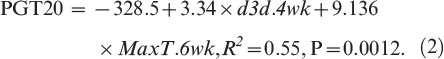Introduction
In many parts of the world where peanuts are grown, including the southeastern US, aflatoxin contamination is a reoccurring problem. Contamination of peanut seed occurs only after pods are colonized by Aspergillus flavus (Link), Aspergillus parasiticus (Speare) or both of these closely related fungi (hereafter referred to as A. flavus or aflatoxigenic fungi). These fungi are common soil inhabitants that readily invade peanut pods during any developmental stage (Diener and Davis, 1977). Aflatoxins are produced by these fungi after seed colonization in as little as five days under optimal conditions (Diener and Davis, 1977).
High temperatures, especially in conjunction with drought, have long been recognized as critical for aflatoxin contamination in peanut (Hill et al. 1983, Wilson and Stansell, 1983, Cole et al., 1985). Wilson and Stansell (1983) reported that moisture stress during the last 40 to 75 d before harvest was more conducive for high aflatoxin concentrations in peanut than drought stress occurring earlier in the cropping season. Hill et al. (1983) observed that aflatoxin contamination occurred only when drought stress was accompanied by high geocarposphere soil temperatures. Cole et al. (1985) noted that geocarposphere temperatures averaging 26.3 to 29.6 C were most conducive to aflatoxin accumulation in peanuts, while temperatures above 31.3 C appeared suppressive. These studies and others provided the information that Chauhan et al. (2010) built into a simulation model that calculates the risk of aflatoxin contamination in peanuts. While this simulation has been shown to be reliable, its use may be limited due its complexity and the need for multiple environmental variable inputs. A simpler means of calculating the risk for aflatoxin contamination in peanuts is desired by growers or in places where appropriate soil measurements are difficult to obtain. The ultimate goal of this work is to develop a model to predict aflatoxin contamination in peanut in order to better manage a peanut crop to minimize this problem. The objective of the current study was to determine specific periods of temperature and moisture conditions prior to harvest that would define environments with high risk for aflatoxin contamination in peanuts.
Materials and Methods
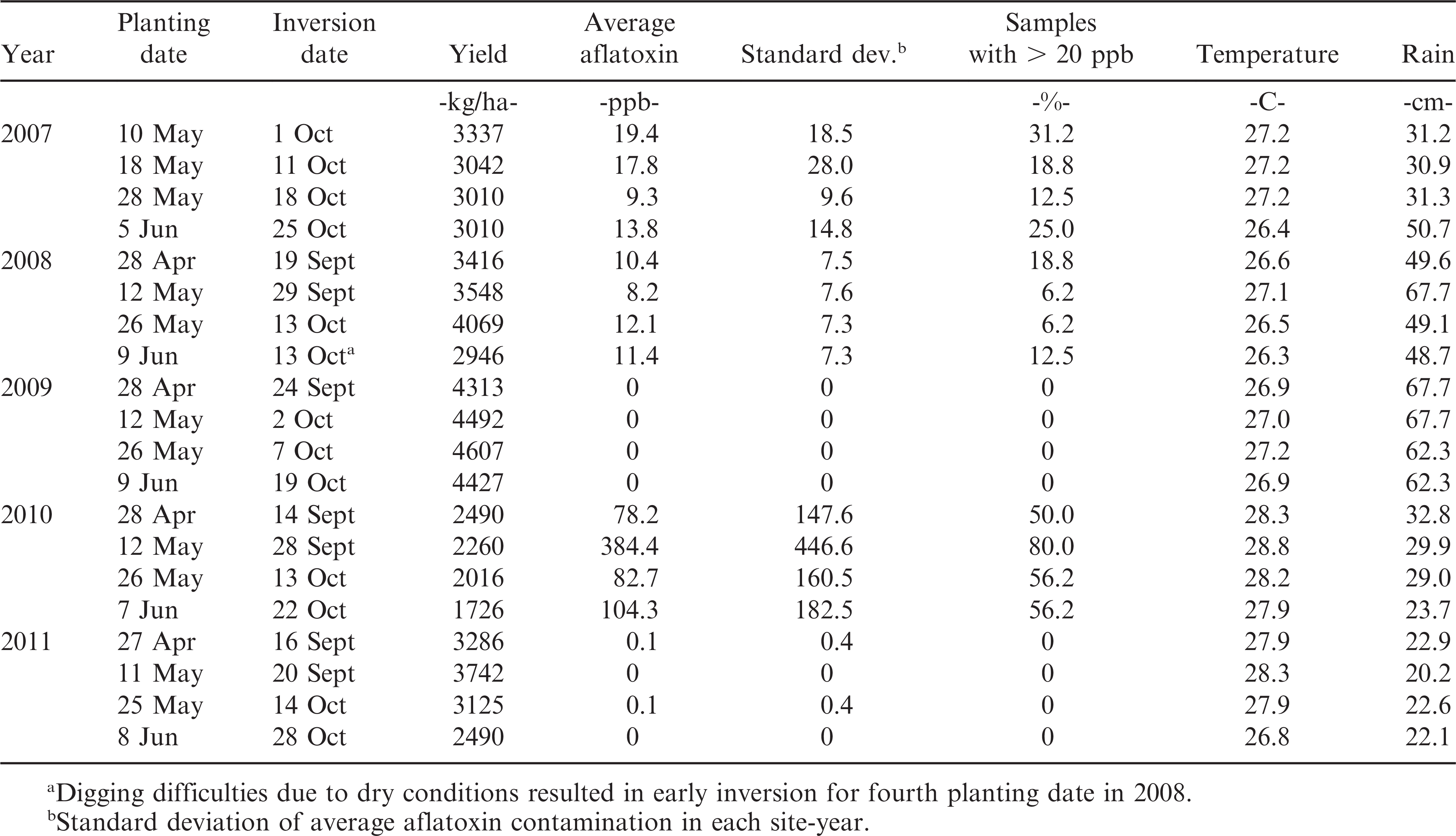
This study was done on a non-irrigated site on the Wiregrass Research and Experiment Center (WREC) near Headland, AL, starting in autumn 2006. The soil type is a Dothan sandy loam soil (fine-loamy, kaolinitic, thermic Plinthic Kandiudults) with <1% organic matter. The study consisted of a 4 × 4 factorial set of treatments, with four planting dates and four winter cover crops, randomized in four replications. Winter cover crops were ‘Harrison’ oats, ‘Wren’s Abruzzi’ rye, ‘GA Gore’ (2006 to 2009, 2011) and ‘SS8641’ (2010, 2012 to 2014) wheat and fallow. Small grains were planted in mid-Nov to mid-Dec and killed with glyphosate in late-Feb to mid-Mar. Peanuts were then strip-tilled into the small grain cover crop plots, while fallow plots were conventionally tilled. The four peanut planting dates were scheduled on 2-wk intervals, starting in late April and ending in early June (Table 1). Peanut cultivars were Georgia-03L (Branch, 2004) in 2007 and 2008, Florida-07 (Gorbert and Tillman, 2009) in 2009 and 2010, Georgia-06G (Branch, 2007) in 2011 and 2012. There have been no reports indicating that these cultivars differ in their resistance to Aspergillus spp. infection or aflatoxin accumulation. With four planting dates in each year, this study provided 20 environments over 5 yr (2007 through 2011) of data collection.
Peanuts were inverted at optimal maturity, based on hull-scrape (Williams and Drexler, 1981), for each planting date (Table 1). Three to 5 d following inversion, pods were harvested from individual plots and yield per plot recorded. Peanuts were shelled and seed samples from each plot of approximately 0.5 kg (∼ 20% of total plot yield) were ground in a Wiley mill (Swedesboro, NJ) using a 6 mm screen. From 2007 through 2010, aflatoxins were analyzed by high performance liquid chromatography (HPLC) as described by Wilson and Romer (1991) with slight modifications. Briefly, ground samples were blended with 90% acetonitrile (J.T. Baker, through VWR, Radnor, PA) then filtered through Whatman No. 1 filter paper (VWR). Filtrate was passed through a Mycosep Multifunctional Cleanup Column (Romer Labs, Inc., Washington, MO), then 200 ml of purified solution was added to 700 ml derivatizing agent (water/trifluoroacetic acid/acetic acid; 70:20:10 (v/v/v)) and incubated at 55 C for 30 min before injection into the HPLC. A commercially available Aflatoxin B and G mixture (Sigma Chemical Co, St. Louis, MO, USA) served as a standard for total aflatoxin quantification (sum of B1, B2, G1, and G2). Beginning in 2011, 10 g of ground peanuts from each plot were assayed using Veratox® and Veratox® HS kits (Neogen Corp., Lansing, MI). These ELISA-based assays are valid for quantifying 5 to 50 ppb and 1 to 8 ppb total aflatoxins, respectively. The high sensitivity kits (HS) were used when <5 ppb was detected in initial assays of samples. When the regular assay indicated levels >50 ppb, the extraction was diluted and re-assayed. In each year, at least 10% of all samples were assayed a second time to confirm assay results.
Temperatures were collected from the Alabama Mesonet unit located approx. 2.4 km from the study site (AWIS, 2014). Rainfall was recorded daily at 7 a.m. from a rain gauge at the field site (B. Gamble, personal communication). Temperature variables that were evaluated included daily minimum, maximum, and average air temperatures. Soil temperatures were initially taken on site but were inconsistent due to frequent equipment failures, often due to animal damage to cables. Total rain, numbers of dry days (<0.25 cm rain) and numbers of dry periods of differing lengths, were also evaluated as variables representing moisture. The differing lengths of dry periods were 2, 3 or 4 consecutive days with ≤0.25 cm rain during any one day. Thus, five days without rain would be equal to four 2-d-dry, three 3-d-dry, or two 4-d-dry periods. Each of the temperature variables were averaged over 2, 3, 4, 5, 6 and 7 wk prior to inversion; precipitation, dry days and each of the dry periods were summed over these same intervals.
Data analysis
Sixteen samples of peanuts were assayed from each planting date (4 replications by 4 cover crops) in each year. Total aflatoxin concentrations (parts per billion, ppb) were averaged over these 16 samples and natural log-transformed (TPPB):
to normalize the data prior to analyses. The proportion of samples with greater than 20 ppb aflatoxins (PGT20) was also determined for each planting date-year environment. Rank-order correlation coefficients were calculated for TPPB and PGT20 with each of the temperature and moisture variables (48 variables) from the first 5 yr of this study (2007-2011; n = 20) to identify which of the environmental variables had the greatest influence on aflatoxin levels or contamination risk. Stepwise regression was done to confirm results of correlation regarding variables that most affected TPPB and PGT20. Significance was set at P = 0.10.
Aflatoxin risk occurrences were grouped according to PGT20 for each planting date-year: ‘no risk’ had TPPB = PGT20 = 0, ‘low’ had TPPB > 0 and PGT20 = 0, ‘moderate’ had TPPB > 0 and PGT20 > 0, and ‘high’ had PGT20 ≥ 30%. These were used for graphical displays of results.
Weather variables selected from the above procedures were used as independent variables in regression analysis for more precise prediction for the risk for aflatoxin contamination. For this analysis, PGT20 was the dependent variable.
Risk thresholds and models were validated using data from the same study site in 2012, 2013 and 2014, and two additional 2010 studies. In the 2013 and 2014 studies used for validation, only three and two planting dates, respectively, were sampled. One of the 2010 studies was done at WREC in a field approximately half way between the Mesonet weather unit and the field site rain gauge (Uppala et al., 2013). Plots of this study were inoculated with A. flavus-infested corn grits. Soil at this site was a Lucy sandy loam (loamy, kaolinitic, thermic Arenic Kandiudults). The other 2010 study was located near Tallassee, AL, in a Cahaba loamy sand (fine-loamy, siliceous, semiactive, thermic Typic Hapludults) (Uppala et al., 2013) and located approx. 4.2 km from an Alabama Mesonet weather recording unit.
Results
Winter cover crops did not have consistent significant effects on aflatoxin levels (data not shown); therefore, the cover crop plots were used as additional replication of planting date treatments. Aflatoxin concentrations in samples from the same planting date-year were highly variable (Table 1), so PGT20 was used to reflect the risk of aflatoxin occurrence in each environment. Twenty ppb was used as a baseline for this variable because it is the USDA-FDA maximum limit for aflatoxin content in peanuts meant for human consumption, immature animals, or when the intended use is not known (USDA, 2002).
Aflatoxin levels differed substantially by year. In 2007 and 2008, aflatoxin concentrations in all samples were 0 to 90 ppb while PGT20 values were 0 to 32% over planting dates; no toxins were detected in 2009 and a few samples had very low toxins in 2011 (Table 1). In 2010, aflatoxin concentrations averaged >70 ppb and PGT20 ≥ 50% for all four planting dates. Over four planting dates in 5yr, the two variables, TPPB and PGT20, were highly correlated to one another (R = 0.93, P < 0.0001).
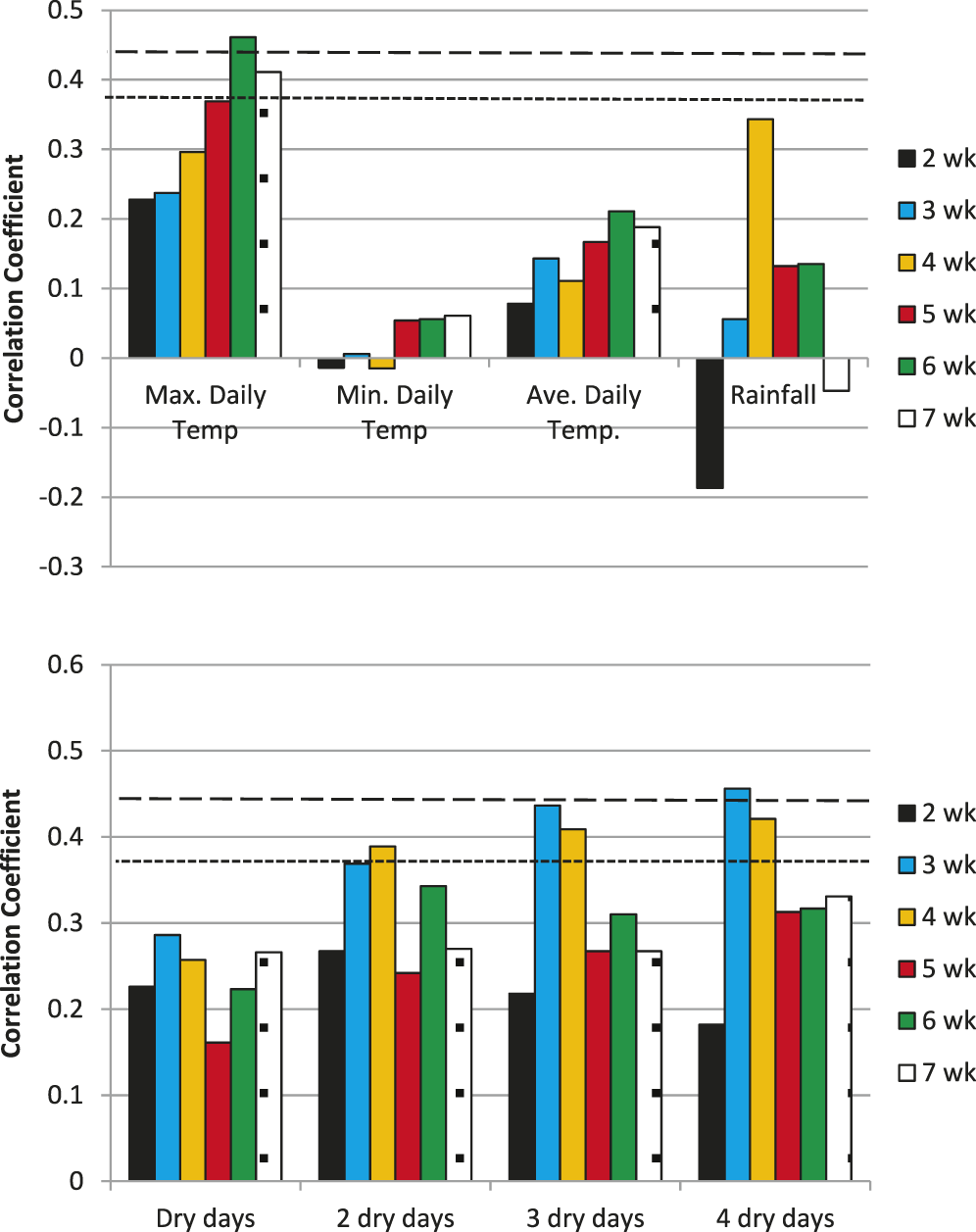
Ambient minimum and average temperatures over any of the six time periods prior to inversion were not correlated to TPPB (data not shown) or to PGT20 (Fig. 1). Only maximum daily temperatures averaged over 6 wk (MaxT.6wk) was significantly correlated to TPPB (R = 0.42, P = 0.06) (data not shown) or to PGT20 (R = 0.46, P = 0.041) (Fig. 1). Total rainfall for any time period was not correlated to TPPB (P > 0.24) or to PGT20 (P > 0.13) (Fig. 1). However, correlation coefficients for dry days were consistently positive, and 3- and 4-d-dry periods summed over 3 and 4 wk prior to inversion were significant for TPPB (R > 0.44, P < 0.055) and PGT20 (R > 0.41, P < 0.075) (Fig. 1).
The first two independent variables selected through stepwise regression for predicting PGT20, from among all temperature and moisture variables, were MaxT.6wk and cumulative number of 3-d-dry periods over 4 wk prior to harvest (d3d.4wk) (R2 = 0.55, P = 0.0012). Each of these variables was highly significant to the model (P < 0.02). The next variable selected through stepwise regression was total rainfall over 3 wk prior to harvest; however, this variable was less significant to the model (P = 0.053). Thus, MaxT.6wk and d3d.4wk were used as variables for defining thresholds for aflatoxin risk.
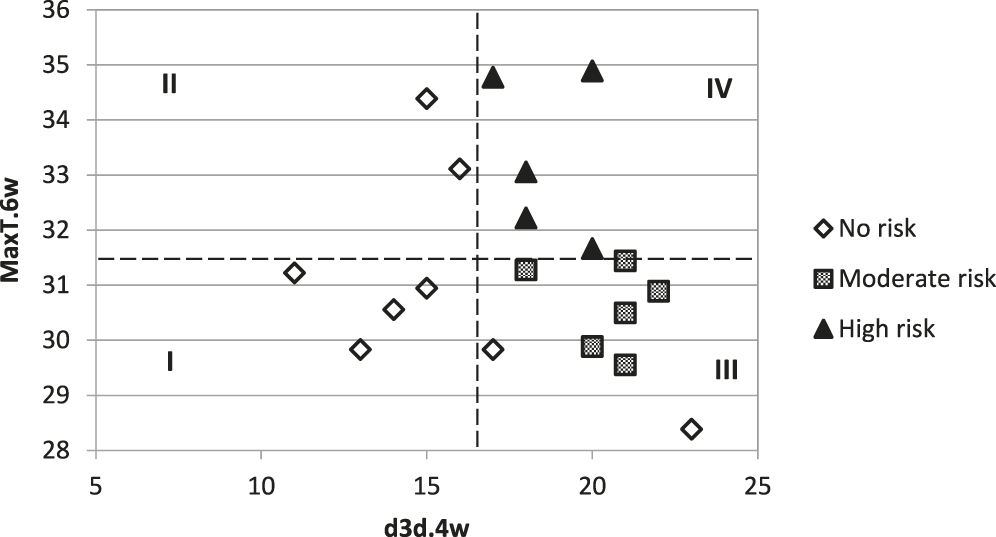
The graph of aflatoxin risk groups on MaxT.6wk and d3d.4wk shows that all occurrences of PGT20 ≥ 30 occurred with MaxT.6wk > 31.5C and d3d.4wk ≥ 17 (Fig. 2). Chi-square analysis confirmed (χ2 < 0.0001) that these values separated risk groups as shown by quadrats on Fig. 2. Seventy-five percent (6 out of 8) of no and low risk events (PGT20 = 0) were in quadrats I and II; the two instances of PGT20 = 0 in quadrat III were from environments with some of the coolest MaxT.6wk observed among all sites. All moderate risk occurrences were in quadrat III; all high risk events were in quadrat IV.
Cumulative number of 3-d-dry periods over 4 wk prior to inversion (d3d.4wk) and maximum daily temperatures averaged 6 wk before inversion (MaxT.6wk) for each of the 20 environments in this study. Markers represent proportion of samples with > 20 ppb (PGT20); where no risk = no aflatoxins detected; low = 0 < PGT20 ≤ 30; and high = PGT20 > 30.
Multivariate regression of the two selected environmental variables on PGT20 resulted in an acceptable model for estimation based on fit statistics:
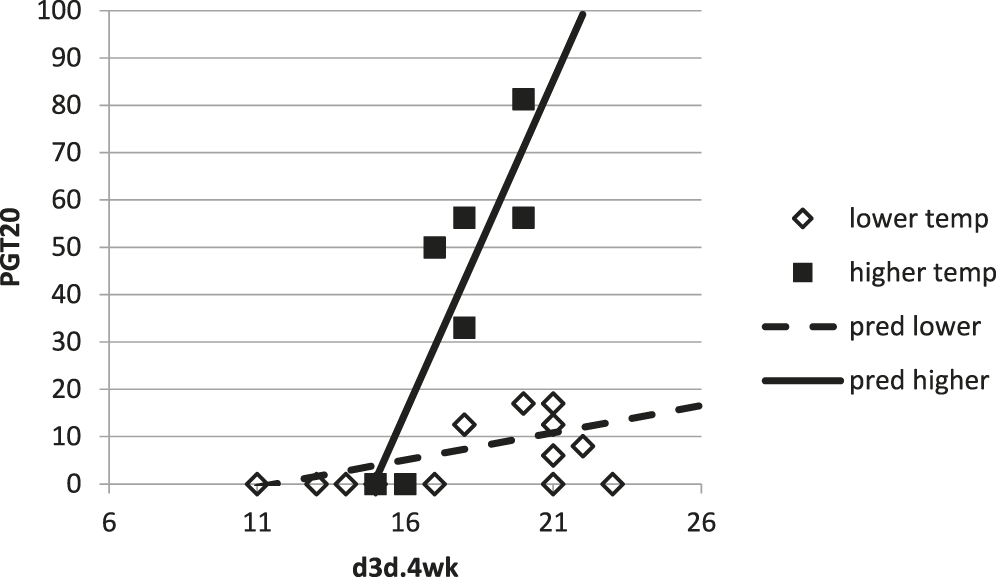
However, this model consistently underestimated PGT20 when MaxT.6wk > 30. Therefore, the threshold MaxT.6wk = 31.5 C was used for calculating two models (one for MaxT.6wk ≤ 31.5, another for MaxT.6wk > 31.5) with PGT20 as the dependent variable and d3d.4wk as the independent variable. The resulting model, when temperatures preceding harvest were ≤31.5C, explained only 25% of the variability of PGT20 values (R2 = 0.25, P = 0.080) (Fig. 3). The model for warmer temperatures explained 77% of the variability of PGT20 (R2 = 0.77, P = 0.0097) (Fig. 3). With all of these models, results were limited to 0 ≤ PGT20 ≤ 100.
Proportion of samples with greater than 20 ppb (PGT20) based on cumulative number of 3-d-dry periods over 4 wk prior to inversion (d3d.4wk) and maximum daily temperatures averaged 6 wk before inversion (MaxT.6wk). Open markers and dotted line had MaxT.6wk ≤ 31.5, PGT20 = 1.15×d3d.4wk – 13.34, R2 = 0.25, P = 0.080. Solid markers and line had MaxT.6wk > 31.5C, PGT20 = 14.03 × d3d.4wk – 209.48, R2 = 0.77, P = 0.0097.
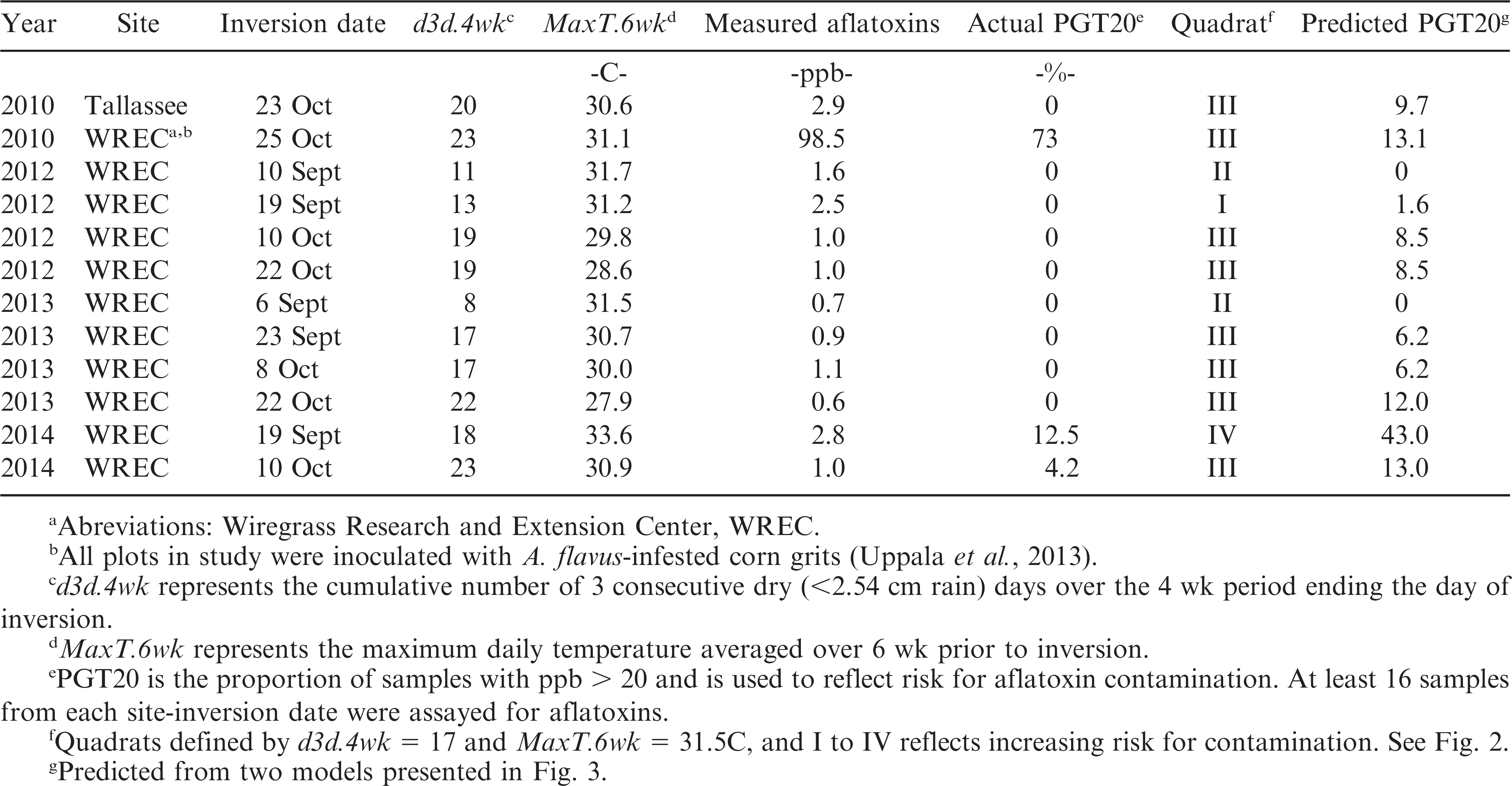
Among environments used for validation of the thresholds for defining aflatoxin risk in peanuts, three had d3d.4wk < 17 and were placed in quadrats I and II (Table 2). All peanut samples from these three environments had low aflatoxin levels (<3 ppb) and PGT20 = 0. Among remaining environments for validation, eight placed in quadrat III and one was placed in quadrat IV (Table 2). Two of the eight environments in quadrat III had PGT20 > 0, and one of these sites had PGT20 = 73, which would be high risk. The site that placed in quadrat IV had PGT20 = 12.5 (Table 2). Predicted risk for aflatoxin occurrence was greater than the observed risk in 11 of the 12 environments used for validation.
Discussion
Previous studies conducted at the National Peanut Research Lab (NPRL) in Dawson, GA, utilizing controlled environment plots with automated roof systems for imposing drought during rain and heating cables in the soil for elevating temperatures, have documented the role of high soil temperatures and moisture stress on aflatoxin contamination in peanut (Blankenship et al., 1984; Sanders et al., 1984; Cole et al., 1985; Sanders et al., 1985). The results of these NPRL studies have shown that soil temperatures of 26.3 to 29.6 C (Cole et al., 1985) in conjunction with a drought period in excess of 20 d before harvest (Sanders et al., 1985) are needed for aflatoxin contamination in peanut.
Soil temperature directly affects aflatoxigenic fungal growth and development (Jaime-Garcia and Cotty, 2010) as well as peanut plant growth and pod fill. Unfortunately, soil temperature is not readily available through weather monitoring services and is difficult for growers to directly monitor. However, soil temperature is highly correlated to air temperature (e.g., Zhang et al., 1993; Brown et al., 2000; Ahmad and Rasul, 2008; Sharma et al., 2010), although it is also affected by moisture, plant cover and other parameters including soil color and texture (Brady, 1974). Air temperatures were used in the current study to predict aflatoxins in peanut, primarily because of difficulties in obtaining soil temperatures.
In the current study, maximum daily air temperatures averaged over a relatively long period prior to harvest (i.e., 6 wk) were correlated to aflatoxin risk and contamination in peanut. According to Davidson et al. (1991), extended periods of high soil temperatures contribute more toward aflatoxin contamination than shorter periods of high temperatures. The current results closely conform to the NPRL studies (Blankenship et al., 1984; Sanders et al., 1985; Cole et al., 1985) where aflatoxin contamination of peanuts was problematic when soil temperature was elevated for 40 to 50 days before harvest.
It is not clear why maximum air temperatures reflected aflatoxin levels or the risk of contamination better than minimum or average air temperatures, particularly since many soil temperature prediction models use average air temperature (e.g., Zhang et al., 1993; Sharma et al., 2010). Average soil and air temperatures (maximum and average) from the Alabama Mesonet station located at WREC in July, August and September, over 4 of the 5 yr of this study, were highly correlated (R > 0.84) to one another. However, averages of maximum air temperatures were generally within 1 C of average soil temperatures while average air temperatures were consistently 4 to 6 C lower than soil temperatures. Thus, at the site of this study, maximum air temperature is a better predictor of soil temperature than is average air temperature.
Long-term average maximum monthly temperatures were ≥31.5C for July, Aug and the first third of Sept in Headland, AL (U.S. Climate Data, 2015), the site for this research and center of southeastern Alabama peanut production. Thus, temperatures are often favorable for aflatoxin contamination of peanuts harvested in Sept. Temperatures generally decline later in Sept and into Oct, which could mean a lower risk of aflatoxin contamination if later planting results in later harvest. In addition, aflatoxin contamination problems do not occur every year because of the mitigating effects of precipitation.
Dry periods were reflective of moisture stress in the current study, and higher occurrences of 3-d-dry periods during the 4 wk prior to inversion (d3d.4wk) would indicate greater moisture stress. Regardless of temperature, no aflatoxin contamination was detected when d3d.4wk < 17, suggesting that moisture is more important than temperature relative to aflatoxin accumulation in peanut. This observation has been made by others. For example, Dorner et al. (1989) observed that drought contributed more to decreases in kernel moisture than did higher temperature. Decreased kernel moisture was linked to decreased phytoalexin content, and phytoalexin production in kernels limits A. flavus growth and aflatoxin contamination (Dorner et al., 1989).
The threshold of d3d.4wk ≥ 17 for higher risk of aflatoxin contamination of peanuts is supported by previous observations. Using automated roof shelters, Sanders et al. (1985) had noted that more than 20 consecutive days of drought prior to harvest were needed for aflatoxin contamination in edible grades of peanuts. Twenty days with no rain is eighteen (18) 3-d-dry periods, which is greater than the threshold determined herein for a higher risk for aflatoxin contamination. Shorter drought durations were not evaluated by Sanders et al. (1985). Waliyar et al. (2003) noted that irrigation intervals of 14 d, but not 7 d, resulted in higher (ppb > 20) aflatoxin contamination of a moderately susceptible peanut cultivar in Niger. Seven- and 14-d-intervals for irrigation would be equivalent to d3d.4wk = 16 and 22, respectively. Thus, this threshold accurately reflects that the 7-d irrigation interval had little or no risk, while the 14-d intervals had higher risk for aflatoxin contamination.
In this study, neither total precipitation nor numbers of rain days prior to harvest correlated to aflatoxin levels. However, following a substantial rain event (e.g., > 1.5 cm), soil at the study site was dry within 2 or 3 d. Thus, regardless of the quantity of rain, the soil would be dry soon thereafter; ‘dry periods’ reflect this. Several dry period variables correlated well to aflatoxin content, but the best were over a relatively short duration before inversion, i.e., 3 and 4 wk.
A model was determined for predicting the risk for aflatoxin contamination from observations with MaxT.6wk > 31.5. While this model had a good fit to the original data, it over-predicted PGT20 for 10 of 11 validation sites. Given that aflatoxin contamination is, potentially, a food safety concern, over-prediction is more desirable than under-prediction. The single validation site which did not fit with the thresholds and risk prediction had been inoculated with A. flavus-infested grits. Arunyanark et al. (2009) noted that inoculation increased aflatoxin contamination regardless of the drought-status of the peanut crop. Specifically, severe drought increased ppb by 175% compared to non-inoculated irrigated treatment (7 ppb compared to 4 ppb), while inoculation plus severe drought increased ppb by 625% (29 ppb). It may be that inoculation can supercede environmental effects, such that the thresholds determined herein would need to decrease in the event of inoculation. Indeed, if the temperature threshold for model choice was MaxT.6wk = 31 (rather than 31.5), predicted aflatoxin risk for the 2010 WREC inoculated site used for validation would be PGT = 100.
Soil temperatures are of critical importance relative to the growth and development of aflatoxigenic fungi and for accumulation of aflatoxin in peanut seed. It is known that soil characteristics, such as permeability, drainage, and even soil color can impact soil temperatures (Brady, 1974). The study site used for the data in this analysis had a Dothan sandy loam and validation studies were on Lucy sandy loam and Cahaba loamy sand. All of these soils are well-drained, have moderate to slow permeability and have similar color (NRCS, 2014). Studies done at NPRL apparently used Tifton loamy sand (fine-loamy, kaolinitic, thermic Plinthic Kandiudults) (Hill et al., 1983; Sanders et al., 1985) which is also well-drained with moderately slow permeability. It is possible that the thresholds and predictive models for aflatoxin contamination presented herein could vary for peanuts grown in substantially different soil types.
The thresholds determined in this study suggest a management option for growers for avoiding excessive aflatoxin contamination in peanuts. This option is to invert peanuts 2 to 6 d early in order to avoid accumulation of d3d.4wk > 17 when maximum daily temperatures average >31.5 C. Previously, Rachaputi et al. (2002) reported that aflatoxin contamination was lower when peanuts were harvested ( = inverted) 2 wk early in an environment with a high risk for aflatoxin. This study did not appear to investigate varying harvest dates nor was aflatoxin risk as precisely defined as in the current study. Early inversion of peanuts for aflatoxin management, when there is a high risk for contamination, needs to be further explored.
Acknowledgements
This project was partially funded by the Alabama Agricultural Experiment Station and the National Peanut Board through the Southeastern Peanut Research Initiative.
Literature Cited
Ahmad M.F. and Rasul G. 2008 Prediction of soil temperature by air temperature; a case study for Faisalabad Pakistan J. Meteor. 5 : 19 – 23 .
Arunyanark A Jogloy S. Wongkaew S. Akkasaeng C. Vorasoot N. Wright G.C. Rachaputi R.C.N. and Patanothai A. 2009 Association between aflatoxin contamination and drought tolerance traits in peanut Field Crops Res. 114 : 14 – 22 .
AWIS 2014 Alabama Mesonet Weather Data On-line at http://www.awis.com/mesonet/index.html. Accessed 19 August 2015 .
Blankenship P.D Cole R.J. Sanders T.H. and Hill R.A. 1984 Effect of geocarposphere temperature on pre-harvest colonization of drought-stressed peanuts by Aspergillus flavus and subsequent aflatoxin contamination Mycopathologia. 85 : 69 – 74 .
Brady N.C. 1974 The Nature and Properties of Soils Macmillan Publ. Co., Inc. , New York, NY .
Branch W.D. 2004 Registration of ‘Georgia-03L’ Peanut Crop Sci. 44 : 1485 – 1486 .
Branch W.D. 2007 Registration of ‘Georgia-06G’ Peanut J. Plant Reg. 1 : 120 .
Brown S.E Pregitzer K.S. Reed D.D. and Burton A.J. 1999 Predicting daily mean soil temperature from daily mean air temperature in four northern hardwood forest stands Forest Sci. 46 : 297 – 301 .
Chauhan Y.S Wright G.C. Rachaputi R.C.N. Holzworth D. Broome A. Krosch S. and Robertson M.J. 2010 Application of a model to assess aflatoxin risk in peanuts J. Agric. Sci. 148 : 341 – 351 .
Cole R.J Sanders T.H. Hill R.A. and Blankenship P.D. 1985 Mean geocarposphere temperatures than induce preharvest aflatoxin contamination of peanuts under drought stress Mycopathologia. 91 : 41 – 46 .
Davidson J.I Blankenship P.D. Henning R.J. Guerke W.R. Smith R.D. and Cole R.J. 1991 Geocarposphere temperature as it relates to Florunner peanut production Peanut Sci. 18 : 79 – 85 .
Diener U.L. and Davis N.D. 1977 Aflatoxin formation in peanut by Aspergillus flavus Ala. Agric. Exper. Sta. Bull. #493, Auburn University .
Dorner J.W Cole R.J. Sanders T.H. and Blankenship P.D. 1989 Interrelationship of kernel water activity, soil temperature, maturity, and phytoalexin production in preharvest aflatoxin contamination of drought-stress peanuts Mycopathologia. 105 : 117 – 128 .
Gorbet D.W. and Tillman B.L. 2009 Registration of ‘Florida-07’ Peanut J. Plant Reg. 3 : 14 – 18 .
Hill R.A Blankenship P.D. Cole R.J. and Sanders T.H. 1983 Effects of soil moisture and temperature on preharvest invasion of peanuts by the Aspergillus flavus group and subsequent aflatoxin development Appl. Environ. Microbiol 45 : 2 : 628 – 633 .
Jaime-Garcia R. and Cotty P.J. 2010 Crop rotation and soil temperature influence the community structure of Aspergillus flavus in soil Soil Biol. Biochem. 42 : 1842 – 1847 .
Natural Resources Conservation Service (NRCS) 2014 Official Soil Series Descriptions On-line at https://soilseries.sc.egov.usda.gov/. Accessed 15 May 2015 .
Rachaputi N Wright G.C. and Krosch S. 2002 Management practices to minimize pre-harvest aflatoxin contamination in Australian peanuts Austral. J. Exper. Agric. 42 : 595 – 605 .
Sanders T.H Blankenship P.D. Cole R.J. and Hill R.A. 1984 Effect of soil temperature and drought on peanut pod and stem temperatures relative to Aspergillus flavus invasion and aflatoxin contamination Mycopathologia. 86 : 51 – 54 .
Sanders T.H Cole R.J. Blankenship P.D. and Hill R.A. 1985 Relation of environmental stress duration to Aspergillus flavus invasion and aflatoxin production in preharvest peanuts Peanut Sci. 12 : 90 – 93 .
Sharma P Shukla M.K. and Sammis T.W. 2010 Predicting soil temperature using air temperature and soil, crop and meteorological parameters for three specialty crops in southern New Mexico Appl. Eng. Agric. 26 : 47 – 58 .
Uppala S.S Bowen K.L. and Woods F.M. 2013 Pre-harvets aflatoxin contamination and soluble sugars of peanut Peanut Sci. 40 : 40 – 51 .
U.S Climate Data 2015 Climate Headland – Alabama On-line at www.usclimatedata.com. Checked 4/1/15.
USDA 2002 Aflatoxin Handbook GIPSA, Federal Grain Inspection Service http://www.gipsa.usda.gov/Publications/fgis/handbooks/aflatoxin/aflatoxin-cvr.pdf .
Waliyar F Traoré A. Fatondji D. and Ntare B.R. 2003 Effect of irrigation interval, planting date, and cultivar on Aspergillus flavus and aflatoxin contamination of peanut in a sandy soil of Niger Peanut Sci. 30 : 79 – 84 .
Williams E.J. and Drexler J.S. 1981 A non-destructive method for determining peanut pod maturity Peanut Sci. 8 : 134 – 141 .
Wilson T.J. and Romer T.R. 1991 Use of the Mycosep multifunctional cleanup column for liquid chromatographic determination of aflatoxins in agricultural products J. Assoc. Anal. Chem. 74 : 951 – 956 .
Wilson D.M. and Stansell J.R. 1983 Effect of irrigation regimes on aflatoxin contamination of peanut pods Peanut Sci. 10 : 54 – 56 .
Zhang E Hunt E.R. and Running S.W. 1993 A daily soil temperature model based on air temperature and precipitation for continental applications Clim. Res. 2 : 183 – 191 .
Notes
- Professor, Department of Entomology and Plant Pathology, Auburn University, AL 36849
- Professor and Extension Specialist, Department of Entomology and Plant Pathology, Auburn University, AL 36849 *Corresponding author Email: bowenkl@auburn.edu
Author Affiliations