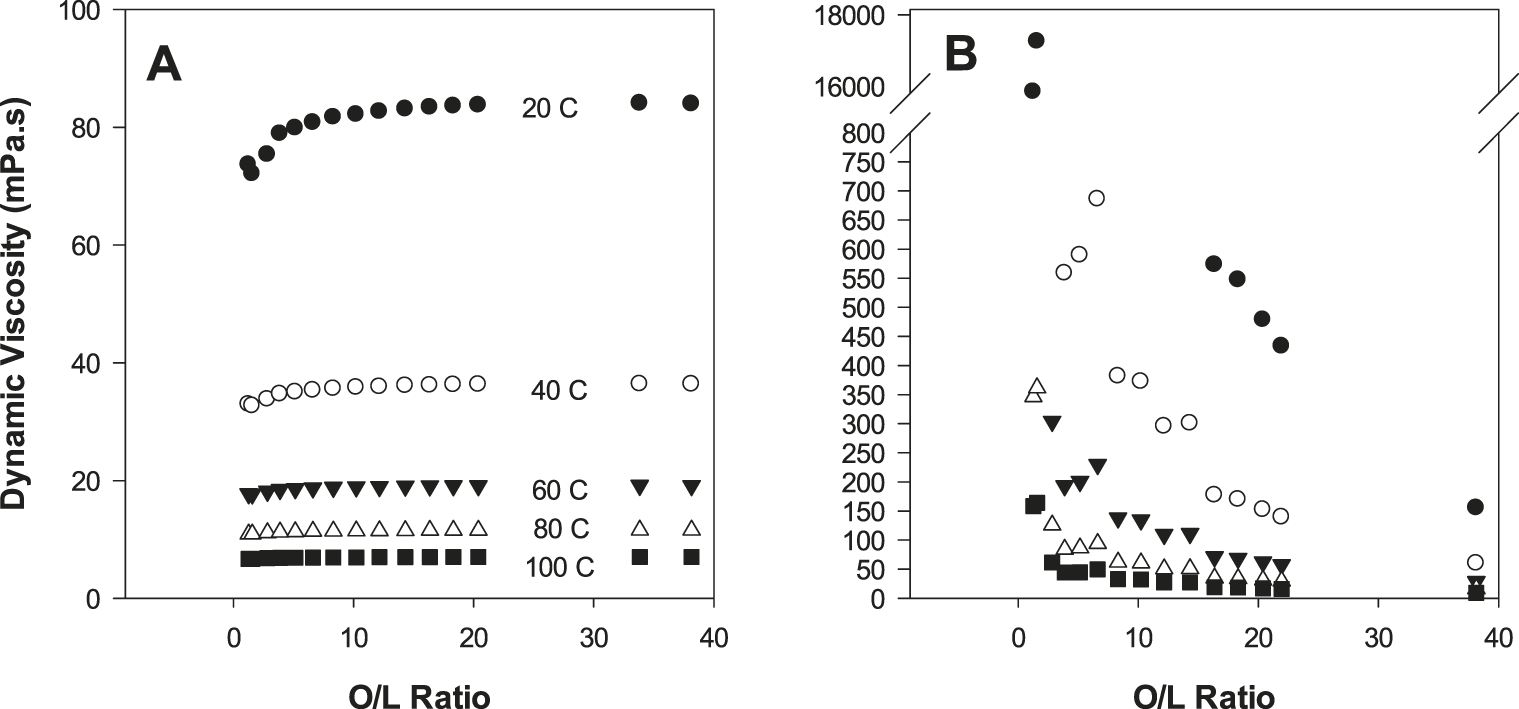
A secondary goal of this study was to collect oil physical property data and evaluate this information from a quality perspective. For density (Figure 4), refractive index (Figure 4) and dynamic viscosity (Figure 5), linear trends were observed for these physical properties as a function of either % oleic acid or % linoleic acid. When considered simultaneously via the O/L ratio, the response of these physical properties to the O/L ratio was curvilinear (Figures 4, 5). Similar data for oil density, refractive index, and viscosity as a function of peanut O/L chemistry has been previously reported (Davis et al. 2008; Davis et al. 2013). These distinct trends are attributable to a combination of three factors: 1) the predominance of oleic and linoleic acid in peanut oil; 2) the strong inverse correlation of these two FA; and 3) the distinct molecular structures of these two FA resulting from their differing levels of unsaturation. Such physical property data has important practical applications. For example, refractive index is commonly used in the peanut industry as a rapid, simple and cost effective screen to determine if a sample is high O/L (Davis et al. 2013). As RI measurements are possible with only a drop of oil, this measurement is especially effective for determining if oil from a single seed is high O/L (assuming appropriate accuracy/precision) during single seed sampling protocols (Davis et al. 2013). Oil density and viscosity have implications in the processing and handling of oils. Due to Stoke’s Law considerations, oil density and viscosity influence the subsequent stability/textural properties of products utilizing these oils, i.e. dressings, or perhaps peanut butter, although no published studies have specifically investigated the textural qualities of the latter product as a function of O/L chemistry.
During oxidation, in addition to off-flavor development, seed oil physical properties are also expected to change; however, there is very little data of this type published specifically for peanut oil. To better understand these oxidative effects, samples were reclaimed after the OSI measurements had been completed and oil refractive index (Figure 6) and dynamic viscosity (Figure 7) measured. Refractive index for fresh (Figure 6A) and highly oxidized (post OSI, Figure 6B) oils was measured at 4 different temperatures, which was programmable and automatically controlled by the instrument. For all samples, as temperature increased, refractive index shifted predictably downwards (Figure 6). This general temperature response is well documented for oils and many liquids (Wang 2002), and demonstrates the importance of having proper temperature control during refractive index measurements to properly utilize this technique for documenting peanut O/L chemistry. After OSI, the refractive index of the highly oxidized oils shifted upwards, with a greater shift for low O/L oils (Figure 6). Increasing refractive index with increasing oxidation is generally established with seed oils; however, such data specifically for peanut oil was heretofore lacking. These data suggests that under carefully controlled conditions and for specific applications, refractive index measurements have potential to serve as a cost effective and rapid means to document peanut oil oxidation. Related measurements of oil viscosity were highly temperature sensitive (Figure 7). For fresh oils, high O/L oils had increased viscosities; however, after OSI, the highly oxidized low O/L oils had viscosities that were orders of magnitudes greater than oxidized high O/L samples (Figure 7). Increased oil viscosity with advanced oxidation results from formation of large molecular weight polymeric compounds (Tseng et al. 1996; Tyagi and Vasishtha 1996), and current data suggest that despite having lower starting viscosities, lower O/L samples are much more apt to develop increased viscosity during thermal abuse.
Conclusion
Extensive shelf life data was collected for peanut oil blends strategically prepared to cover a range of industrially relevant O/L ratios. Overall, both oxidative stability index measurements of oil blends, which is a relative predictor of shelf life, and peroxide value/descriptive flavor analyses collected over 6 months of storage at 24 C at 50% relative humidity demonstrated that substantial shelf life benefits were realized across the entire O/L range examined: 1.3 to 33.8. As only seemingly minor changes (< 3%) in concentrations of oleic acid and linoleic acid occur at O/L values greater than 15, the continued benefit thru the entire range was not expected. Overall, data generally supports the current industry norm of selecting and O/L = 9.0 as the threshold for classifying samples as high O/L.
Disclaimer
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Acknowledgements
Mr. Jim Schaefer for his assistance with tocopherol measurements.
Literature Cited
Ahmed E.H. and Young C.T. 1982 Composition, nutrition and flavor of peanut . Pages 655 - 687 . In Pattee H.E. and Young C.T. (eds). Peanut Science and Technology , American Peanut Research and Education Society, Inc. , Yoakum, TX . Pages 825 .
Andersen P.C. Hill K. Gorbet D.W. and Brodbeck B.V. 1998 Fatty acid and amino acid profiles of selected peanut cultivars and breeding lines J. Food Comp. Anal. 11 : 100 - 111 .
Barkley N.A. Chenault Chamberlin K.D. Wang M.L. and Pittman R.N. 2011 Genotyping and fatty acid composition analysis in segregating peanut (Arachis hypogaea L.) populations Peanut Sci. 38 : 11 - 19 .
Bolton G.E. and Sanders T.H. 2002 Effect of roasting oil composition on the stability of roasted high-oleic peanuts J. Amer. Oil Chem. Soc. 79 : 129 - 132 .
Braddock J.C. Sims C.A. and O’Keefe S.F. 1995 Flavor and oxidative stability of roasted high oleic acid peanuts J. Food Sci. 60 : 489 - 493 .
Chamberlin K.D. Melouk H.A. Madden R.D. Dillwith J.W. Bannore Y.C. El Rassi Z. and Payton M.E. 2011 Determining the oleic/linoleic acid ratio in a single peanut seed: A comparison of two methods Peanut Science. 38 : 78 - 84 .
Choe E. Lee J. and Min D.B. 2005 Chemistry for oxidative stability of edible oils . Pages 558 - 590 . In Akoh C.C. and Lai O-M. (eds). Healthful Lipids AOCS Press , Urbana, IL . Pages 670.
Chu Y. Holbrook C.C. and Ozias-Akins P. 2009 Two alleles of ahFAD2B control the high oleic acid trait in cultivated peanut Crop Sci. 49 : 2029 - 2036 .
Coppin E. and Pike O. 2001 Oil stability index correlated with sensory determination of oxidative stability in light-exposed soybean oil J. Amer. Oil Chem. Soc. 78 : 13 - 18 .
Davis J.P. Dean L.L. Price K.M. and Sanders T.H. 2010 Roast effects on the hydrophilic and lipophilic antioxidant capacities of peanut flours, blanched peanut seed and peanut skins Food Chem. 119 : 539 - 547 .
Davis J.P. Dean L.O. Faircloth W.H. and Sanders T.H. 2008 Physical and chemical characterizations of normal and high-oleic oils from nine commercial cultivars of peanut J. Amer. Oil Chem. Soc. 85 : 235 - 243 .
Davis J.P. Geller D. Faircloth W.H. and Sanders T.H. 2009 Comparisons of biodiesel produced from unrefined oils of different peanut cultivars J. Amer. Oil Chem. Soc. 86 : 353 - 361 .
Davis J.P. Sweigart D.S. Price K.M. Dean L.L. and Sanders T.H. 2013 Refractive index and density measurements of peanut oil for determining oleic and linoleic acid contents J. Amer. Oil Chem. Soc. 90 : 199 - 206 .
Dean L.L. Davis J.P. and Sanders T.H. 2011 Groundnut (Peanut) Oil . Pages 225 - 239 . In: Gunstone F. D. (ed). Vegetable Oils in Food Technology: Composition, Properties and Uses, Second Edition Wiley-Blackwell , West Sussex, UK . Pages 337 .
Firestone D. 2004 Official Methods and Recommended Practices of the AOCS Champaign, IL, American Oil Chemists’ Society .
Grimm D.T. Sanders T.H. Pattee H.E. Williams D.E. and Sanchez-Dominguez S. 1996 Chemical composition of Arachis hypogaea L. Subsp hypogaea Var. hirsuta peanuts. Peanut Sci. 23 : 111 - 116 .
Hashim I.B. Koehler P.E. Eitenmiller R.R. and Kvien C.K. 1993 Fatty acid content and Tocopherol content of drought stressed Florunner peanuts Peanut Sci. 20 : 21 - 24 .
Isleib T.G. Wilson R.F. and Novitzky W.P. 2006 Partial dominance, pleiotropism, and epistasis in the inheritance of the high-oleate trait in peanut Crop Sci. 46 : 1331 - 1335 .
Johnsen P.B. Civille G.V. Vercellotti J.R. Sanders T.H. and Dus C.A. 1988 Development of a lexicon for the description of peanut flavor J. Sens. Stud. 3 : 9 - 17 .
Knauft D.A. Gorbet D.W. Norden A.J. inventors; University of Florida Research Foundation, Inc., assignee. 2000 May 16 , 2000 Enhanced peanut products and plant lines. US Patent 6063984 .
Knothe G. and Dunn R.O. 2003 Dependence of oil stability index of fatty compounds on their structure and concentration and presence of metals J. Amer. Oil Chem. Soc. 80 : 1021 - 1026 .
Moore K.M. and Knauft D.A. 1989 The inheritance of high oleic acid in peanut J. Hered. 80 : 252 - 253 .
Mugendi J.B. Sims C.A. Gorbet D.W. and O’Keefe S.F. 1998 Flavor stability of high-oleic peanuts stored at low humidity J. Amer. Oil Chem. Soc. 75 : 21 - 25 .
Neta E.R. Sanders T. Drake M.A. 2010 Understanding Peanut Flavor: A Current Review . Pages 985 - 1022 . In: Hui Y. H. (ed). Handbook of Fruit and Vegetable Flavors John Wiley & Sons, Inc. , New York, NY . Pages 1095 .
Norden A.J. Gorbet D.W. Knauft D.A. and Young C.T. 1987 Varability in oil quality among peanut cenotypes in the Florida Breeding Program Peanut Science 14 : 7 - 11 .
O’Brien R. 2002 Cottonseed Oil . Pages 203 - 230 . In: Gunstone F. D. (ed). Vegetable Oils in Food Technology: Composition, Properties and Uses CRC Press LLC , Boca Raton, FL . Pages 337 .
O’Keefe S.F. Wiley V.A. and Knauft D.A. 1993 Comparison of oxidative stability of high-oleic and normal-oleic peanut oils J. Amer. Oil Chem. Soc. 70 : 489 - 492 .
Pattee H.E. Johns E.B. Singleton J.A. and Sanders T.H. 1974 Composition changes of peanut fruit parts during maturation Peanut Sci. 1 : 57 - 62 .
Shin E-C. Craft B.D. Pegg R.B. Phillips R.D. and Eitenmiller R.R. 2010 Chemometric approach to fatty acid profiles in Runner-type peanut cultivars by principal component analysis (PCA) Food Chem. 119 : 1262 - 1270 .
Shin E-C. Huang Y-Z. Pegg R.B. Phillips R.D. and Eitenmiller R.R. 2009 Commercial Runner peanut cultivars in the United States: Tocopherol composition J. Agric. Food Chem. 57 : 10289 - 10295 .
Singkham N. Jogloy S. Kesmala T. Swatsitang P. Jaisil P. and Puppala N. 2010 Genotypic variability and genotype by environment interactions in oil and fatty acids in high, intermediate, and low oleic acid peanut genotypes J. Agric. Food Chem. 58 : 6257 - 6263 .
Tseng Y.C. Moreir R. and Sun X. 1996 Total frying-use time effects on soybean-oil deterioration and on tortilla chip quality Inter. J. Food Sci.Tech. 31 : 287 - 294 .
Tyagi V.K. and Vasishtha A.K. 1996 Changes in the characteristics and composition of oils during deep-fat frying J. Amer. Oil Chem. Soc. 73 : 499 - 506 .
Warner K. and Nelsen T. 1996 AOCS collaborative study on sensory and volatile compound analyses of vegetable oils J. Amer. Oil Chem. Soc. 73 : 157 - 166 .
Williams J.P. Duncan S.E. Williams R.C. Mallikarjunan K. Eigel W.N. and O’Keefe S.F. 2006 Flavor fade in peanuts during short-term storage J. Food Sci. 71 : S265 - S269 .
Worthington R.E. Allison J.R. and Hammons R.O. 1972 Varietal differences and seasonal effects on fatty acid composition and stability of oil from 82 peanut genotypes J. Agric. Food Chem. 20 : 727 - 730 .
Notes
- USDA ARS Market Quality & Handling Research Unit, Raleigh, NC 27695
- Dept. of Food, Bioprocessing & Nutrition Sciences, North Carolina State University, Raleigh, NC 27695
- Technical center, The Hershey Company, Hershey, PA 17033 *Corresponding author is now with JLA International: jackdavis@jlaglobal.com
Author Affiliations