Introduction
Palmer amaranth is a C4 summer annual native to the southwest United States and Mexico (Ehleringer 1983). The potential yield loss from Palmer amaranth competition has been well documented in several crops, including corn, cotton, and soybean (Massinga et al. 2001; Rowland et al. 1999; Klingaman and Oliver 1994). In peanut, yield loss of 28% was associated with Palmer amaranth season long competition, and peanut canopy cover was also reduced (Burke et al. 2007). Palmer amaranth is ranked as the most troublesome weed in peanut production in Florida, Georgia, and South Carolina (Webster 2009). The rapid growth rate of Palmer amaranth, when compared with other Amaranthus spp., combined with seed production of up to 1.2 billion seed ha−1 (greater than 250,000 seeds per plant), necessitate the need for effective control of this weed to economically grow peanut (Burke et al. 2007; Sellers et al. 2003).
In-season weed management in peanut often relies on postemergence herbicides. However, many of these do not provide season-long control of Palmer amaranth. For example, Norsworthy et al. (2008) noted paraquat to be highly effective, but the herbicide is only labeled for use 28 days after peanut emergence and possesses no soil activity (Anonymous 2012a, Senseman 2007). The acetolactate synthase (ALS)-inhibiting herbicide imazapic also controls Palmer amaranth from both foliar and soil activity while having a more flexible application criteria (Grichar 2007). However, due to development of ALS resistance in Florida and neighboring states, the efficacy of this herbicide cannot be guaranteed (Heap 2013). The protoporphyrinogen oxidase (PPO) inhibiting herbicides aciflourofen and lactofen are often the only postemergence options for growers attempting to control Palmer amaranth in peanut. But considering the contact activity of these herbicides, aciflourofen and lactofen require good coverage of young, actively growing weeds for maximum weed control (Anonymous 2012b).
Spray technology has evolved toward faster moving spray equipment and lower carrier volumes in an effort to reduce fuel costs from transporting large quantities of water and the need to cover more hectarage per tank-load (Etheridge 1999). In an effort to reduce the drift potential of these faster moving sprayers, many growers employ the use of drift-reducing nozzles. Air induction (AI) nozzles, for example, produce larger droplets, which are less susceptible to drift, than extended range (XR) nozzles at the same pressure (Etheridge 1999; Miller and Lane 1999; Ellis et al. 2002). Although these larger droplets reduce drift, spray coverage may be reduced. Knoche (1994) reported that smaller droplets from XR nozzles were more effective than larger droplets when applying postemergence herbicides at a constant carrier volume. Recent research reported that control of velvetleaf (Abutilon theophrasti Medik.) and common lambsquarters (Chenopodium album L.) with fomesafen, a PPO herbicide, was improved as carrier volume was increased for both XR and AI nozzles (Sikkema et al. 2008). However, Ramsdale and Messersmith (2001) noted that paraquat provided effective grass control regardless of nozzle type or carrier volume. Other studies have shown that nozzle type, carrier volume, and spray pressure provide varying levels of control that are herbicide- and weed species-specific (Buhler and Burnside 1984; Brown et al. 2007; Sikkema et al. 2008).
Numerous studies have documented that herbicide application to small weeds increases control. Common waterhemp (Amaranthus rudis Sauer) was controlled more effectively at 5 cm than at 10 cm with several herbicides (Hager et al. 2003). Mayo et al. (1995) demonstrated that Palmer amaranth control with lactofen herbicide declined from 99% control 14 days after planting (DAP) to 56% control 28 DAP. This trend continued with other contact herbicides tested. Acifluorfen provided 84% control 21 DAP and declined to 24% control when applied 35 DAP (Mayo et al. 1995). The lactofen registration label (Anonymous 2012b) limits application to Palmer amaranth having a maximum of 6 leaves, which Dotray et al. (1996) reported to be approximately 4 to 8 cm in height. Since Palmer amaranth can grow up to 5 cm/day, making applications with these herbicides before plants exceed the registration labeled height can be challenging (Sellers et al. 2003).
Current information is limited on the effects of application practices on Palmer amaranth control with contact herbicides. Although importance of weed size at application has been well documented, nozzle type and carrier volume have not been thoroughly investigated. The objective of this research was: (1) evaluate the spray coverage area for XR and AI nozzles to determine the range of carrier volumes and (2) conduct a field study that investigated the effect of application timing, carrier volume, and nozzle selection, and possible interactions, on Palmer amaranth control in peanut.
Materials and Methods
Spray coverage
XR Teejet flat fan nozzles (Spraying Systems Company, Wheaton, IL, USA) and AI Teejet flat fan nozzles (Spraying Systems Company, Wheaton, IL, USA) were calibrated to deliver application volumes of 94, 187, and 281 L/ha. Water sensitive cards were placed 50 cm below the spray nozzles. Water was sprayed over each card using each nozzle and carrier volume combination. The cards were then allowed to completely dry before being stored in zip-type plastic bags. A high-resolution flatbed scanner and color identification software program, WinCAM (Regent Instruments Inc., Canada), were used to evaluate the cards for percent spray coverage. Each nozzle and carrier volume combination was replicated across four cards. Percent spray coverage, as indicated by WinCam analysis, was subjected to analysis of variance using SAS (ver. 9.2; SAS Institute Inc.; Cary, NC) to determine significant treatment effects and interactions. Means were separated using Fisher's Protected LSD at P ≤ 0.05.
Field experiment
Field studies were conducted in 2008 and 2012. In 2008, studies were conducted at Sandlin Farms near Williston, Florida on Candler fine sand with less than 1% organic matter. In 2012, studies were conducted in Tifton, Georgia on Tifton loamy sand with 1.3% organic matter. Palmer amaranth was present in natural populations or seed was dispersed prior to the study to achieve a density of 10 to 40 plants/m. Peanut was planted between April 26 and May 10 at both sites with 18 seed per m of row. The study was multifactorial with nozzle type, carrier volume, and Palmer amaranth size as factors. All treatments were replicated four times and were arranged as a randomized complete block. Nozzle types used were the Teejet XR and the air induction Teejet AI to represent two levels of spray coverage. Carrier volumes evaluated were 94, 187, and 281 L/ha. Palmer amaranth size was measured at each location until average heights of 5 to 10 cm or 15 to 20 cm in height was reached. Lactofen (0.21 kg/ha plus crop oil concentrate at 1% v/v) was applied at the appropriate carrier volume, nozzle type, and plant height combinations.
Palmer amaranth control was estimated visually 7, 14 and 21 d after treatment (DAT) using a scale of 0 to 100% with 0 = no control and 100 = plant death (Frans et al. 1986). All data were subjected to analysis of variance and combined when appropriate. Means were separated with Fisher's Least Significant Difference (LSD) test at P ≤ 0.05.
Results and Discussion
Spray coverage
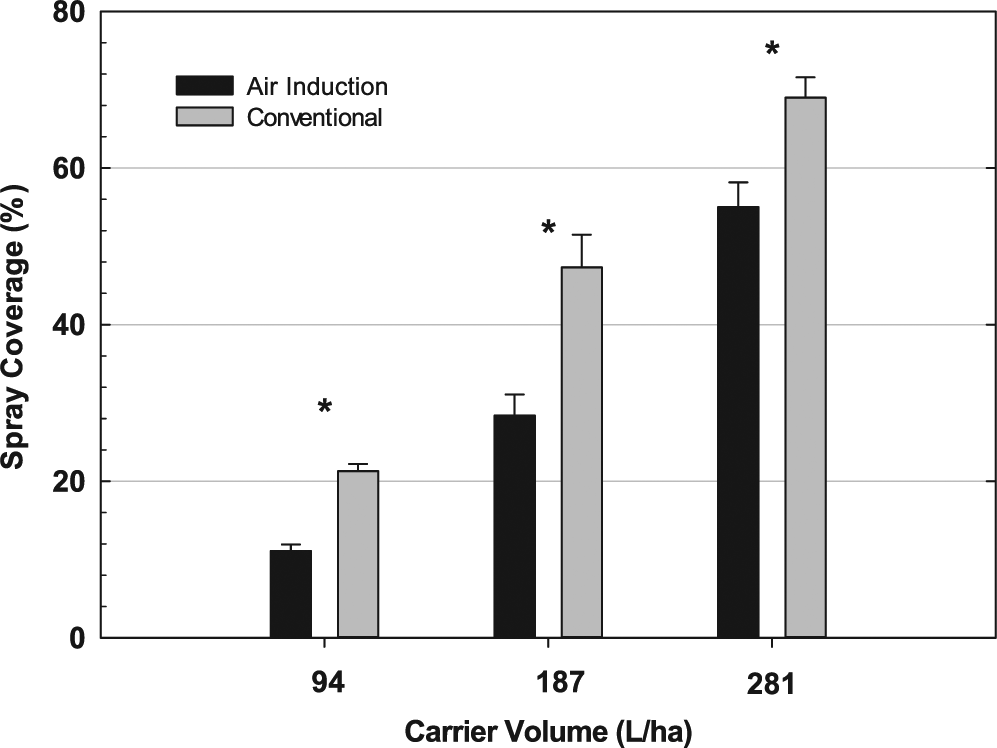
The interaction between nozzle type and carrier volume was significant, therefore data are presented for both nozzle type and carrier volume. At a carrier volume of 94 L/ha, 21% coverage was achieved with the use of XR nozzles while AI nozzles provided only 11% coverage (Figure 1). Increasing carrier volume to 187 L/ha resulted in significant differences with XR and AI nozzles providing 47% and 28% coverage, respectively. The trend continued at a carrier volume of 281 L/ha with XR nozzles providing 69% coverage and AI nozzles providing 55%. These data are similar to those reported by Ramsdale and Messersmith (2001). XR nozzles provided greater coverage at each carrier volume tested which could possibly lead to greater weed control when applying contact herbicides.
Field experiment
Nozzle type and location were not significant as a main effect or in any interaction. Therefore, data were combined across both nozzle type and location. The lack of interaction for nozzle type was unexpected since the spray coverage study indicated consistent differences in percent coverage. In some cases, as with application at 94 L/ha, coverage with AI nozzles was about half that observed with XR nozzles (Figure 1). However, it is consistent with results reported by Sikkema et al. (2008) who reported that no differences existed between XR flat fan and AI nozzles in control of common lambsquarters, velvetleaf, and common ragweed (Ambrosia artemisiifolia L.) when using fomesafen.
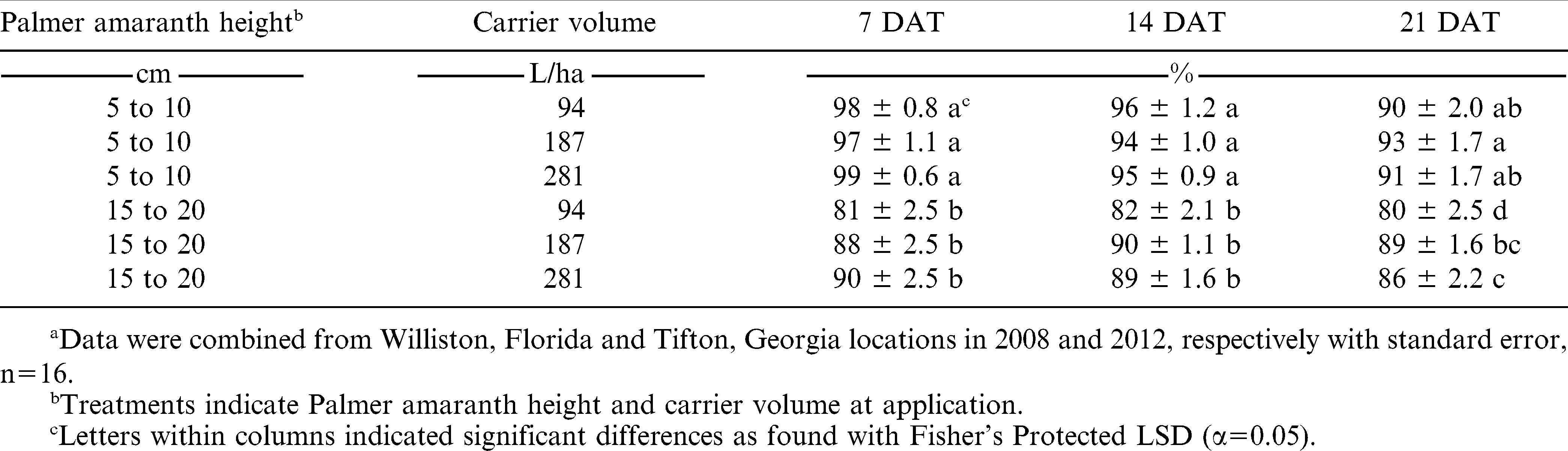
The main effects of carrier volume and Palmer amaranth height at application, as well as the interaction, were significant (P < 0.0001 for each factor). All applications made at 5 to 10 cm in height were 94 to 99% control when rated 7 and 14 DAT (Table 1). Applications made to 15 to 20 cm tall plants were less effective at 81 to 90% control. This was expected since previous research indicated that lactofen provided 92% control of Palmer amaranth when applied to 5 to 10 cm tall plants, but only 48% control when applied to 15 to 20 cm tall plants (Grichar 2007). Mayo et al. (1995) noted that Palmer amaranth control due to lactofen application decreased as time after crop planting increased. Hager et al. (2003) noted that applications of lactofen on 5 cm tall common waterhemp resulted in 9% greater control than applications made on 10 cm common waterhemp.
The influence of carrier volume was not significant within either application stage when rated at 7 or 14 DAT. By 21 DAT, applications made to 5to 10 cm tall plants were also quite effective resulting in greater than 90% control. However, applications made at 15to 20 cm tall with a carrier volume of 94 L/ha provided 80% control, which was statistically less than all other treatments tested. This reduction in control at 21 DAT was due to regrowth of Palmer amaranth plants.
Conclusions
Adequate coverage is essential for maximizing efficacy with lactofen. The results of this research demonstrate that AI nozzles do provide less coverage than XR nozzles, but this difference in coverage does not translate to differences in lactofen efficacy on Palmer amaranth. Applications made at 94 L/ha resulted in reduced control when treating 15–20 cm tall Palmer amaranth plants. But of greater importance is the fact that greater than 90% control was achieved, regardless of nozzle type or carrier volume, when 5–10 cm tall plants were targeted. Therefore, it is essential that peanut producers regularly scout fields to monitor Palmer amaranth height if contact herbicides such as lactofen are to be used. Applications should be made to small weeds to ensure greater control; if larger Palmer amaranth must be treated, increased carrier volume should be used.
Literature Cited
Anonymous 2012a Gramoxone SL 2.0 herbicide label. EPA Reg. No. 100-1431. Syngenta Pub. No. SCP 1431A-L1A 0812 4076806, Greensboro, NC: Syngenta Crop Protection, LLC .
Anonymous 2012b Cobra herbicide label. EPA Reg. No. 59639-34. Valent Pub. No. 2012-COB-0001, Walnut Creek, CA: Valent USA Corporation .
Brown L Soltani N Shropshire C Spieser H and Sikkema P.H 2007 Efficacy of four corn (Zea mays L.) herbicides when applied with flat fan and air induction nozzles Weed Biol. Mang. 7 : 55 – 61 .
Buhler D.D and Burnside O.C 1984 Effect of application factors on postemergence phytotoxicity of fluazifop-butyl, haloxyfop-methyl, and sethoxydim Weed Sci. 32 : 574 – 583 .
Burke I.C Schroder M.S Thomas W.E and Wilcut J.W 2007 Palmer amaranth interference and seed production in peanut Weed Technol. 21 : 367 – 371 .
Dotray P.A Keeling J.W Henniger C.G and Abernathy J.R 1996 Palmer amaranth (Amaranthus palmeri) and devil's-claw (Proboscidea lousianica) control in cotton (Gossypium hirsutum) with pyrithiobac Weed Technol. 10 : 7 – 12 .
Ehleringer J 1983 Ecophysiology of Amaranths palmeri, a sonoran desert summer annual Oceologia. 57 : 107 – 112 .
Ellis M.C.B Swam T Miller P.C.H Waddelos S Bradley A and Tuck C.R 2002 Design factors affecting spray characteristics and drift performance of air induction nozzles Biosyt. Eng. 82 : 289 – 296 .
Etheridge R.E Womac A.R and Mueller T.C 1999 Characterization of the spray droplet spectra and patterns of four venture-type drift reduction nozzles Weed Technol. 13 : 765 – 770 .
Frans R Talbert R Marx D and Crowley H 1986 Experimental design and techniques for measuring and analyzing plant responses to weed control practices, In: Camper N.D (ed.) Research Methods in Weed Science, 3rd ed., Champaign, IL.: Southern Weed Science Society. Pp. 29 – 46 .
Grichar W.J 2007 Horse purslane (Trianthema portulacastrum), smellmelon (Cucumis melo), and Palmer amaranth (Amaranthus palmeri) control in peanut with postemergence herbicides Weed Technol. 21 : 688 – 691 .
Hager A.G Wax L.M Bollero G.A and Stoller E.W 2003 Influence of diphenylether herbicide application rate and timing on common waterhemp (Amaranthus rudis) control in soybean (Glycine max) Weed Technol. 17 : 14 – 20 .
Heap I 2013 The international survey of herbicide resistant weeds Online. Internet. Thursday, July 11, 2013. Available www.weedscience.com .
Klingaman T.E and Oliver L.R 1994 Palmer amaranth (Amaranthus palmeri) interference in soybeans (Glycine max) Weed Sci. 42 : 523 – 527 .
Knoche M 1994 Effect of droplet size and carrier volume on performance of foliage-applied herbicides Crop Prot. 13 : 163 – 178 .
Massinga R.A Currie R.S Horak M.J and Boyer J 2001 Interference of Palmer amaranth in corn Weed Sci. 49 : 202 – 208 .
Mayo C.M Horak M.J Peterson D.E and Boyer J.E 1995 Differential control of four Amaranthus species by six postemergence herbicides in soybean (Glycine max) Weed Technol 9 : 141 – 147 .
Miller P.C.H and Lane A.G 1999 Relationship between spray characteristics and drift risk into field boundaries of different structure Aspects Appl. Biol. 45 : 45 – 51 .
Norsworthy J.K Griffith G.M Scott R.C Smith K.L and Oliver L.R 2008 Confirmation and control of glyphosate resistant Palmer amaranth (Amaranthus palmeri) in Arkansas Weed Technol. 22 : 108 – 113 .
Ramsdale B.K and Messersmith C.G 2001 Drift-reducing nozzle effects on herbicide performance Weed Technol. 15 : 453 – 460 .
Rowland M.W Murray D.S and Verhalen L.M 1999 Full-season Palmer amaranth (Amaranthus palmeri) interference with cotton (Gossypium hirsutum) Weed Sci. 47 : 305 – 309 .
Sellers B.A Smeda R.J Johnson W.G Kendig J.A and Ellersick M.R 2003 Comparative growth of six Amaranthus species in Missouri Weed Sci. 51 : 329 – 333 .
Senseman S (ed.) 2007 Herbicide Handbook, 9th Edition Weed Science Society of America , Lawrence, KS. 458 pp.
Sikkema P.H Brown L Shropshire C Spieser H and Soltani N 2008 Flat fan and air induction nozzles affect soybean herbicide efficacy Weed Biol. Mang. 8 : 31 – 38 .
Webster T.M 2009 Weed survey- southern states Proc. South. Weed. Sci. Soc. 62 : 510 – 525 .
Notes
- First and third authors:, Graduate Student and Associate Professor, Agronomy Dept., University of Florida, Gainesville, FL 32611, Second author:, Technology Development Representative, Seminis Vegetable Seeds, Hahira, GA 31632, Fourth author:, Research agronomist, USDA-ARS, Tifton, GA 31794 * Corresponding author’s E-mail: sberger@ufl.edu
Author Affiliations