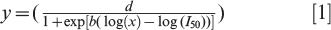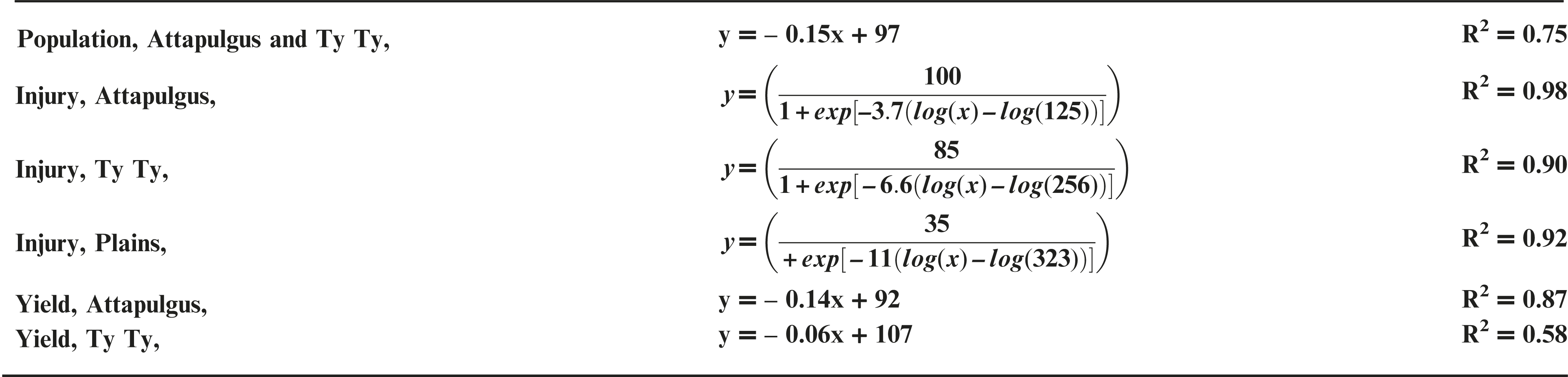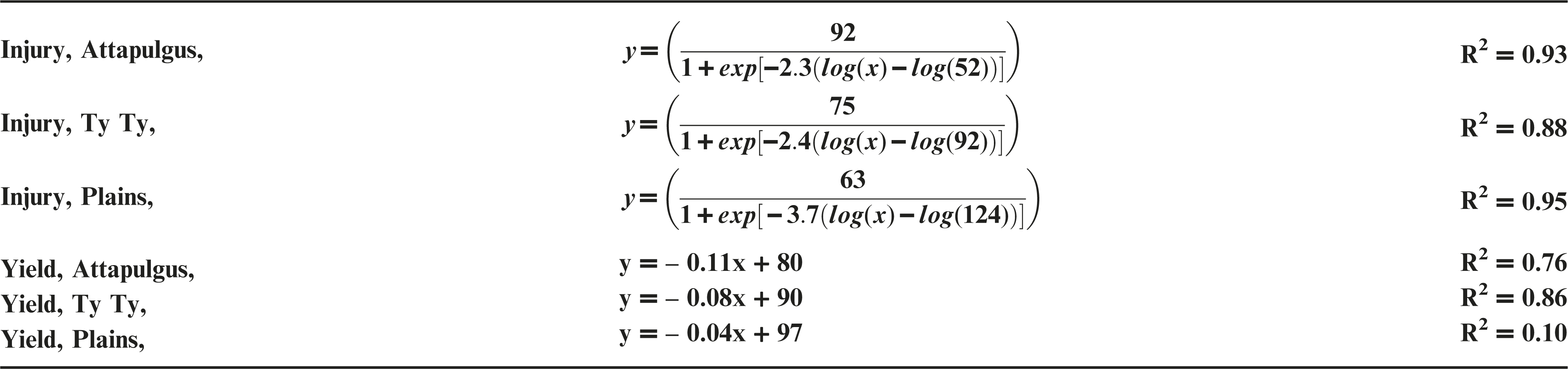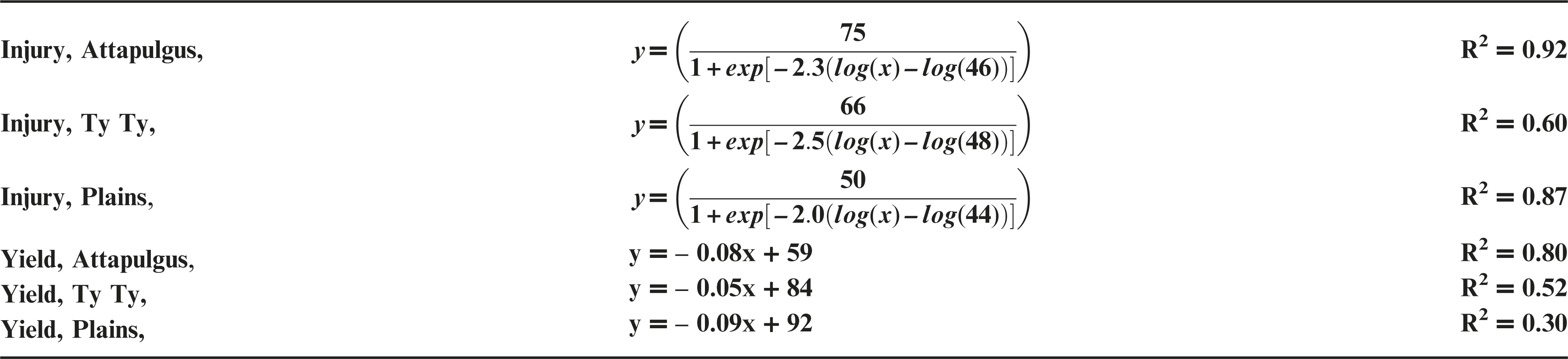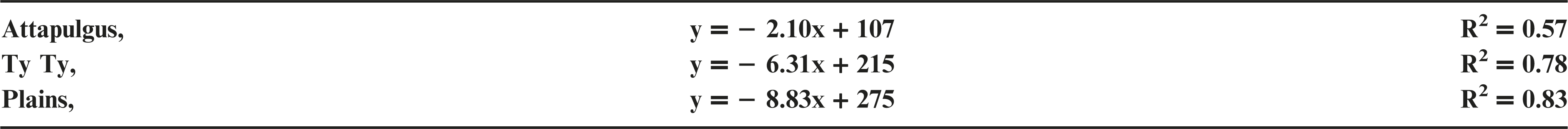Introduction
Since 1964, dicamba has been an active ingredient on herbicide registered for topical use in grass crops and preplant in other agronomic crops (Keller, 2014). Dicamba is currently registered for use in soybean [Glycine max (L.) Merr.], cotton (Gossypium hirsutum L.), corn (Zea mays L.), pasture, and small grains. Cotton and soybean require 15 to 30 d between preemergence (PRE) dicamba application and planting, along with 2.5 cm of irrigation/rainfall to reduce the chance of significant injury to the crop. Currently only monocot crops tolerate topical applications of dicamba. Dicamba has proven performance for control of numerous annual broadleaf weeds, and is very helpful in controlling problematic glyphosate-resistant Palmer amaranth (Amaranthus palmeri S. Wats.) (Edwards et al., 2013).
Herbicide-resistant weeds, especially with resistance to acetolactate synthase (ALS) inhibiting (Wise et al., 2009) and glyphosate herbicides (Culpepper et al., 2006), have lessened the effectiveness of numerous weed management systems. Therefore, there is an interest in engineering other types of herbicide-resistant crops (Johnson et al., 2012b; Subramanian et al., 1997). Monsanto Company and BASF Corporation, major agricultural seed and chemical companies, have responded by developing alternative weed management systems that use dicamba preemergence (PRE) and postemergence (POST) on dicamba-resistant crops including cotton and soybean (Behrens et al., 2007; Griffin et al., 2013; Johnson et al., 2012b). Dicamba-resistant crops have been genetically altered so that they metabolize the dicamba herbicide more readily, thus no injury occurs from application (Subramanian et al., 1997). The proposed dicamba-resistant crops will also include herbicide-resistance technology for other herbicide mechanisms of action (MOA) (Griffin et al., 2013). This advantage will allow the use of several MOA as an approach to reducing the selection pressure on a single MOA (Vencill et al., 2012), as well as assist in controlling current herbicide-resistant weeds.
Georgia leads the US with 239,271 ha of peanut (Arachis hypogaea L.) harvested in 2014. Peanut is commonly grown in the proximity of cotton and soybean across the southeast (Lassiter et al., 2007; Prostko et al., 2011; Prostko et al., 2013). Herbicide resistance technology is used in most cotton and soybean cultivars for weed management solutions, and the increased use of glyphosate and glufosinate herbicides throughout the growing season increased the occurrence of accidental injury to sensitive crops like peanut (Grey and Prostko, 2010; Johnson et al., 2012a; Lassiter et al., 2007). Peanut foliage injury can occur from herbicide residue remaining in the spray system when it is not properly cleaned prior to the next application in peanut (Grey and Prostko, 2010; Prostko et al., 2011).
Herbicide drift occurs when the herbicide becomes suspended in air, never reaching the target site, then moving onto a sensitive species nearby (Auch and Arnold, 1978; Grey and Prostko, 2010). Herbicide drift can be linked to many different factors: nozzle type, boom height, wind speed, pressure, spray formulation, sprayer speed, volume per area, and other environmental variables (Wolf et al., 1993). Grover et al. (1978) reported that herbicide drift amounts can range from 1 to 8%, and Wolf et al. (1993) indicated drift, without spray nozzle shielding, can reach 16% off target.
Research has indicated that peanut injury from glyphosate at 240, 320, and 470 g ai ha−1 caused significant yield reduction of 12 to 36% when applications were made 75, 90, and 105 days after planting (DAP) (Grey and Prostko, 2010). In another study, glyphosate applied to peanut at 560 g ai ha−1, 4 weeks after planting, resulted in yield loss greater than 50% and visual estimates of injury correlated with yield loss (Lassiter et al., 2007). Glufosinate also caused yield loss to peanut when applied less than a normal rate of 538 g ai ha−1. Glufosinate at 135 and 269 g ai ha−1 applied three weeks after emergence (WAE) caused yield loss of 14 and 51% respectively in North Carolina (Jordan et al., 2011). Visual estimates of peanut injury and peanut yield indicated a significant negative correlation.
Dicamba is known to cause significant injury to sensitive broadleaf crops when off-target exposure occurs as a result of tank contamination, herbicide drift, and movement due to volatilization (Behrens and Lueschen, 1979; Egan et al., 2014; Sciumbato et al., 2004). There is great concern with the introduction of dicamba-resistant crops, as the use of dicamba in amplified quantities throughout the growing season would likely increase the occurrence of accidental crop injury to sensitive broadleaf species grown in the same vicinity, including peanut (Egan et al., 2014; Egan and Mortensen, 2012; Johnson et al., 2012b; Leon et al., 2014; Prostko et al., 2011). The dicamba registration for use in common crops of the southeast can be approximately 560 g ae ha−1, and 16% of that rate is 90 g ha−1. If 90 g ha−1 of dicamba can move off-target in a controlled experiment, higher rates of dicamba could drift off-target if applicators did not follow all herbicide label directions. Dicamba exposure rates from tank contamination could range considerably.
Research conducted in 2008 with dicamba treatments applied 30, 60, and 90 days after planting (DAP) indicated peanuts to be more sensitive at the 30 and 60 DAP treatments (during the early reproductive stages of growth) when compared to 90 DAP treatments. Overall, yield loss was 2% to 100% with rates of dicamba at 40 to 560 g ai ha−1 (Prostko et al., 2011). Another study conducted in 2009 and 2010 included dicamba treatments at 0.6 to 140 g ae ha -1 applied three weeks after emergence (WAE) to peanut field plots. Visual estimates of injury were 30 to 80% at 2 weeks after treatment (WAT), and regression analysis indicated significant responses for yield when plotted against herbicide rate at three of the four locations (Johnson et al., 2012a). Leon et al. (2014) indicated that dicamba at 35 to 560 g ae ha−1 caused 20 to 78% injury to peanut from applications made 21 and 42 DAP, and the highest rate reduced peanut yield by 65%.
These studies are of great benefit to growers that face the possibility of peanut crop injury from off-target dicamba exposure, because in such an unfortunate situation the grower, extension agents, and crop consultants would be able to make an informed decision about the appropriate plan of action (Leon et al., 2014; Prostko et al., 2011). In an effort to link current data, it is necessary to quantify the injury and yield response of peanut after dicamba applications across various rates at preemergence up to beginning bloom (R1). Therefore, research was conducted to determine the sensitivity of peanut, in terms of plant injury and crop yield, to six rates of dicamba at four early-season application timings during the vegetative growth stages.
Materials and Methods
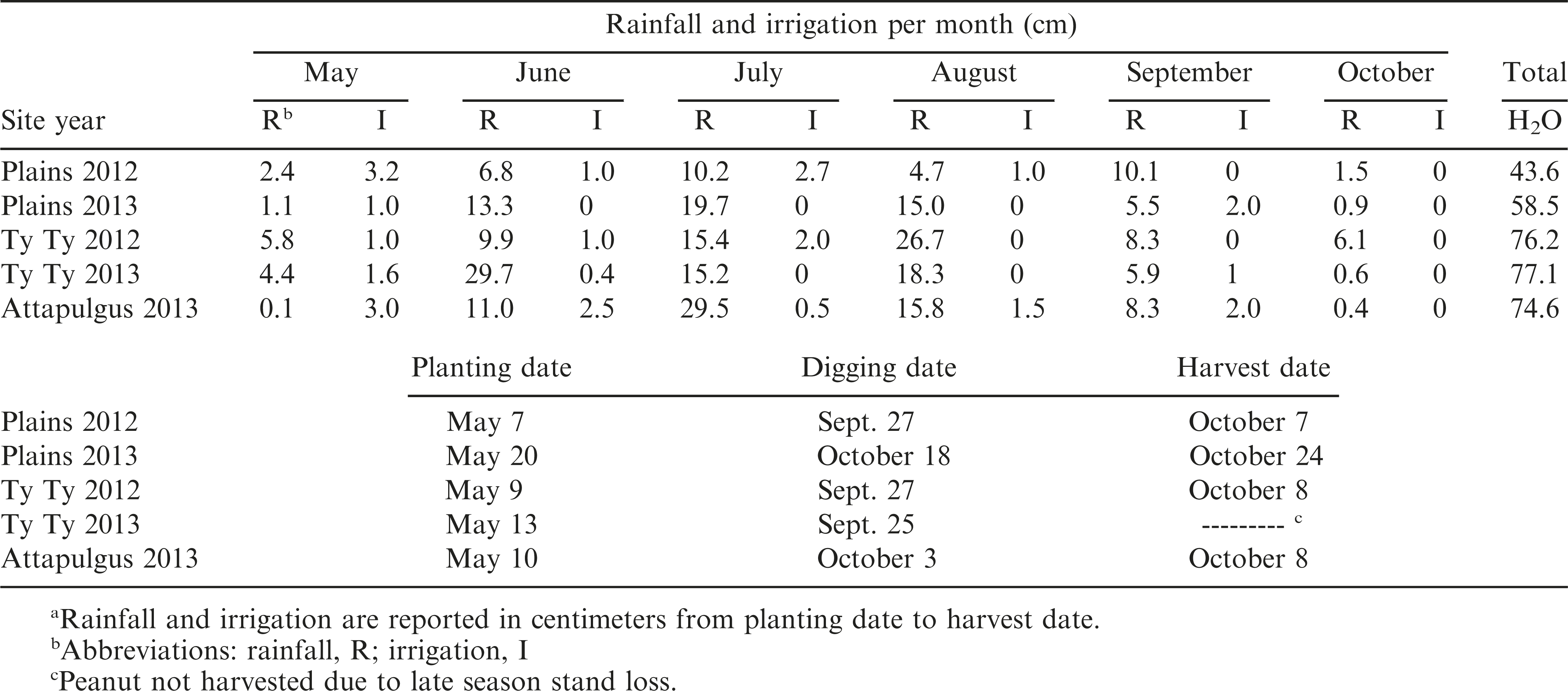
Field trials were conducted during 2012 and 2013 at three locations that represent the peanut growing regions in Georgia. The first location was the University of Georgia (UGA) Southwest Georgia Research and Education Center in Plains, GA, which had a Greenville sandy loam (fine, kaolinitic, thermic Rhodic Kandiudult) soil with 3.8% organic matter (OM), 60% sand, 10% silt, and 30% clay; the second location was the UGA Tifton Campus Ponder Farm in Ty Ty, GA, which had a Tifton loamy sand (fine-loamy, kaolinitic, thermic Plinthic Kandiudult) soil with 1% OM, 90% sand, 6% silt, and 4% clay; and the third location was the UGA Attapulgus Research and Education Center in Attapulgus, GA, which had an Orangeburg loamy sand (fine-loamy, kaolinitic, thermic typic kandiudult) soil with 1.5% OM, 86% sand, 6% silt, and 8% clay. Soil pH was 5.56, 5.63, and 6.0, respectively. The soil was prepared using a tillovator to loosen the soil and make it more suitable for planting. Peanut cultivar ‘Georgia-06G’ (Branch, 2007) was planted when soil conditions were warm enough for proper germination (around the 2nd week of May in Georgia). Specific planting dates are shown in Table 1. Peanuts were planted into rows spaced 91cm apart, two rows per plot, using vacuum style planters adjusted to 20 seed m−1 row−1. Plots were 1.8 m wide and 7.6 m or 9.1 m long with the same size boarder on either side of each plot.
Preemergence herbicides were applied to suppress weeds early in the season, which included 1066 g ai ha−1 of pendimethalin (Prowl H2O®, 456 g ai L−1, BASF Corporation, 26 Davis Drive, Research Triangle Park, NC), 71.4 g ai ha−1 of flumioxazin (Valor®, 51% ai w/w, granule, Valent U.S.A. Corporation, P.O. Box 8025, Walnut Creek, CA), and 26.5 g ai ha−1 of diclosulam (Strongarm®, 84% ai granule, Dow AgroSciences LLC, 9330 Zionsville Road, Indianapolis, IN). PRE treatments were applied within three d of planting. Irrigation was applied to incorporate the herbicide into the soil and to firm up the soil around the peanut seed. Applications of POST herbicides were used as needed throughout the season which included acifluorfen (Ultra Blazer®, 240 g ai L−1, United Phosphorus, Inc., 630 Freedom Business Center, Suite 402, King of Prussia, PA), clethodim (Select Max ®, 116 g ai L−1, Valent U.S.A. Corporation), and imazapic (Cadre®, 240 g ai L−1, BASF Corporation). Hand-weeding was used as necessary. Supplemental irrigation was applied throughout the growing season as needed, which is shown in Table 1 with monthly rainfall received. Fertilizer, fungicides, and insecticides were applied by on-site farm management for the duration of the trials based on University of Georgia recommendations (UGA, 2013).
Trials were conducted using a randomized complete block design with 4 treatment timings and 6 herbicide rates, which included a non-treated control. Dicamba (Clarity® 4SC, 480 g ae L−1, BASF Corporation) at rates of 0, 35, 70, 140, 280, and 560 g ae ha−1 was applied preemergence (PRE) immediately following crop planting, 10, 20, and 30 DAP. Treatments were replicated 4 times at Plains and Ty Ty and 3 times at Attapulgus. All treatments were applied using a CO2 pressurized backpack sprayer calibrated to deliver 140 L ha−1 at 152 kPa using TeeJet® XR8002VS nozzles (TeeJet® Technologies, Spraying Systems Company, Wheaton facility, P.O. Box 7900, Wheaton, IL).
The 10, 20, and 30 DAP treatments coincided with V2, V3, and V5 peanut vegetative growth stages respectively (Boote, 1982). The treatments will be discussed as the PRE, V2, V3, and V5 treatments. When the V5 treatments were applied, 25% of the peanut plants had blooms, making them very close to what is considered beginning bloom for a population of peanut plants or the first reproductive stage (R1) (Boote, 1982).
Peanut populations were recorded 20 d after planting. Visual estimates of injury were determined based on a combination of plant chlorosis, necrosis, stem epinasty, stem swelling, leaf strapping, leaf cupping, plant stunting, and lack of emergence with a scale of 0 (no injury relative to NTC) to 100% (plant death). Crop injury was evalauted throughout the growing season every 10 d until 80 DAP. At 130 DAP, non-dicamba-treated sections of peanut were checked for maturity using the hull-scrape method (Williams and Drexler, 1981) and the Peanut Profile Board (Developed by Jay Williams). Based on maturity tests, peanuts were dug and inverted. Digging dates and harvest dates are presented in Table 1. Peanuts were partially dried in bright sunlight for 5 to 10 d, and then each peanut plot crop was harvested with a two-row peanut harvester, bagged, and weighed. Moisture content was recorded and adjusted to 10% for final yield determination. Yield data were not collected for the Ty Ty 2013 site year due to late season stand loss. Peanut pod samples (500 g) from each plot were stored in small paper or mesh bags. Within three weeks, each plot sample was shelled with a mechanical peanut sheller, and then kernel weight of 100 random whole seed was measured from each sample.
Data were subjected to ANOVA using PROC GLMMIX (SAS Institute, Inc. 2012) to determine interactions between main factors (α = 0.05). Application timing and herbicide rate were considered fixed effects, whereas random effects included locations, years, replications and the associated interactions. Peanut injury estimates, crop population density, peanut canopy diameter, and peanut yield were each regressed on dicamba rate and fit to linear and nonlinear models.
The relationships between peanut injury and dicamba rate using raw replicate data were fit to log-logistic regression models,
where y is the estimate of peanut injury (%), d is the upper limit of the regression, x is the rate of dicamba (g ae ha−1), I50 is the rate of dicamba that resulted in 50% of the maximum injury, and b is the slope of the regression at I50 (Ritz et al., 2013; Seefeldt et al., 1995). Differences among parameters between regression models were evaluated using t-test (Glantz and Slinker, 2001).
Results and Discussion
Significant effects were observed for location by dicamba rate (P < 0.0002) and location by dicamba application timing (P < 0.004). Based on those findings, peanut population, crop injury, and peanut yield were analyzed by location and treatment timing by herbicide rate.
Preemergence Treatments
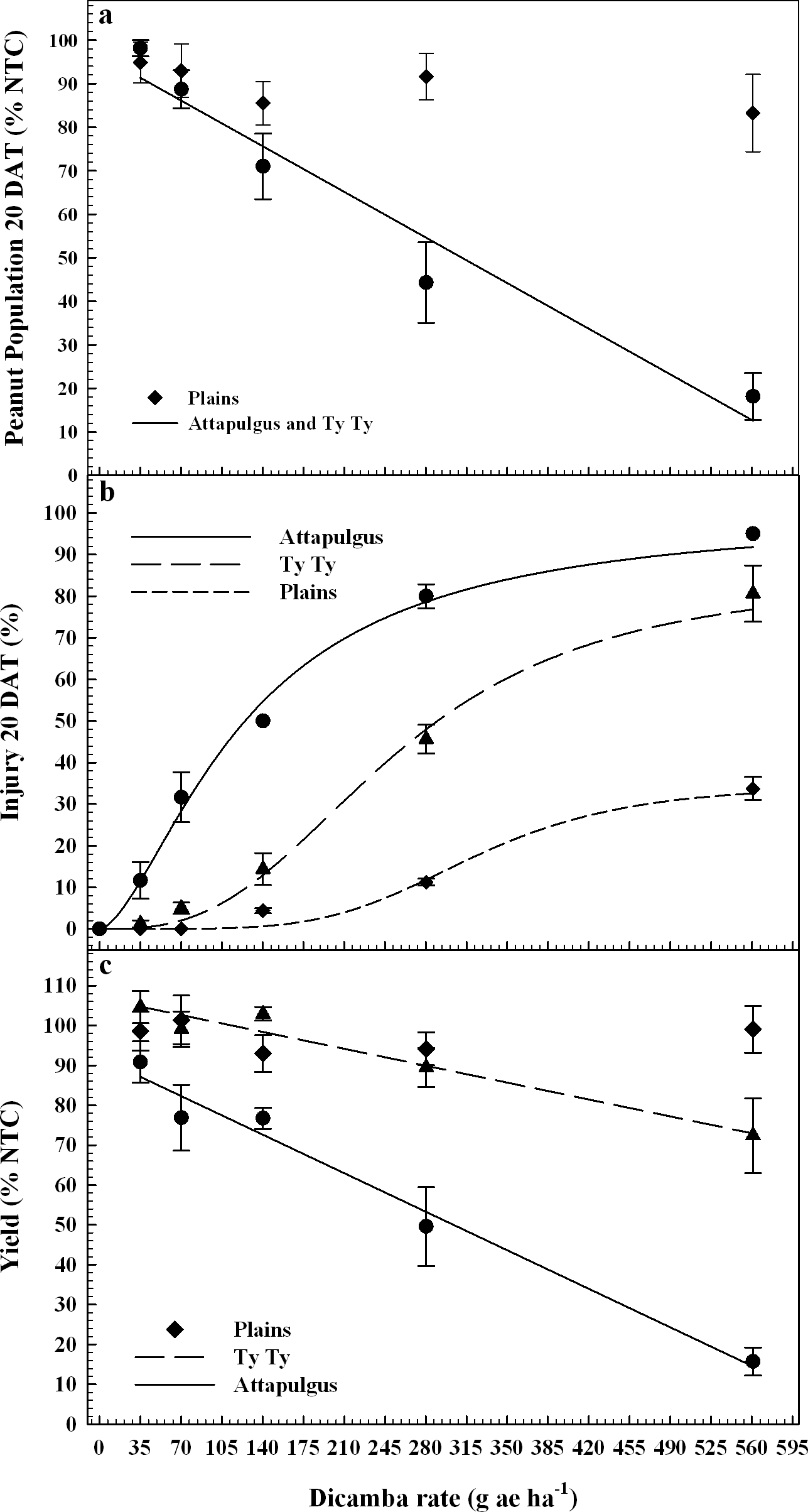
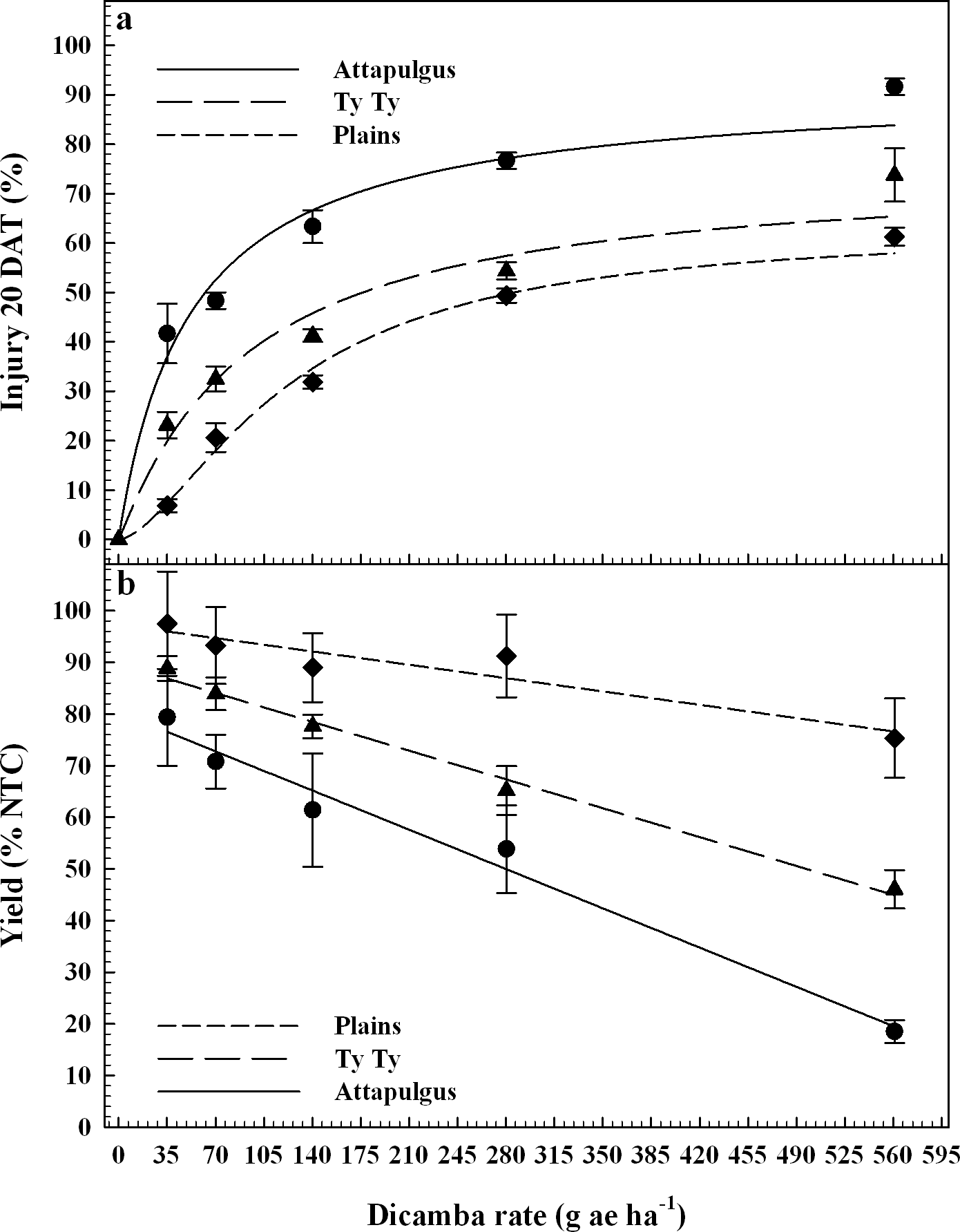
Peanut response varied by location, possibly due to differences in edaphic characteristics (e.g. soil characteristics, microbial activity, temperature, etc.) or other environmental factors. PRE dicamba treatments caused a reduction in peanut emergence relative to the nontreated control (NTC), with a linear relationship between crop population and dicamba rate at Attapulgus and TyTy (Figure 1a). Peanut populations at Attapulgus and TyTy were reduced 15% for every 100 g/ha of dicamba, up to a maximum of 81% reduction at the highest tested dicamba rate (560 g ha−1). However, there was no peanut population response to rate of dicamba at Plains.
Previous research demonstrated that dicamba has limited adsorption to soil particles, but studies have shown it moves with the farthest penetration of water through soil profile in an 18 hr period, suggesting some adsorption (Friesen, 1965). Dicamba is degraded under warm and moist aerobic conditions with half-lives of 1 to 6 wk, depending on soil microbial activity and soil texture (Taylor, 1983). In the present study, the Greenville soil (Plains) had a finer texture with 60% sand, 30% clay, and higher organic matter (OM) of 3.8 %; compared to the Tifton soil (Ty Ty) with 90% sand, 4% clay, and 1.0% OM; and the Orangeburg soil (Attapulgus) with 86% sand, 8% clay, and 1.5% OM.
Data analysis indicated that 20 DAT was the ideal post-application interval to evaluate peanut injury. This coincides with the timing of decisions that a grower must make concerning the future of an injured peanut crop (i.e. replant or continue with existing crop), and supports the conclusions of Leon et al. (2014). Visual estimates of peanut injury at 20 DAT were regressed on dicamba rate and fit to log-logistic regression models (Figure 1b). Maximum peanut injury (parameter d of the log-logistic regression model) occurred at the highest dicamba rate in the study (560 g ha−1) and was 35% at Plains, 85% at TyTy and 100% at Attapulgus. The log-logistic regression model estimated an I50 of 125 g ha−1 of dicamba for Attapulgus peanut injury, which was a lower (P = 0.0003) dicamba rate than the I50 of 256 g ha−1 at TyTy and 323 g ha−1 at Plains (P = 0.00044). Prostko et al. (2003) reported peanut was injured 10 to 30% at 2 WAT when 300 g ha−1 of dicamba was applied 0 or 7 days before planting (DBP) of peanut. They concluded that peanut requires a 15 d planting interval when applying dicamba preplant to minimize the potential for peanut injury (Prostko et al., 2003); dicamba in the current experiment was applied PRE immediately after peanut planting.
Peanut yield data were regressed on dicamba rate and fit to linear regression models for TyTy and Attapulgus (Figure 1c). Maximum peanut yield loss at TyTy was 28% from 560 g ha−1 dicamba, with a 6% rate of yield loss with each 100 g ha−1 dicamba. The rate of peanut yield loss at Attapulgus was more than double that at TyTy, with 14% yield loss for each 100 g ha−1 dicamba, with maximum yield loss of 84%. In contrast to the other locations, there were no detectable differences in peanut yield losses at Plains related to PRE dicamba treatments. We hypothesize that this lack of yield response at Plains can be attributed, in part, to the consistent peanut populations at this location across all rates of dicamba (Figure 1a). Prostko et al. (2003) determined that 300 g ha−1 dicamba reduced peanut yield when applied at planting in Texas in 2000 and Attapulgus, GA in 2001, however other site-years did not have yield loss.
V2 Growth Stage Treatments
Dicamba treatments were applied at the V2 vegetative growth stage of peanut, approximately 10 DAP. Peanut injury data were regressed on dicamba rates and fit to log-logistic regression models (Figure 2a). At Plains, maximum peanut injury at 20 DAT from V2 applications of dicamba was nearly double the injury from PRE applications (P <0.0003). At TyTy and Attapulgus, maximum peanut injury from V2 dicamba treatments was >75%, consistent (P >0.44) with injury from PRE dicamba treatments at both of the locations. Attapulgus and Plains peanut injury I50 estimates of 52 and 124 g ha−1 of dicamba, respectively, were significantly different (P = 0.001) from one another, whereas Ty Ty peanut injury I50 estimate of 92 g ha−1 of dicamba was no different from Attapulgus or Plains (P > 0.24). This demonstrates the greater sensitivity of emerged peanut to dicamba, as I50 values from V2 applications were less than half those from PRE applications, indicating a lower rate of dicamba was sufficient to elicit 50% injury.
Peanut yield declined in a linear manner as rate of dicamba applied at V2 growth stage increased (Figure 2b). Maximum yield loss varied among locations, with 560 g ha−1 causing 24% yield loss at Plains, 54% at TyTy, and 82% at Attapulgus. The rate of yield loss varied among locations, with 4, 8, and 11% peanut yield loss for each 100 g ha−1 of dicamba at Plains, TyTy, and Attapulgus, respectively.
V3 Growth Stage Treatments
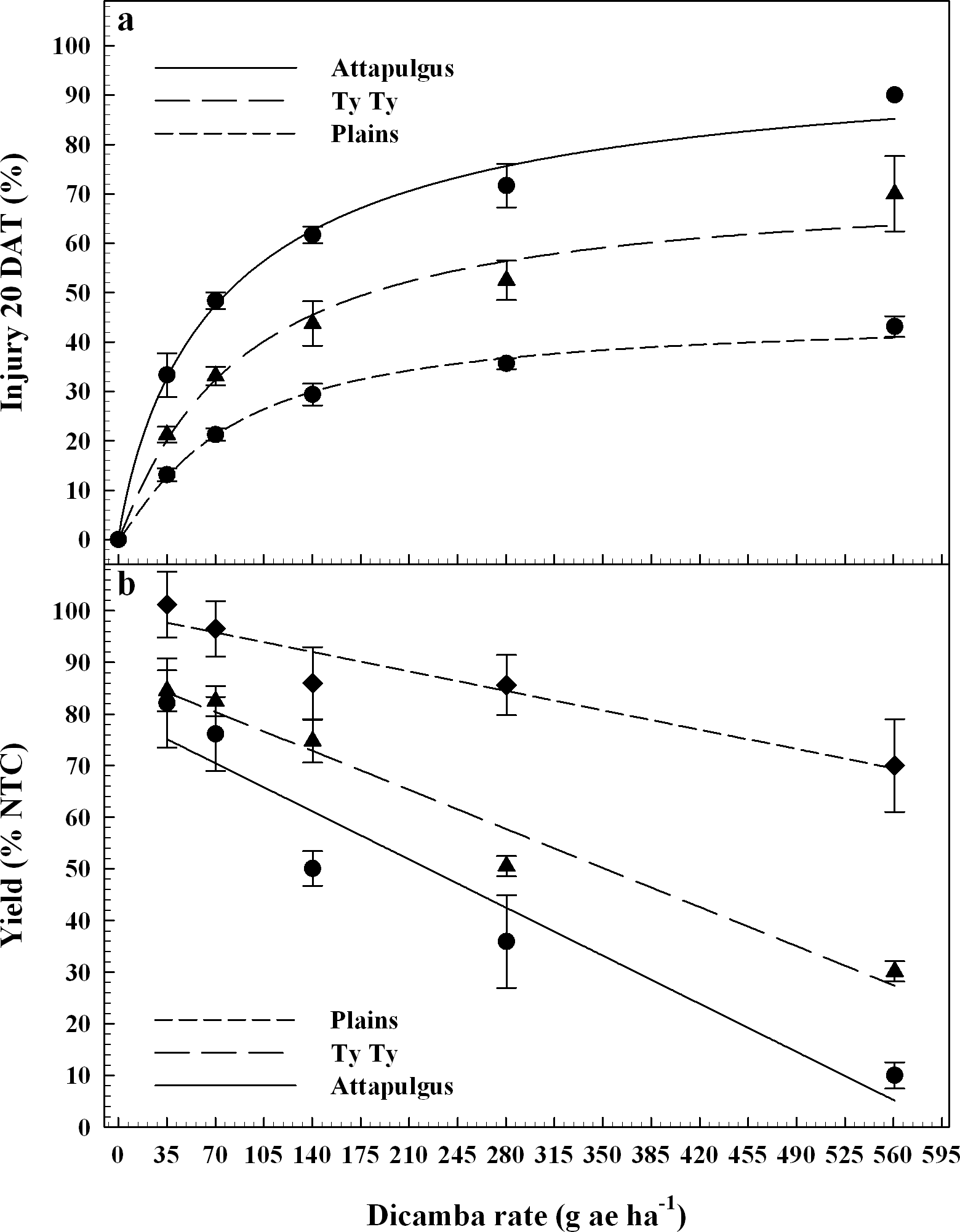
Dicamba treatments were applied at the V3 vegetative growth stage of peanut, approximately 20 DAP. Peanut injury data evaluated 20 DAT were regressed on dicamba rate and fit to log-logistic regression models (Figure 3a). Maximum peanut injury 20 DAT (parameter d) from V3 applications of dicamba was 45% at Plains, 72% at Ty Ty, and 100% at Attapulgus; this maintained the trend of greater injury (P = 0.0037) at Attapulgus relative to Plains, with Ty Ty as intermediate and similar to both (P >0.14). There were no differences (P > 0.85) in peanut injury I50 estimates among the locations (78 to 85 g ha−1 dicamba). Griffin et al. (2013) determined that soybean, another legume, is sensitive to dicamba during the V3 and V4 growth stages. By 7 DAT, they estimated soybean injury was 20 to 89% from 4 to 280 g ha−1 of dicamba, and by 14 DAT the predicted soybean injury for 140 g ha−1 had increased an additional 19%, and the 280 g ha−1 had increased to 97% injury (Griffin et al., 2013). Leon et al. (2014), reported 20 to 78% injury to peanut from 35 to 560 g ha−1 of dicamba, when applications were made at 21 and 42 d after planting.
Peanut yield data was regressed on rate of dicamba and fit to linear models by location (Figure 3b). There were differences (P < 0.004) in the rate of yield reduction per unit of dicamba between Plains (5% yield loss for each additional 100 g ha−1 of dicamba) and both Ty Ty and Attapulgus (10 and 13% yield loss for each additional 100 g ha−1, respectively). The maximum yield loss ranged from 30% at Plains to 94% at Attapulugus. Griffin et al. (2013) reported soybean yield loss of 85% for the 140 g ha−1 rate of dicamba. Leon et al. (2014) indicated 20% peanut yield loss at 35 g ha−1, and 65% yield loss at 560 g ha−1 of dicamba 21 and 45 DAP.
V5 Growth Stage Treatments
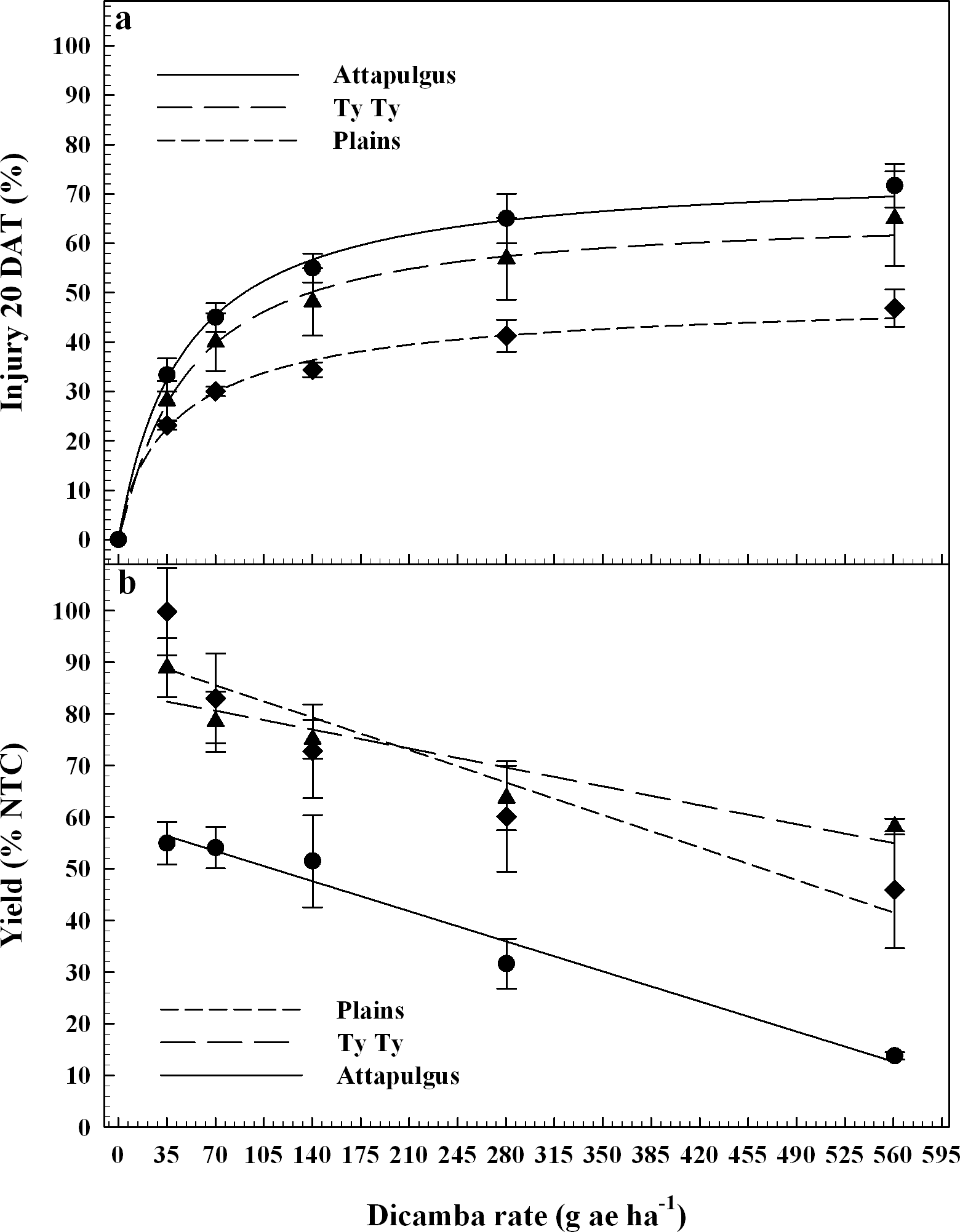
The final application of dicamba treatments were applied at the V5 vegetative growth stage of peanut, approximately 30 DAP when 25% of the plants were in the early stages of flowering. According to Boote (1982), the first reproductive growth stage is beginning bloom (R1), which is characterized by 50% of the peanut population with blooms. Therefore, while the peanut trials had not entered the R1 growth stage at the time of dicamba applicaiton, they did just days later. Peanut injury was regressed on dicamba rate and fito to log-logistic models (Figure 4a). Maximum peanut injury from dicamba was lower (P = 0.04) at Plains (50%) relative to Attapulgus (75%), with Ty Ty (66%) similar to both (P > 0.33). There were no differences (P > 0.89) between peanut injury I50 estimates (44 to 48 g ha−1) among the locations. A previous study documented that peanut treated with 140 g ha−1 of dicamba approximately 3 wk after crop emergence incurred 80% injury 2 WAT (Johnson et al., 2012a).
Similar to each of the other application timings, peanut yield data declined in a linear manner as dicamba rate increased at V5 applications (Figure 4b). There were no detectable differences (P > 0.11) in the rate of yield reduction per unit of dicamba (5 to 9% yield loss for each additional 100 g ha−1 of dicamba) among locations. However, there were differences in the y-intercept (representing the response as dicamba dose approached zero) between Attapulgus and both Ty Ty (P = 0.0001) and Plains (P = 0.0005). These differences among locations were maintained at the highest tested dicamba rate (560 g ha−1), with 86% yield loss at Attapulgus, 58% at Plains, and 44% at Ty Ty. At the same Plains location, Prostko et al. (2011) reported an estimated peanut yield loss of 7% from 40 g ha−1 of dicamba applied 30, 60, and 90 DAP. In the current study, peanut yield loss associated with 40 g ha−1 dicamba ranged from 0 to 44% when applied PRE, 10 (V2), 20 (V3), and 30 DAP (V5), with a yield loss of 15% when averaged over locations and application timings (Figures 1c, 2b, 3b, 4b). In a previous study, peanut at the Ty Ty location had an estimated yield loss of 93% at 560 g ha−1 when applied 30 DAP (Prostko et al., 2011). The current study confirms the potential for this level of peanut yield loss from 560 g ha−1 when applied PRE (0 to 86% yield loss), 10 DAP (24 to 82%), 20 DAP (30 to 94%), and 30 DAP (45 to 87%). However, the variability among locations in peanut yield loss suggests that additional factors may potentially confound accurate predictions of yield loss from off-target movement or misapplicaitons of dicamba; additional research to further investigate this variability is needed.
Summary and Conclusions
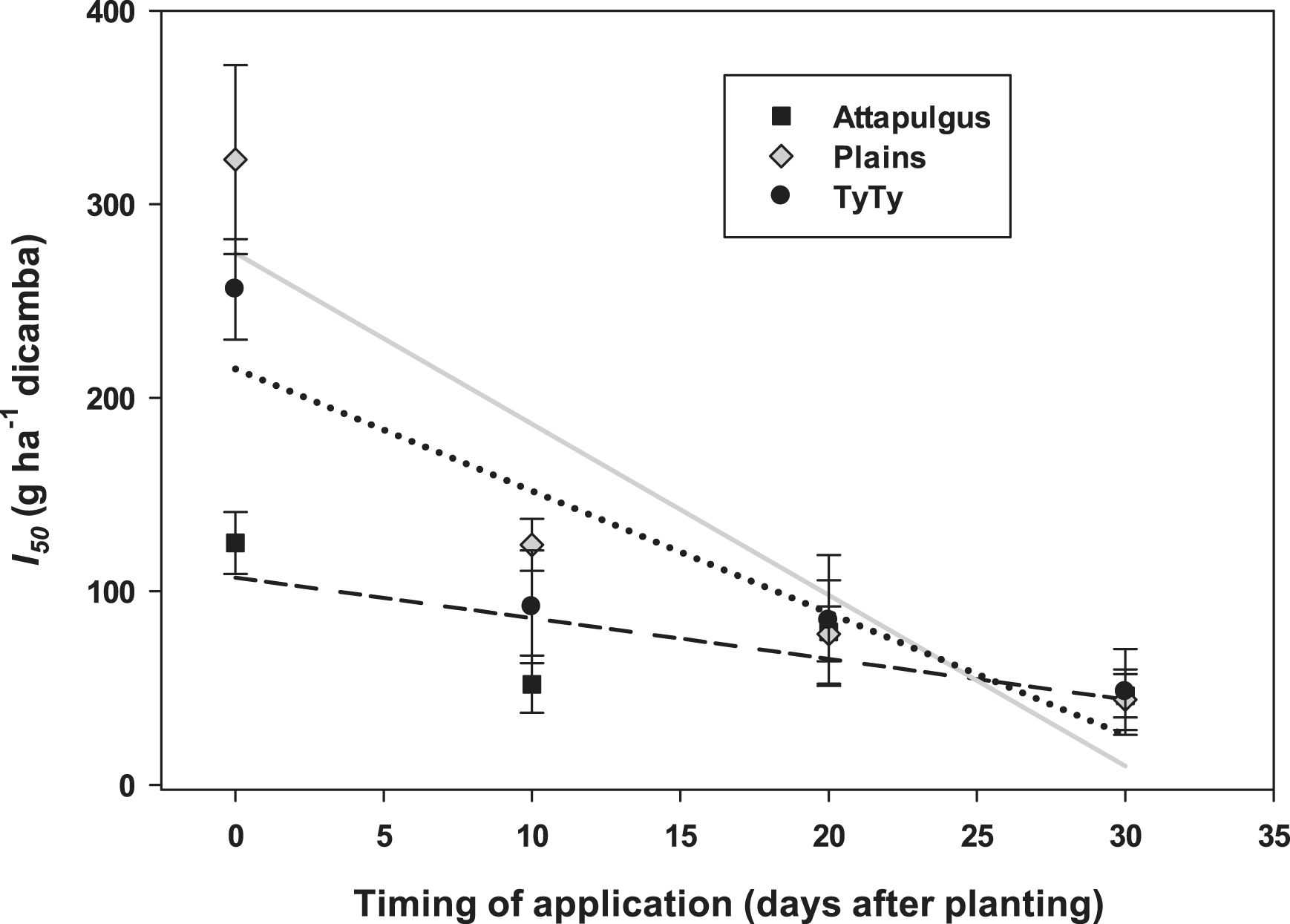
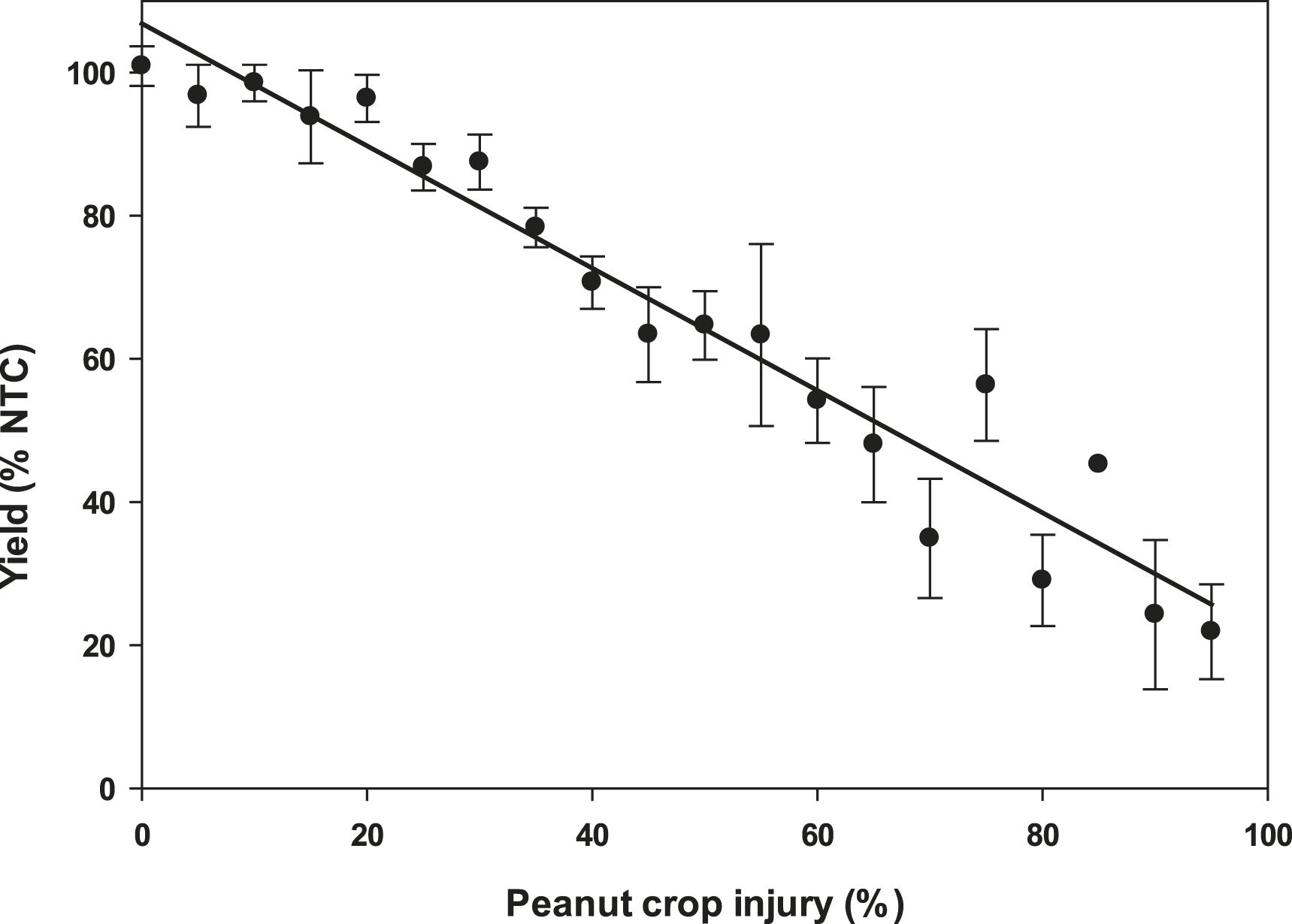
Plant injury increased with dicamba rate when applied to peanut in vegetative growth stages. Due to the detrimental injury (leaf chlorosis, necrosis, stem epinasty, leaf strapping, stem swelling, and plant stunting) peanut plant growth was reduced or stopped, resulting in reduced peanut yield. Peanut injury and yield loss was quite variable among locations. Differences in soil textures and other edaphic conditionspotentially contributed to variable responses by peanut to dicamba. Generally, dicamba was more injurious to peanut as plants approached initial bloom at 30 DAP, the treatment applied to the most mature plants evaluated in this study. There was a linear relationship between I50 values for peanut injury and timing of application by location, with a decline in dicamba rate needed to produce a 50% response in peanut injury over time (Figure 5). This demonstrated the greater peanut sensitivity to dicamba as applications were made to older plants. Across all treatments and locations, there was also a negative linear relationship between peanut yield and peanut crop injury, with a decline of 8.5% yield for every 10% increase in crop injury (Figure 6).
Based on the rapid adoption of previous herbicide resistant crop technology (Webster and Sosnoskie 2010), dicamba resistant crops have the potential for widespread use in the near future. Growers utilizing this technology will need to be aware of potential for off-target movement of dicamba to peanut fields, implementing practices to prevent dicamba drift, sprayer contamination, and volatilization. Due to the sensitivity of peanut to dicamba, growers and applicators should take caution when applying dicamba in the proximity of peanut throughout all vegetative and early reproductive growth stages. In the unfortunate situation where peanut injury from accidental dicamba exposure occurs, these data could assist the grower in determining peanut yield loss estimates and a possible plan of action.
Acknowledgements
This research was partially supported through a grant provided by the Georgia Peanut Commission. Thanks are given to Sidney Cromer, Fritz Turpin, Steve Li, Brittney Cromer, Jody Thompson, Haydon Davis, and all the UGA farm crews for their technical assistance.
Literature Cited
Auch DE Arnold WE ( 1978 ) Dicamba use and injury on soybeans (Glycine max) in South Dakota Weed Sci. 26 : 471 – 475
Behrens MR Mutlu N Chakraborty S Dumitru R Jiang WZ LaVallee BJ Herman PL Clemente TE Weeks DP ( 2007 ) Dicamba resistance: Enlarging and preserving biotechnology-based weed management strategies Science 316 : 1185 – 1188
Behrens R Lueschen WE ( 1979 ) Dicamba volatility Weed Sci. 27 : 486 – 493
Boote KJ ( 1982 ) Growth stages of peanut (Arachis hypogaea L.) Peanut Sci. 9 : 35 – 40
Branch WD ( 2007 ) Registration of ‘Georgia-06G' peanut J. Plant Reg .: 120
Culpepper AS York AC Brown SM Hanna WW Davis JW Vencill WK Grey TL Webster TM Kichler JM ( 2006 ) Glyphosate-resistant Palmer amaranth (Amaranthus palmeri) confirmed in Georgia Weed Sci. 54 : 620 – 626
Edwards CB Eubank TW Shaw DR Steckel LE ( 2013 ) Preemergence and postemergence efficacy of dicamba on glyphosate resistant Palmer amaranth (Amaranthus palmeri S. Wats.) and optimum rate for control Mississippi Soybean Promotion Board Project 20-2012 online at http://mssoy.org/wp-content/uploads/2013/08/20-2012-FINAL-REP-MSSOY.pdf
Egan JF Barlow KM Mortensen DA ( 2014 ) A meta-analysis on the effects of 2,4-D and dicamba drift on soybean and cotton Weed Sci. 62 : 193 – 206
Egan JF Mortensen DA ( 2012 ) Quantifying vapor drift of dicamba herbicides applied to soybean Environ. Tox. Chem. 31 : 1023 – 1031
Friesen HA ( 1965 ) The movement and persistence of dicamba in soil Weeds 13 : 30 – 33
Glantz SA Slinker BK ( 2001 ) Primer of applied regression and analysis of variance 2nd edn New York : McGraw-Hill, Medical Pub. Division . Pp. 25 – 28
Grey TL Prostko EP ( 2010 ) Physiological effects of late season glyphosate applications on peanut seed development and germination Peanut Sci. 37 : 124 – 128
Griffin JL Bauerle MJ Stephenson Miller DK Boudreaux JM ( 2013 ) Soybean response to dicamba applied at vegetative and reproductive growth stages Weed Technol. 27 : 696 – 703
Grover R Yoshida K Maybank J ( 1978 ) Spray drift from agricultural pesticide applications J. Air Pol. Cont. Assoc. 28 : 1009
Johnson VA Fisher LR Jordan DL Edmisten KE Stewart AM York AC ( 2012a ) Cotton, peanut, and soybean response to sublethal rates of dicamba, glufosinate, and 2,4-D Weed Technol. 26 : 195 – 206
Johnson WG Hallett SG Legleiter TR Whitford F Weller SC Bordelon BP Lerner BR ( 2012b ) 2,4-D and dicamba-tolerant crops- some facts to consider Purdue Extension : 1 – 7
Jordan DL Johnson VA Fisher LR ( 2011 ) Peanut response to simulated drift rates of glufosinate Crop Manage. 0802-02-RS
Keller R ( 2014 ) Engenia specific for dicamba-resistant crops http://www.agprofessional.com/news/Engenia-specific-for-dicamba-resistant-crops-257392081.html
Lassiter BR Burke IC Thomas WE Pline-Srnic WA Jordan DL Wilcut JW Wilkerson GG ( 2007 ) Yield and Physiological response of peanut to glyphosate drift Weed Technol. 21 : 954 – 960
Leon RG Ferrell JA Brecke BJ ( 2014 ) Impact of exposure to 2,4-D and dicamba on peanut injury and yield Weed Technol. 28 : 465 – 470
Prostko EP Grey TL Johnson WC Jordan DL Grichar WJ Besler BA Brewer KD Eastin EF ( 2003 ) Influence of preplant applications of 2,4-D, dicamba, tribenuron, and tribenuron plus thifensulfuron on peanut Peanut Sci. 30 : 18 – 22
Prostko EP Grey TL Marshall MW Ferrell JA Dotray PA Jordan DL Grichar WJ Brecke BJ Davis JW ( 2011 ) Peanut Yield response to dicamba Peanut Sci. 38 : 61 – 65
Prostko EP Webster TM Marshall MW Leon RG Grey TL Ferrell JA Dotray PA Jordan DL Grichar WJ Brecke BJ ( 2013 ) Glufosinate application timing and rate affect peanut yield response Peanut Sci. 40 : 115 – 119
Ritz C Pipper CB Streibig JC ( 2013 ) Analysis of germination data from agricultural experiments Europ. J. Agronomy 45 : 1 – 6
SAS Institute I ( 2012 ) SAS/STAT ® 9.2 User's Guide: World Headquarters SAS Institute Inc 100 SAS Campus Drive, Cary, NC
Sciumbato AS Chandller JM Senseman SA Bovey RW Smith KL ( 2004 ) Determining exposure to auxin-like herbicides II. Practical application to quantify volatility. Weed Technol. 18 : 1135 – 1142
Seefeldt SS Jensen JE Fuerst EP ( 1995 ) Log-logistic analysis of herbicide dose-response relationships Weed Technol. 9 : 218 – 227
Subramanian MV Tuckey J Patel B Jensen PJ ( 1997 ) Engineering dicamba selectivity in crops: a search for appropriate degradative enzyme(s) Ind. Microbiol. Biotechnol. 19 : 344 – 349
Taylor RJ ( 1983 ) Chemical fact sheet for: dicamba . Pages 1–4 Washington, DC : Environmental Protection Agency
UGA ( 2013 ) Georgia Pest Management Handbook - Commercial Edition: College of Agricultural & Environmental Sciences . Pp. 848
Vencill WK Nichols RL Webster TM Soteres JK Smith CM Burgos NR Johnson WG McClelland MR ( 2012 ) Herbicide resistance: Toward an understanding of resistance development and the impact of herbicide-resistant crops Weed Sci. 60 : 2 – 30
Webster TM Sosnoskie LM ( 2010 ) The loss of glyphosate efficacy: a changing weed spectrum in Georgia cotton Weed Sci. 58 : 73 – 79
Williams EJ Drexler JS ( 1981 ) A non-destructive method for determining peanut pod maturity Peanut Sci. 8 : 134 – 141
Wise AM Grey TL Prostko EP Vencill WK Webster TM ( 2009 ) Establishing the geographical distribution and level of acetolactate synthase resistance of Palmer amaranth (Amaranthus palmeri) accessions in Georgia Weed Technol. 23 : 214 – 220
Wolf TM Grover R Wallace K Shewchuk SR Maybank J ( 1993 ) Effect of protective shields on drift and deposition characteristics of field sprayers Canadian J. Plant Sci. 73 : 1261 – 1273 .
Notes
- Former Graduate Research Assistant, Professor and Professor, respectively. Department of Crop and Soil Sciences, The University of Georgia, 2360 Rainwater Drive, Tifton, GA 31793
- Research Agronomist, USDA-ARS – Crop Protection and Management Research Unit, Tifton, GA 31794 *Corresponding author Email: tgrey@uga.edu
Author Affiliations