Introduction
Aflatoxin has carcinogenic, hepatotoxic, and immunosuppressive properties that have caused high mortality and reduction of productivity in livestock as well as reduced immunity and liver cancer in humans (Chu, 1991, JEFCA, 2001, Swindale, 1989). Due to these risks to human and livestock health, aflatoxin contamination is regularly monitored in peanut (Arachis hypogaea L.), corn and cotton (Wilson, 1995). In the United States and many other countries, peanuts with aflatoxin contents lower than 20 ppb are accepted for direct human and livestock consumption, while those with aflatoxin contents with 20 ppb and above are rejected (FAO, 2004, Whitaker et al., 2005).
Several mechanical and chemical methods for the detection, extraction, and quantification of aflatoxin have been developed. These include Fourier transform near-infrared spectroscopy (Tripathi and Mishra, 2009), a fluorometric method (Holbrook et al., 2000), high performance liquid chromatography (HPLC) (Manetta et al., 2005), liquid chromatography-tandem mass spectrometry (LC-MS) (Edinboro and Karnes, 2005), and enzyme-linked immunosorbent assay (ELISA) (Li et al., 2009b). These methods are accurate, selective, sensitive, and effective. However, they are usually costly, largely intended for laboratory scientific research, and require special equipment and training (Zhou et al., 2009). In addition, most of these technologies are not accessible to developing countries where aflatoxin is of greater concern (Pitt et al., 2012, Waliyar et al., 2008). The improvement of standards of living in developing countries demands increased attention to food safety and monitoring of aflatoxin. There is a need in the food and feed sectors of both developed and developing countries to develop rapid aflatoxin testing methods that are low-cost, easy to handle, usable on-site, independent of other instruments, and that can be easily integrated into the production process (Shim et al., 2007). Immunochromatographic test strips, also known as lateral flow test strips, have been developed and are now firmly integrated into routine quality-monitoring procedures. These test strips are easily operated following simple manufacturer’s procedures, produce immediate results, and do not require expensive instruments (Li, et al. 2009a, Zhang et al., 2011). In addition, they do not require refrigeration, thus, facilitating use in developing countries.
A list of test strips, including AflaCheckTM (Vicam) and AgraStrip® (Romer Labs) which were used in this study, have been approved by USDA Grain Inspection Packers and Stockyards Administration (GIPSA) for qualitative aflatoxin testing (USDA-GIPSA, 2014). These test strips have an expiration date of 1 - 1½ years when kept under the manufacturer-recommended storage temperatures of 15 - 30 C and 2 - 25 C for AflaCheckTM and AgraStrip®, respectively. In many peanut production areas, however, storage within the range of temperatures may not be feasible. Considering these conditions, this study addressed two objectives: (1) To assess the sensitivity and accuracy of immunochromatographic test strips in the qualitative detection of peanut aflatoxin at a 20 ppb cut-off limit as compared to the quantitative fluorometry method; and, (2) To evaluate the effect of continuous high and fluctuating temperatures to the sensitivity and accuracy of the test strips.
Materials and Methods
Performance of the Test Strips in Comparison to the Vicam Fluorometric Method
Sample Collection and Preparation
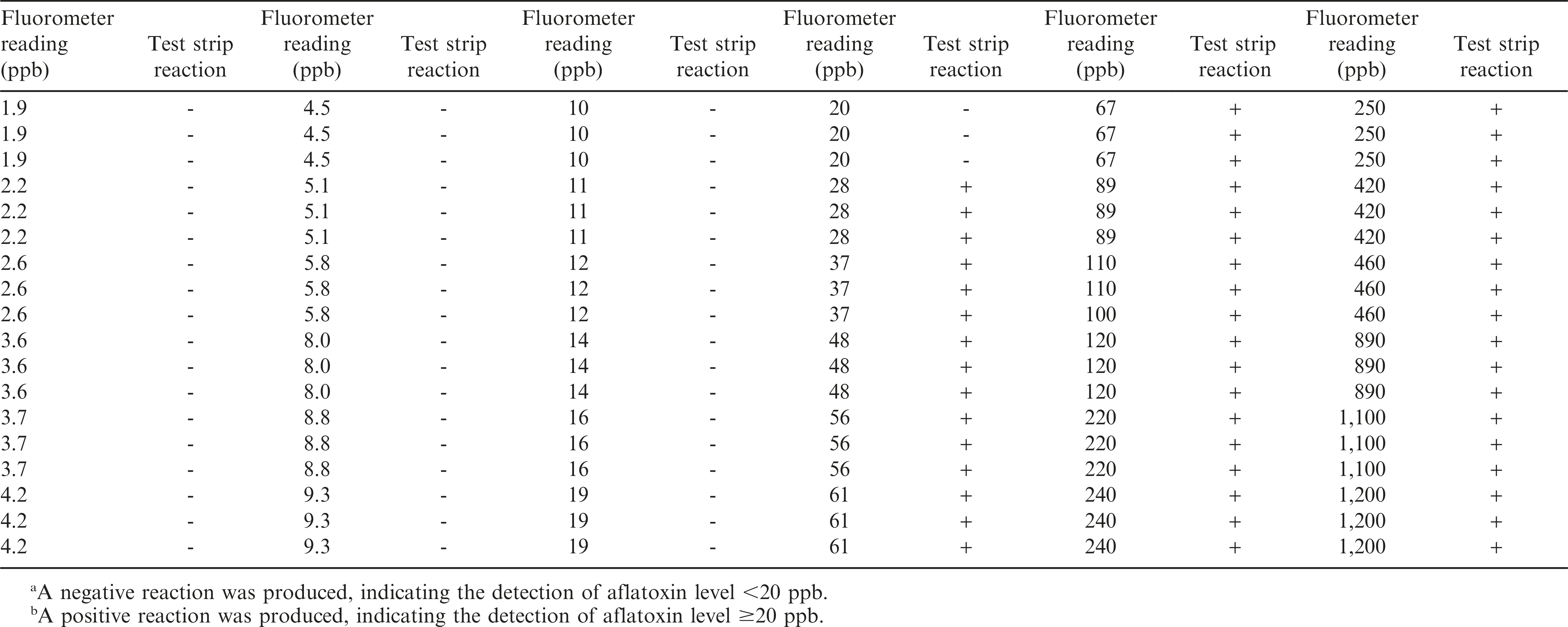
Peanut samples were collected from different fields during the summer of 2013. Peanut samples were collected from field trials that had been inoculated and others not inoculated with aflatoxigenic fungi in order to acquire a good range of aflatoxin contamination. These samples were subjected to aflatoxin extraction and quantification using the Vicam fluorometry method. Briefly, representative samples (100 g) of shelled peanuts were added with 10 g of NaCl and 200 ml of methanol/water (80:20 v/v), homogenized using a Waring blender at high speed for 1 minute and filtered through Whatman paper. Five ml of the filtrate was diluted with 20 ml HPLC water then re-filtered. Ten ml filtrate was purified with Vicam immunoaffinity columns (Vicam Aflatest, MA) containing aflatoxin-specific (B1, B2, G1 and G2) monoclonal antibodies and washed with 10 ml HPLC water before the aflatoxin was eluted with 1 ml methanol. The eluted fraction was diluted twice with HPLC water and measured with the Vicam fluorometer (Vicam Series 4EX Fluorometer). All procedures were done according to the manufacturer’s instructions. Results of the fluorometer readings showed aflatoxin levels ranging from 1.9 - 1,200 ppb (Table 1).
A total of 108 AflaCheckTM (Vicam, MA) test strips were used to test the aflatoxin level of the same peanut samples quantified using the fluorometer (Table 1). Fifty four test strips were used to test peanut samples with fluorometer readings <20 ppb (1.9 to 19 ppb) and another 54 to test those with ≥20 ppb (20 to 1,200 ppb). The number of test strips used was based on the suggested number of not less than 50 positive and 50 negative samples for qualitative assay studies (CLSI/NCCLS, 2008).
Accuracy, Sensitivity and Specificity Validation
The AflaCheckTM test strip used in this study was designed to qualitatively detect aflatoxin at a cut-off limit of 20 ppb. A positive reaction indicating the detection of aflatoxin level ≥20 ppb is displayed by the production of one visible line (Figure 1). A negative reaction indicating the detection of aflatoxin level <20 ppb is displayed by two visible lines. The absence of any line is indicative of an invalid result. The accuracy, sensitivity and specificity of the test strips to detect aflatoxin levels were evaluated by comparing the observed results to the fluorometer readings. The calculation of accuracy, sensitivity, specificity and Fisher’s exact test were completed using the PROC FREQ procedure in SAS ver. 9.3 (SAS Institute, Cary, NC).
Detection reactions of the chromatographic test strips (left = AflaCheckTM from Vicam; right = AgraStrip® from Romer Labs). Production of one visible line indicates detection of aflatoxin level ≥20 ppb (A); two visible line indicates detection of aflatoxin level <20 ppb (B); while no line is an invalid result (C).
Performance of the Immunochromatographic Test Strips when Exposed to Continuous High or Fluctuating Temperatures at Increasing Time Duration
Incubation Setup
Immunochromatographic test strips from two companies, AflaCheckTM (Vicam, MA) and AgraStrip® (Romer Labs, MO), were stored in three temperature regimes: T0 = room temperature (approximately 25 C); T1 = high temperature (34 C); and, T2 = fluctuating at 34 C (8 hours) and room temperature (16 hours) daily. High temperature (34 C) was imposed by warming the test strips inside an incubator. Fluctuating temperatures were imposed by incubating the test strips for 8 hr at 34 C then bringing them out at room temperature for 16 hr overnight until the next day.
Incubation Stability Test
While the test trips were continually incubated under the three temperature regimes, 10 test strips were taken from each treatment at certain time durations. These test strips were used to test the following solutions prepared from calibrated aflatoxin standards: (a) 50 ppb (≥20 ppb), to examine the test strips for positive results; (b) 10 ppb (<20 ppb), to examine the test strips for negative results; and, (c) 0 ppb (distilled water), as blank control.
The test strips were incubated and tested for a maximum duration of 53 wk (∼1 year). Any problems such as inability to detect aflatoxin or production of results contrary to what was expected were recorded. The data were analyzed using PROC ANOVA procedure in SAS version 9.3 to compare the performance of AflaCheckTM and AgraStrip®. Means were compared using Fisher LSD at P≤0.05.
Results and Discussion
Performance of the Test Strips in Comparison to the Vicam Fluorometry Method
Aflatoxin contamination of peanut is a worldwide concern. Adequate knowledge and several methodologies are currently available to control aflatoxin in food and food products. However, most of these technologies are only readily available in developed countries which have the capability to establish analytical methods to screen for toxins and establish strong regulatory controls. The techniques used in developed countries require sophisticated infrastructure, stable electricity, readily available supplies, and experienced technicians. Most developing countries lack the resources, infrastructure, sustainable supplies, and personnel for efficient regulatory system (Pitt et al., 2012, Waliyar et al., 2008). The use of relatively affordable simple-to-use materials in these developing countries would be a great advantage.
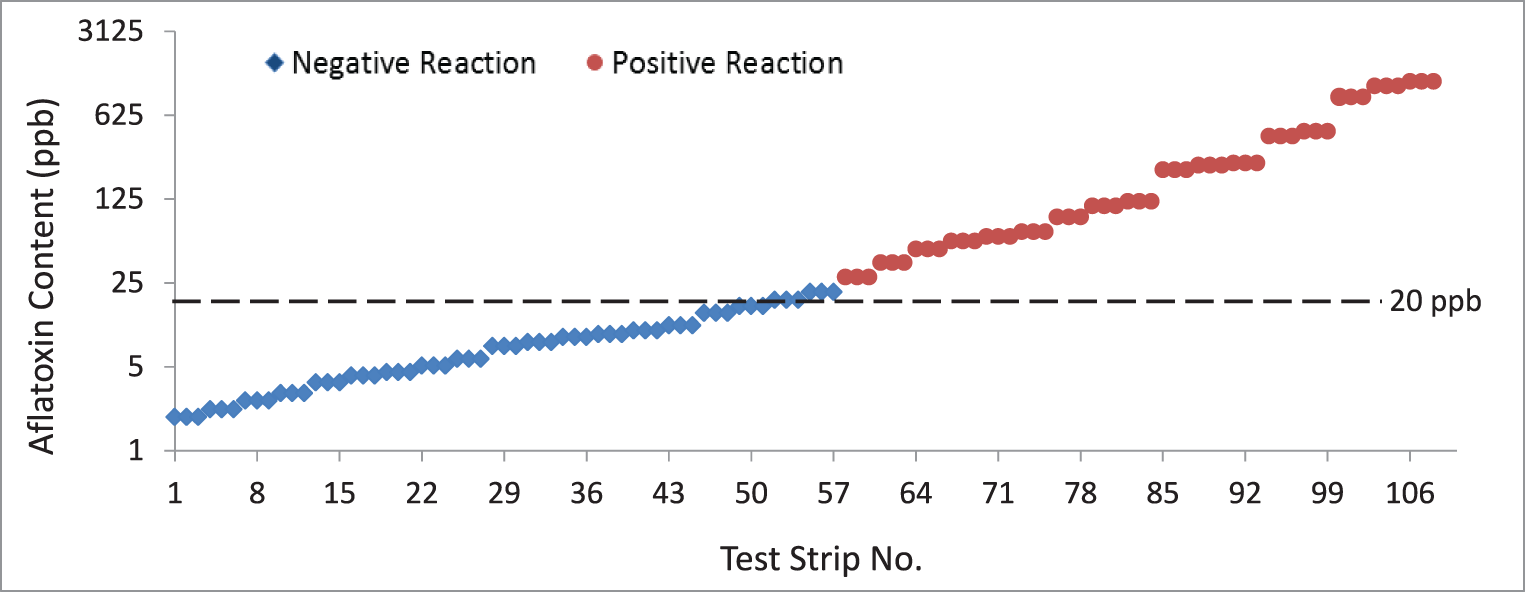
The aflatoxin content of the peanut samples quantified through the Vicam fluorometry method ranged from 1.9 to 1,200 ppb (Table 1, Figure 2). Samples with aflatoxin contents <20 and ≥20 ppb were separated and tested in triplicates. Results from the assay showed that all samples with aflatoxin contents <20 ppb as read by the fluorometer yielded a negative reaction in the test strips. Similarly, all samples with aflatoxin contents >20 ppb as read by the fluorometer yielded a positive reaction in the test strips. Only three samples with exactly 20 ppb (borderline for negative and positive reaction) yielded negative instead of the expected positive reaction. This gave the test strips an overall accuracy of 97.2%, sensitivity of 94.4% and specificity of 100% (P<0.0001, Table 2), indicating that the test strips can be a good option when a fluorometer is not available. Even if the results of the test strips are only qualitative, the negative reaction for aflatoxin detection <20 ppb would be useful in determining that the sampled peanut lot is safe for human consumption. On the other hand, the positive reaction for aflatoxin detection ≥20 ppb will allow for the rejection of the peanut lot for human consumption.
Results of the immunochromatographic test strip assay. A total of 108 test strips were used (54 each for samples with aflatoxin contents <20 and ≥20 ppb). These were used to test peanut samples containing <20 and ≥20 ppb of aflatoxin as measured using the Vicam fluorometer. Results show that the test strips yielded positive reactions when tested on samples containing <20 ppb while samples with aflatoxin contents >20 ppb had negative reactions. Only three samples containing exactly 20 ppb of aflatoxin content, as quantified by the fluorometer, yielded negative reactions instead of the manufacturer-claimed positive reaction if used with the test strips.
Performance of the Test Strips when Exposed to Continuous High and Fluctuating Temperatures at Increasing Time Duration
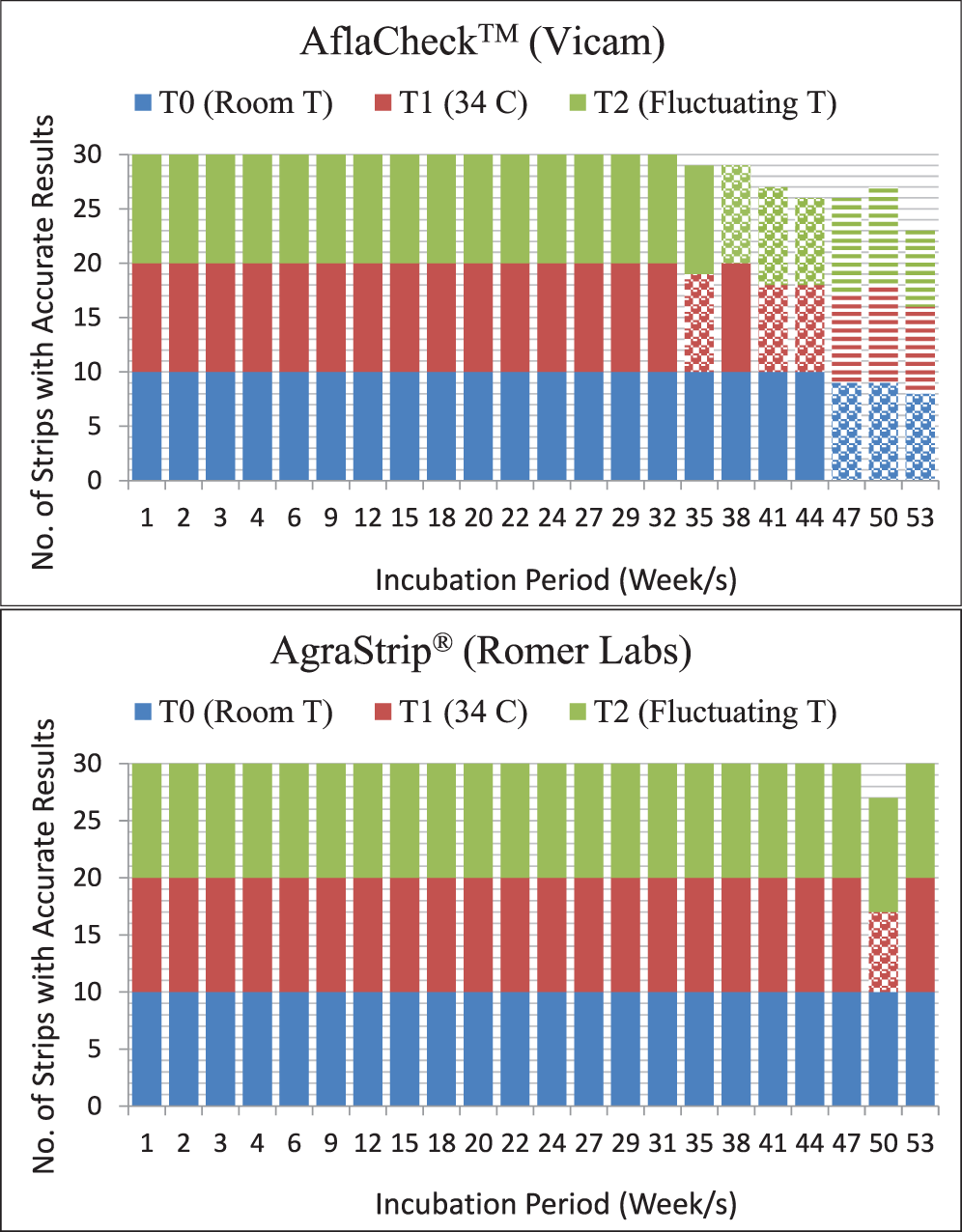
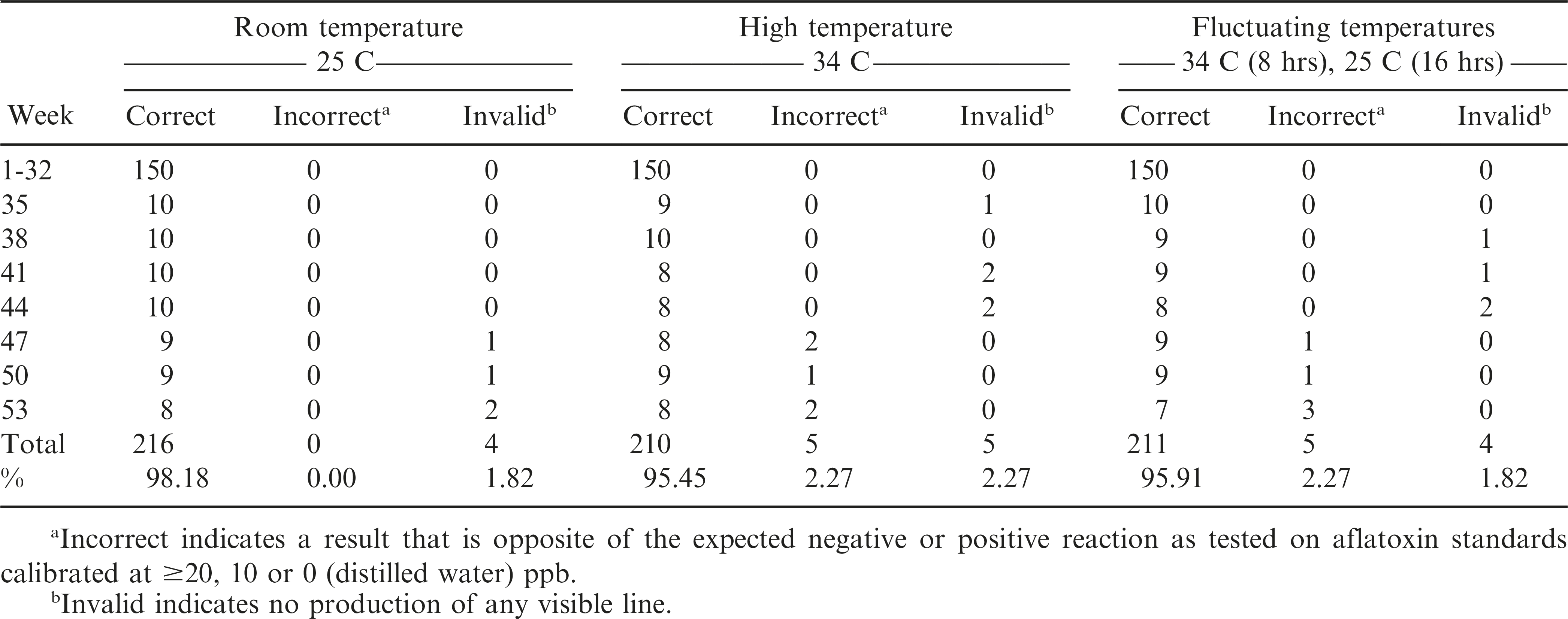
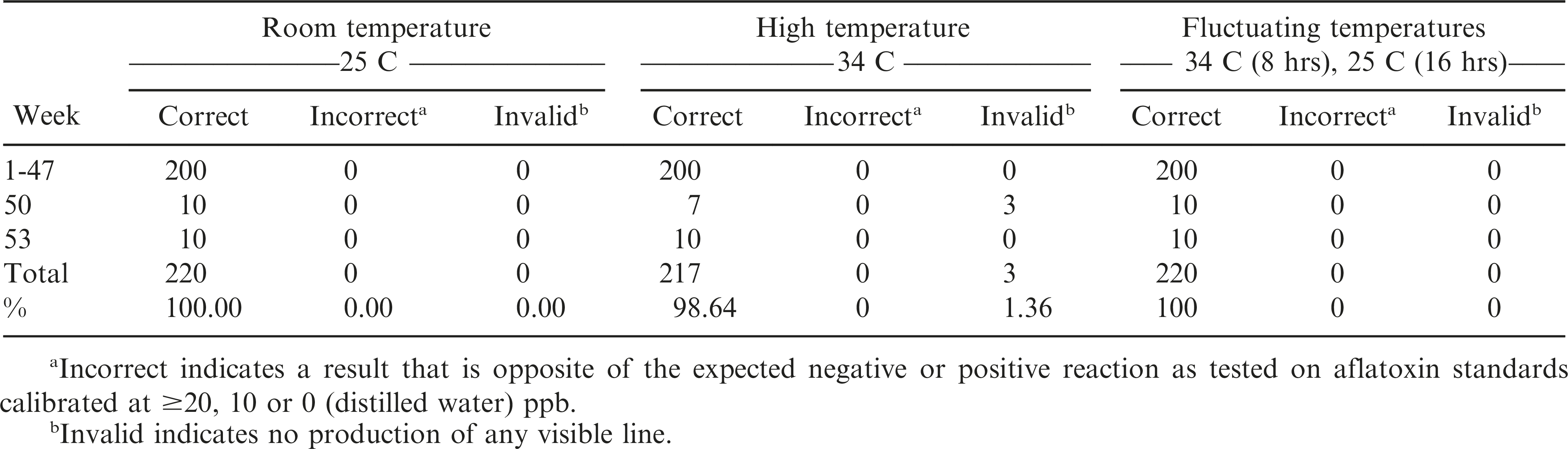
The AflaCheckTM (Vicam) test strips correctly detected the aflatoxin levels of the standard solutions calibrated at ≥20, 10 and 0 ppb for up to 32 wks regardless of incubation at room (T0 = approximately 25 C), continuous high (T1 = 34 C) or fluctuating (T2 = 34 C for 8 hours, 25 C for 16 hours daily) temperatures (Table 3, Figure 3). However, starting at wk 35 and 38, invalid results (no bands formed) were obtained from the test strips incubated at continuous high and fluctuating temperatures, respectively. It was also observed that some of these test strips had slower absorbing flow rate of the liquid towards the pad and/or produced blurry pink-dyed pads as the liquid was absorbed. At 47 wk, the test strips incubated under both temperature regimes began to yield results that were contradictory to what were expected. Test strips maintained at room temperature started yielding invalid results in the same week. In comparison, the AgraStrip® (Romer Labs) test strips yielded correct positive and negative results up to 47 wk of incubation under all temperature regimes (Table 4, Figure 3). This indicated a considerably longer shelf life of AgraStrip® as compared to AflaCheckTM. The only observed problem occurred at wk 50 when three (1.4% occurrence) invalid results were obtained from test strips incubated at continuous high temperature. Data analysis on the detection reaction of the test strips also showed that AgraStrip® had significantly higher number of test strips producing correct results over the 53 wk of incubation than AflaCheckTM (Table 5).
Reaction of the immunochromatographic test strips as affected by high and fluctuating temperature in increasing incubation duration. A total of 30 test strips were tested per wk (10 for each temperature regime). Each treatment was tested on five solutions calibrated to contain aflatoxin level of 50 ppb, three solutions containing 10 ppb, and two solutions with no aflatoxin (distilled water). Specific problems observed are indicated by dotted (due to invalid result as no line was produced) and horizontal (positive result instead of a negative result and vice versa) lines.
For both AflaCheckTM and AgraStrip®, room temperature was within the manufacturer-recommended storage temperatures. High temperature was purposely imposed to mimic the usual temperature in a tropical environment. Exposing the test strips to fluctuating temperatures mimicked two natural conditions in the field: fluctuating day and night storage temperatures; or the usage of the test strips in the field during the day then removed from the vehicle at night to be stored at room temperature when the test strips are not in use. Due to the ability of AflaCheckTM and AgraStrip® to remain stable for detecting aflatoxin levels under continuous high or fluctuating temperatures, the use of these test strips in the absence of a fluorometer or other technology for aflatoxin contamination in field locations that exhibit constant high or fluctuating temperatures is appropriate. Given that the test strips were incubated at temperatures beyond the manufacturer’s recommendation, the accurate performance of the test strips is economically significant.
For future research, it would be beneficial to test the effect of sudden or short term temperature changes in the performance of the test strips, especially at temperatures that exceed 34 C. This could answer what would happen if the test strips are left in a hot car or under the sun during field testing. It is also suggested to test if additional factors such as humidity would have an effect in the storage of the test strips.
Conclusions
This study assessed the performance of immunochromatographic test strips to detect aflatoxin levels at a 20 ppb cut-off limit. Results revealed a significant association (P<0.0001) between the use of the test strips and the fluorometry method, showing that the test strips are highly accurate (97%), sensitive (94%) and specific (100%) in detecting aflatoxin. In addition, incubating the test strips at continuous high (34 C) and fluctuating (34 C for 8 hr, around 25 C for 16 hr daily) temperatures did not alter its efficiency in yielding accurate results for 32 and 47 wk (around 8 and 12 months) for AflaCheckTM and AgraStrip®, respectively. These test strips may, therefore, be used for the qualitative detection of aflatoxin at a 20 ppb cut-off limit in peanut production areas or clinical laboratories that lack specialized equipment like the fluorometer or in tropical locations where refrigeration is not a part of normal storage practice.
Literature Cited
Chu F.S 1991 Mycotoxins: food contamination, mechanisms, carcinogenic potential and preventative measures Mutation Res 259 : 291 – 306 .
CLSI/NCCLS 2008 User protocol for evaluation of qualitative test performance, Approved guideline , 2nd ed., CLSI document EP12-A2 Clinical and Laboratory Standards Institute , Wayne, PA .
Edinboro L.E and Karnes H.T 2005 Determination of aflatoxin B1 in sidestream cigarette smoke by immunoaffinity column extraction coupled with liquid chromatography/mass spectrometry J. Chromatogr. A 1083 : 127 – 32 .
FAO , 2004 Food and Agricultural Organization: Worldwide regulations for mycotoxins for food and feeds in 2003 FAO Food and Nutrition Paper 81, FAO, Vialle Della Terme di Caracalla 00100 , Rome, Italy .
Holbrook C.C Kvien C.K Rucker K.S Wilson D.W and Hook J.E 2000 Preharvest aflatoxin contamination in drought tolerant and intolerant peanut genotypes Peanut Sci 117 : 258 – 64 .
JECFA 2001 Joint FAO/WHO expert committee on food additives, 56th meeting, Geneva, Switzerland, Feb , 6-15 .
Li P.W Zhang Q and Zhang W 2009a Immunoassays for aflatoxin Trends Anal. Chem 28 : 1115 – 1126 .
Li P.W Zhang Q Zhang W Zhang J.Y Chen X.M Jiang J Xie L.H and Zhang D.H 2009b Development of a class-specific monoclonal antibody-based ELISA for aflatoxins in peanut Food Chem 115 : 313 – 317 .
Manetta A.C di Giuseppe L Giammarco M Fusaro I Simonella A Gramenzi A and Formigoni A 2005 High-performance liquid chromatography with post-column derivatisation and fluorescence detection for sensitive determination of aflatoxin M1 in milk and cheese J. Chromatogr. A 1083 : 219 – 22 .
Pitt J.I Wild C.P Baan R.A Gelderblom C.A Miller J.D Riley R.T and Wu F (eds). 2012 Improving public health through mycotoxin control Lyon, France : International Agency for Research on Cancer 151 pp.
Shim W.B Yang Z.Y Kim J.S Kang S.J Woo G.J Chung Y.C Eremin S.A and Chung D.H 2007 Development of immunochromatography strip-test using nanocolloidal gold-antibody probe for the rapid detection of aflatoxin B1 in grain and feed samples J. Microbiol Biotechnol 17 : 1629 – 37 .
Swindale L.D 1989 A general overview of the problem of aflatoxin contamination of groundnut, In D. Mcdonald and V.K. Mehan (eds). Aflatoxin Contamination of Groundnut, ICRISAT: Proceedings of the International Workshop , 6-9 Oct 1987 , ICRISAT Center, Patancheru, India .
Tripathi S and Mishra H.N 2009 A rapid FT-NIR method for estimation of aflatoxin B1 in red chili powder Food Control 20 : 840 – 6 .
USDA-GIPSA 2014 GIPSA performance verified mycotoxin test kits . Available online from www.gipsa.esda.gov/fgis/tech-servup/metheqp/GIPSA_Approved_Myctoxin_Rapid_Test_Kits.pdf.
Waliyar F Siambi M Jones R Reddy S.V Chibonga D Kumar P.L and Denloye S 2008 Institutionalizing mycotoxin testing in Africa , pp. 359 – 68. In J.F. Leslie, R. Bandyopadhyay and A. Visconti (eds) Mycotoxins: Detection, Methods, Management, Public Health and Agricultural Trade. CAB Int. , Wallingford, UK .
Whitaker T.B Dorner J.W Lamb M and Slate A.B 2005 The effect of sorting farmers' stock peanuts by size and color on partitioning aflatoxin into various shelled peanut grade sizes Peanut Sci 32 : 103 – 18 .
Wilson D.M 1995 Management of mycotoxins in peanut , pp. 87 – 92. In H. Melouk and F. Shokes (eds) Peanut Health Management. APS , St. Paul, MN .
Zhang D Li P Yang Y Zhang Q Zhang W Xiao Z and Ding X 2011 A high selective immunochromatographic assay for rapid detection of aflatoxin B1 Talanta 85 : 736 – 42 .
Zhou Y Pan F.G Li Y.S Zhang Y.Y Zhang J.H Lu S.Y Ren H.L and Liu Z.S 2009 Colloidal gold probe-based immunochromatographic assay for the rapid detection of brevetoxins in fishery product samples Biosens. Bioelectron 24 : 2744 – 2747 .
Notes
- Grad Res. Asst., Dept. of Plant Pathology, University of Georgia, Tifton, GA 31794.
- Prof. and Ext. Specialist, Dept. of Plant Pathology, University of Georgia, Tifton, GA 31794. *Corresponding author's email: pmarian@uga.edu
Author Affiliations