Introduction
Peanut (Arachis hypogaea L.) is a rich source of edible oils, protein, and minerals such as phosphorus, calcium, magnesium, potassium, and vitamins (Savage and Keenan, 1994). In the U.S., peanut is an important cash crop and is largely used for peanut butter and snacks while it is used primarily as an oil source in most other peanut production areas of the world.
Seed oxidative stability is closely associated with oil and fatty acid composition and is an important quality attribute regardless of whether it is used as food or oil. The two predominant fatty acids in peanut seed oil are oleic acid (18∶1) and linoleic acid (18∶2) which together comprise about 80% of the total fatty acid composition (Ahmed and Young, 1982). Typically, peanut oleic acid to linoleic acid ratio, commonly referred to as O/L ratio, is about 1.5 to 2.0 (approximately 50% oleic acid to 30% linoleic acid). Oleic acid, the 18-carbon monounsaturated fatty acid and precursor to linoleic acid, is less reactive with oxygen and as a result, more stable than linoleic acid. Therefore, peanut seeds with high oleic acid content and high O/L ratio have improved stability against lipid oxidation that lead to adverse flavors (Mugendi et al., 1998; O'Keefe et al., 1993) and nearly twice the shelf-life as those with normal O/L ratio (Braddock et al., 1995; Branch et al., 1990; James and Young, 1983). Virginia-type peanut cultivars naturally produce oil with slightly lower linoleic percentage and therefore have greater oil stability than other market types (Norden et al., 1982).
High-oleate peanut trait in a runner breeding line, F 435, was found to have 80% oleic acid and 2% linoleic acid (Norden et al., 1987). The relationship between the two fatty acids is inversely related so that O/L ratio accurately reflects the relative contents of these two fatty acids in peanut (Knauft et al., 1993).
Developing Virginia-type cultivars with higher levels of oleic acid and depressed levels of linoleic acid is one of several important goals in public peanut breeding programs in the US. High-oleate peanut lines were developed through backcrosses using the high-oleate line F 435 as the source of the trait and large-seeded Virginia cultivars such as NC-V 11 (Wynne et al., 1991) and Gregory (Isleib et al., 1999) as the recurrent parents. High-oleate lines were selected after two to four backcrosses to the commercial cultivar. The program has resulted in several advanced high-oleate peanut lines with 80 to 85% oleic acid content in their seed oil (Isleib, personal communication, 2012).
A uniform stand of healthy, vigorous seedlings is essential if growers are to achieve the yield and quality needed for profitable peanut production. Spears et al. (2002) reported that peanut seed quality can be separated into five related components: germination, vigor, genetic purity, crop purity and health. Germination and vigor have the greatest impact on seedling emergence and survival. Germination tests are standardized throughout the United States. Vigor tests, however, are common only for major commodities and high value crops.
Seed quality and vigor are greatly influenced by environmental factors that occur during seed growth, development, and maturation (Copeland and McDonald, 2001; TeKrony et al., 1984). High temperature during development has been shown to reduce soybean seed germination and vigor (Egli et al., 2005; Keigley and Mullen. 1986; Spears et al., 1997). Spears et al. (1997) reported that soybean seeds grown in 27/22 C day/night temperature had higher germination and accelerated aging germination and lower EC than those grown at 38/33 C high temperature stress environment. Temperature also influences vegetative and reproductive growth rates (Leong and Ong, 1983). High temperature reduces the numbers of pegs and pods (Ketring, 1984) and the reductions in pod and seed dry weight were reportedly due to reductions in total dry matter and harvest index (Craufurd et al., 2002).
In addition to influencing seed planting quality, production environment can also affect peanut seed chemical composition. Dwivedi et al. (1993) reported significant genotype, environment and genotype-by-environment interaction effects on oil content, individual fatty acid content, and derived oil quality in peanut. Change in seed chemical composition has been identified as one of the factors that can influence seed germination and vigor (Copeland and McDonald, 2001).
While much of the peanut industry's concern has been about peanut dietary oil quality, seed technologists know that lipids are an important source of energy for germination. Consequently, altering peanut seed fatty acid or total lipid composition could influence germination rate, seed and seedling vigor, and seedling survival, especially if seeds are planted in stressful soil conditions. Alterations of seed lipid fatty acid composition brought by traditional or molecular techniques could also change membrane lipid composition and therefore affect membrane function and permeability. Membrane integrity is linked to seed quality, seedling energy, and tolerance to environmental stress during germination and emergence. However, there is little information available in the literature on the effect of altering peanut seed lipid on seed membrane function, germination, or vigor of peanut.
With the increasing number of high-oleate peanut cultivars, there is a need to analyze quality of high-oleate peanut seed produced in various environments. The objectives of this study were to 1) determine the influence of high oleic acid on peanut seed germination and vigor; 2) evaluate planting date and harvest date affect on normal and high-oleate peanut seed germination and vigor; and 3) investigate the effect of temperature on seed oil quality of high-oleate and normal Virginia-type peanut cultivars.
Materials and Methods
Influence of Planting and Digging Date on Seed Quality in the Field
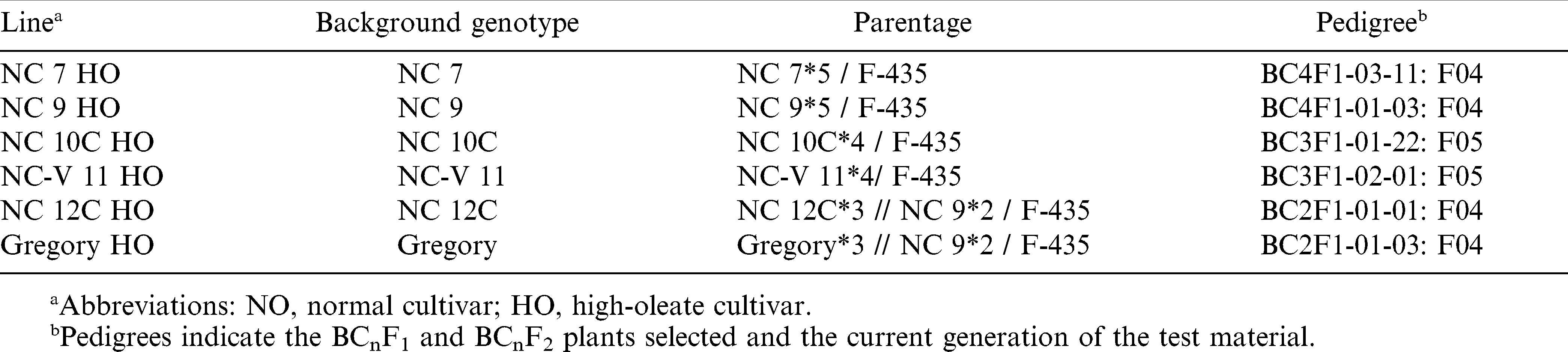
High-oleic peanut lines NC 7 HO, NC 9 HO, NC 10C HO, NC-V 11 HO, NC 12C HO, and Gregory HO were developed by backcrossing the high-oleate trait from F 435 into six large-seeded Virginia-type cultivars: NC 7 (Wynne et al., 1979), NC 9 (Wynne et al., 1986), NC 10C (Wynne et al., 1991), NC-V 11 (Wynne et al., 1991), NC 12C (Isleib et al., 1997), and Gregory (Isleib et al., 1999) (Table 1). These six large-seeded Virginia-type cultivars have 50 to 55% oleic acid content in the seed oil. High-oleic lines were selected after two, three, or four backcrosses to the cultivar and their seed oils contain 80 to 85% oleic acid.
Experiments were conducted at the Peanut Belt Research Station near Lewiston-Woodville, North Carolina during 2003 and 2004 (36.07N, −77.11W). The treatment design included two planting dates, early May and early June, and two harvest dates early October and late October. A split-plot experimental design was used with 2 by 2 factorial combinations of planting and harvest date serving as whole plot treatments. The factorial combination for two peanut oleic acid levels and six cultivars were subplot. Each subplot consisted of two rows, each on a raised bed spaced on 91-cm centers and 10 m in length. Experimental test plots were established according to the treatment design of the experiment and standard production practices appropriate for the region were followed. Soil was a Goldsboro sandy loam (fine-loamy, kaolinitic, thermic typic Kandiudults) with 1.8% organic matter content and pH 6.1.
At harvest, plants were dug with a commercial digger/inverter and pods were removed by machine 3 to 4 days after digging, placed in mesh bags, and dried over non-heated forced air to approximately 8% seed moisture content. Sound mature seeds were collected from each plot, placed in plastic bags, and stored at 12 C until evaluation. Seed quality evaluation included standard germination (SG), cool germination (CG), and electrical conductivity (EC) (ISTA, 1995).
Prior to SG evaluation, seeds were treated with a commercial seed treatment consisting of 45% captan, 15% PCNB, and 10% carboxin (Vitavax PC, Gustafson LLC, Plano, TX). Standard germination tests were conducted by placing four 25-seed subsamples in rolled towels at alternating 30/20 C (day/night) with 16 h at 20 C. The standard germination test procedure followed Association of Official Seed Analysts Rules for Testing Seeds (AOSA, 2002; McDonald, 1994). Seedlings were evaluated at 8 days after planting and germination was assessed as the percentage of seeds producing a normal seedling with a radical at least 3.5 cm long.
Seeds were randomly sampled from the same seed lot used for SG and pretreated with the commercial blend of captan, PCNB, and carboxin. Cool germination tests were conducted on four 25-seed subsamples placed in rolled towels at 18 C (± 0.5 C). The test was conducted in the dark. Seedlings were evaluated at 9 days after planting and only those seedlings with radicals 2.5 cm or longer were considered to have germinated.
Seed vigor was also evaluated by electrical conductivity of seed soak water. Seeds were adjusted to about 7.5% moisture content in a chamber (Model 3020 Water Jacketed Incubator, VWR Scientific, Inc., Suwanee, GA) at 20 C and near 95% RH prior to measuring seed leakage. Fifty seed were weighed and placed in 250 ml deionized water at 25 C. Containers (Fisher Scientific Pyrex Erlenmeyer Flask, Fisher Scientific, Suwanee, GA) were covered with foil and held at 25 C (±0.5 C) for 24 h (±0.5 h). Following incubation, the containers were gently swirled and the conductivity of the seed soak water was measured using a Cole Palmer Conductivity meter model 1481-60 (Cole-Palmer Instrument Company, Niles, IL). Conductivity is reported as µohms cm−1 g−1 of seed. Two randomized subsamples from each field plot as two replications and a completely randomized design (CRD) were used in this experiment.
Prior to statistical analysis, SG and CG percentages were transformed using the angular (arcsin of square root) transformation and EC values were transformed using natural log. Analysis of variance was conducted using the general linear models procedure (PROC GLM) of SAS (SAS Institute, Inc., Cary, NC). Years and replications were considered random effects while planting and harvest dates, oleic acid level, and cultivar were considered fixed effects. Treatment means were separated by t-test using a significance level P = 0.05.
Influence of Temperature Regime on Seed Quality in the Greenhouse
Studies were established during 2003 and 2004 in greenhouses located at the Southeastern Plant Environment Laboratory at North Carolina State University main campus. (35.77N, −78.68W) Four peanut cultivars, NC-V 11 (Wynne et al., 1991), high-oleate NC-V 11 (NC-V 11 HO), Gregory (Isleib et al., 1999), and high-oleate Gregory (Gregory HO) were used in this experiment. A split plot experimental design was used, with greenhouse as the whole plot (three levels) and cultivar as subplot (four levels). All greenhouses were maintained at 80% relative humidity and natural photoperiod plus 3 h night interruption (11pm to 2 am).
The three temperature levels in this study were 22/18 C, 26/22 C, and 30/26 C day/night with 12 hours of high and low temperatures. Single plants were grown in 45-cm diameter pots filled with three parts sand (steam sterilized) and one part peat. Plants were watered twice daily with a complete nutrient solution (Downs and Thomas, 1991). In each greenhouse, randomized complete block designs were used with seven blocks in 2003 and five blocks in 2004. The four cultivars were randomly arranged within each block. Hull scrape and peanut maturity profile method were used to determine maturity and implement harvest (Williams and Drexler, 1981). Following harvest, pods were dried with unheated forced air, shelled by hand, and the seeds stored at 10 C until analyzed for oil quality.
Ten sound mature kernels and 15 embryos of each of the four lines produced under three environments in 2003 and 2004 were analyzed for fatty acid profile using the technique of Zeile et al. (1993). Kernel mass was not determined. Samples were extracted for 12 h in 1 ml of solvent (chloroform: hexane: methanol, 8:5:2 volume) in 16 by 100 mm stoppered test tubes (Fisherbrand Disposable Culture Tubes, Fisher Scientific, Suwanee, GA). Fatty acid methyl esters of the lipid extracts were prepared using sodium methoxide. The samples were analyzed by gas chromatography using an HP 5890 Series II GC (Agilent Technologies, Inc., 2850 Centerville Rd., Wilmington, DE) equipped with an AT-Silar 30 m by 0.53 mm column (same source). Operating conditions were 1 µl injection volume, a 20∶1 split ratio, and He carrier gas flow of 6 ml min−1. Temperatures were 250 C, 200 C, and 275 C for the injector, oven and flame ionization detector (FID), respectively. Chromatograms were analyzed using HP ChemStation software (Agilent Technologies, Santa Clara, CA).
For statistical analysis, data were subjected to analysis of variance over two years and three temperatures based on a mixed linear model with temperature and cultivar as fixed effects of variance and year, replication (year) as random effects were used. Analyses were conducted using the general linear models procedure (PROC GLM) with random statement of SAS (SAS Institute, Inc., Cary, NC). Treatment means were separated by t-test using significance level of P = 0.05.
Results and Discussion
Influence of Planting and Digging Date on Seed Quality in the Field
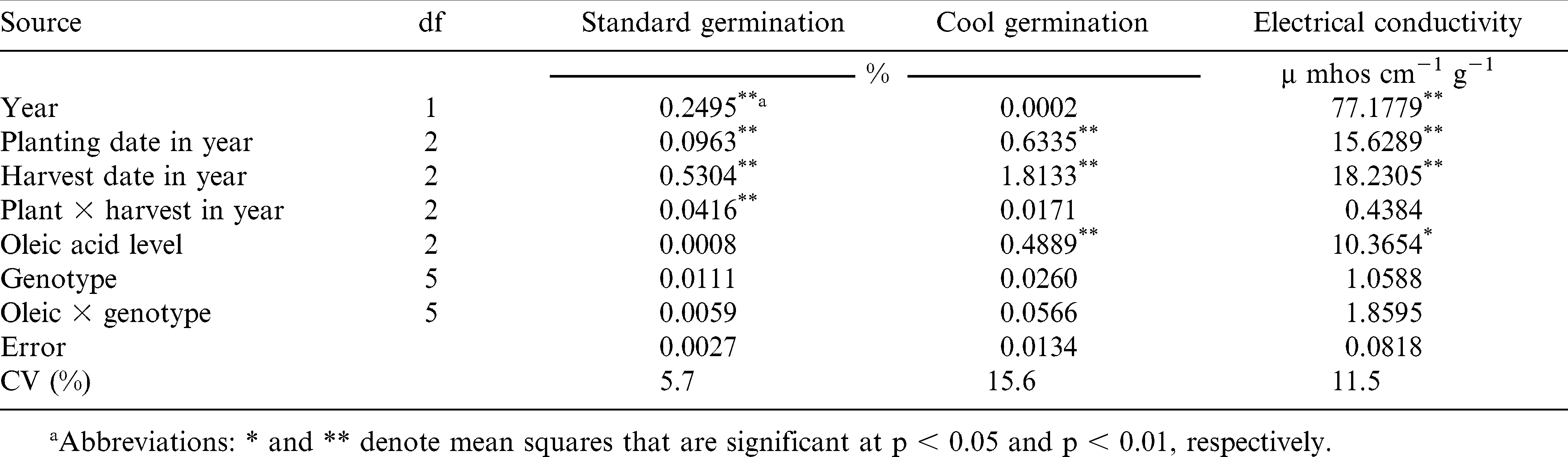
There was no correlation between SG and CG or between SG and EC. However, CG and EC were negatively correlated (r = −0.73, p < 0.01, data not shown). Although they measure different aspects of seed vigor, the two vigor tests appear to be in agreement when evaluating the seed lot vigor potential for the seed used in our study. It is interesting that the two outlying points are NC 10C and NC 10C HO. Why this cultivar pair does not fit into the pattern of the other five cultivar pairs is not known. Further tests might reveal any genetic influence on subsequent seed quality.
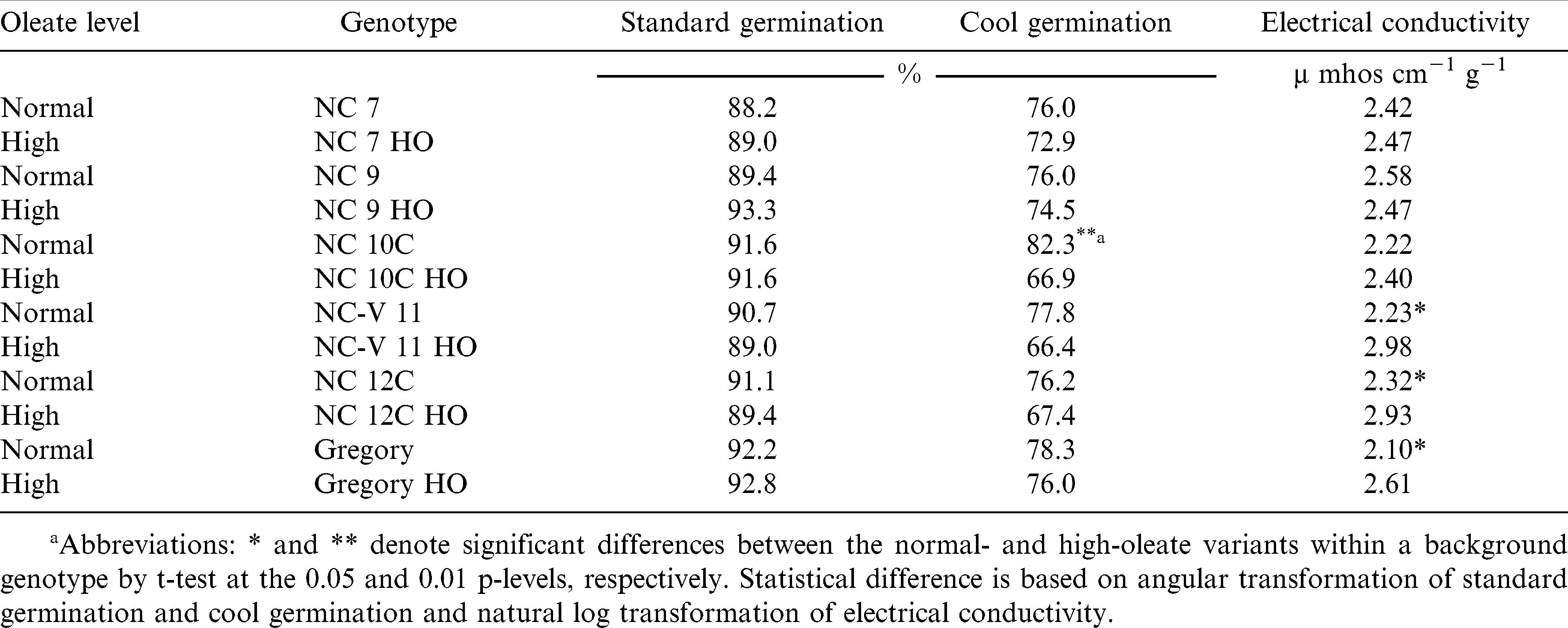
The interaction of oleic acid level by cultivar was not significant for any of the measured traits (Table 2). Likewise, peanut seed oleic acid level had no effect on SG, but did influence CG and EC. All 12 lines, regardless of production environment or oleic acid level, had standard germination percentages above 88% (adjusted mean) and there were no differences for SG between the paired normal and high-oleate lines (Table 3). These data suggest that SG is not affected by the elevated oleic acid content found in the high-oleate lines.
When averaged across production environments, peanut seed CG was lower than SG for each cultivar used in this study (Table 3). The differences between SG and CG were larger for high-oleate lines than those corresponding normal lines. The high-oleate lines had adjusted means ranging from 66.4 to 76.0% while the normal lines had values ranging from 76.2 to 82.3%. Three high-oleate lines, NC 10C HO, NC-V 11 HO, and NC 12C HO had lower CG (66.9, 66.4, and 67.4%) than their paired NC 10C, NC-V 11, and NC 12C parents (82.3, 77.8, and 76.2%).
Oleic acid level also influenced peanut seed EC (Table 3). Three high-oleate lines NC V11 HO, NC 12C HO and Gregory HO had higher EC (2.98, 2.93, and 2.61 µmhos cm−1 g−1) than their three paired normal lines (2.23, 2.32, and 2.10 µmhos cm−1 g−1).
The standard germination test is designed to provide the maximum germination percentage for the seed lot tested (AOSA, 2002; McDonald, 1994). If seeds are planted in fields with near ideal conditions, SG will provide a good estimate of seedling establishment. However, soil environments are rarely ideal and the SG test may not accurately reflect potential seedling emergence and survival (Guerke, 2005; Spears et al., 2002). Seed vigor tests were developed to help practitioners evaluate seed lot field emergence potential. For this research, seed vigor of four of the six paired high-oleate, normal lines (NC 10C, NC-V 11, NC 12C and Gregory) was reduced when the high-oleate trait was incorporated into the cultivar.
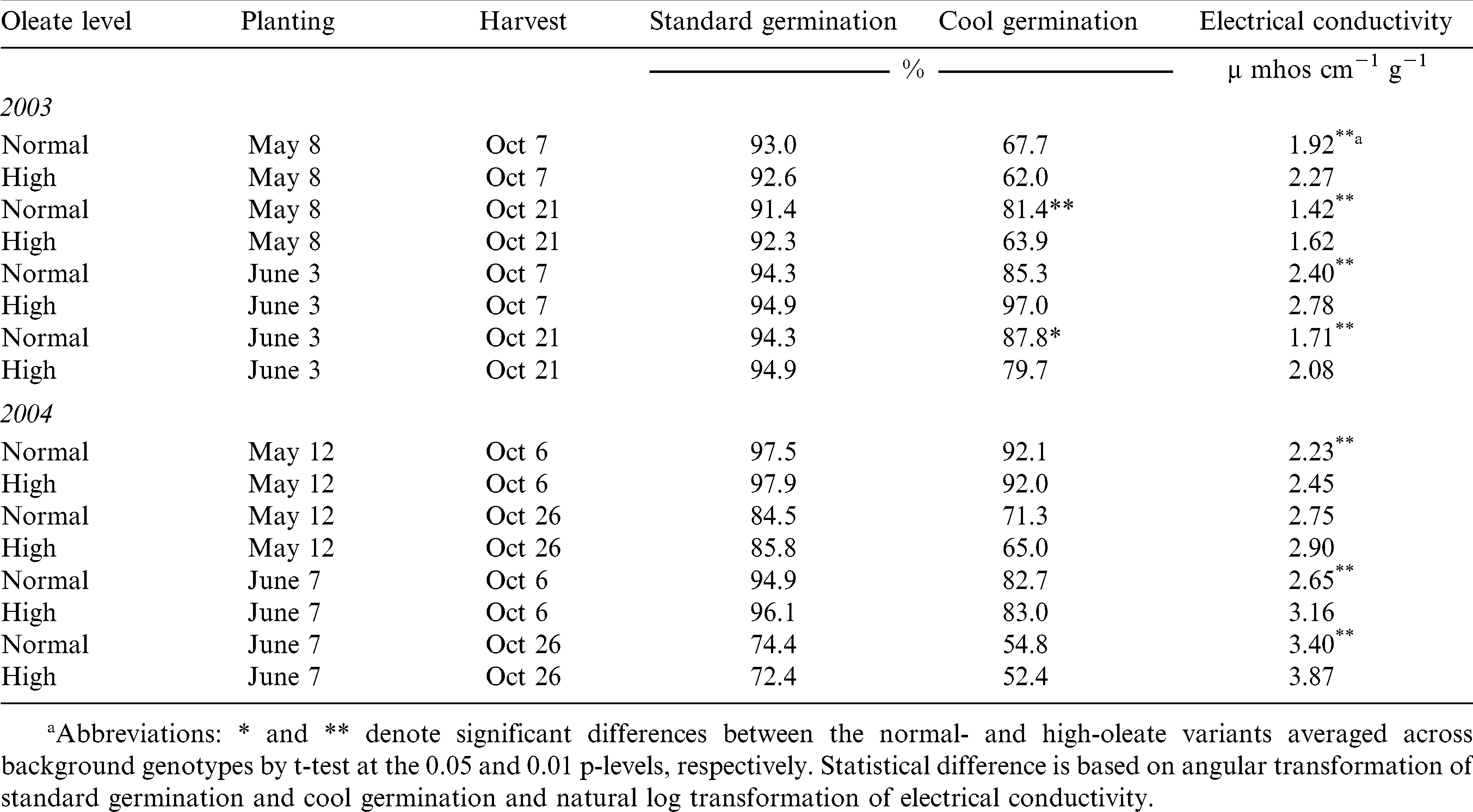
Planting and harvest date influenced SG, CG and EC but the genotype by oleic interaction was not significant (Table 2). Because of the wide temperature and rainfall pattern variations seen in 2003 and 2004, data are presented as eight environments (two years by two planting dates by two harvest dates) for mean comparisons.
In 2003, there was no difference in peanut SG for planting date or for harvest date (Table 4). Germination was above 90% in all production environments. In 2004, however, germination of both normal and high-oleate peanut seed harvested on October 26 was significantly lower than those harvested 20 days earlier. In addition, peanut seed planted in June and harvested on Oct26 had lower SG than those planted in May and harvested on October 26.
In the 2003 production environments, CG for normal cultivars was significantly higher than that of the high-oleate lines, with the exception of CG for peanuts planted on May 8 and harvested on October 7 (Table 4). Delaying harvest of peanut planted in May resulted in a large increase on CG for the normal cultivars (67.7 to 81.4%) but only a slight increase in CG for the high-oleate lines (62.5 to 63.9%). When peanuts were planted in June, delaying harvest date resulted in a large increase in CG for the high-oleate lines (67.0 to 79.7%) but only a slight increase for the normal cultivars (85.3 to 87.8%). The highest CG value for the high-oleate lines (79.7%) occurred when peanuts were planted in June and harvested on October 21.
Electrical conductivity was lower in normal cultivars compared to the paired high-oleate line in 2003 for all planting and harvest dates (Table 4). Electrical conductivity values of May and June planted normal and high-oleate peanuts decreased when the harvest date was delayed. One would predict that the peanut seed with the most time to develop (planted in May and harvested October 21) would have the highest vigor potential. The lowest EC, 1.42 and 1.62 µmhos cm−1 g−1 for normal and high-oleate lines, respectively, was found in these peanuts. However, highest CG was found in peanuts, both normal and high-oleate, planted in June and harvested on October 21. Electrical conductivity and CG tests measure different mechanisms associated with seed vigor. It is possible that the components of seed vigor reach maximum potential at different times in peanut seed development or that they vary in their response to production environment.
In 2004, there were no differences between the CG of normal and high-oleate lines for any planting date/harvest date combination (Table 4). Electrical conductivity of the normal cultivars was significantly lower than that of the high-oleate lines with the exception of the May planting, October 26 harvest. For both planting dates, CG was reduced when harvest date was delayed and the lowest CG (54.8 and 52.4% for normal and high-oleate, respectively) and highest EC (3.40 and 3.87 µmhos cm−1 g−1) occurred when peanuts were planted in June and harvested on October 21. Highest CG values (92.0% for both normal and high-oleic) and lowest EC (2.23 and 2.45 µmhos cm−1 g−1) occurred when peanuts were planted in May and harvested on October 6.
Seed lot quality of most species is influenced by production environment. The two planting and harvesting dates for 2003 and 2004 in this experiment represented diverse production environments (data not shown). Cold and wet weather occurred between the first and second harvest dates in 2004, which resulted lower SG, CG and higher EC for seeds harvested on October 26 compared to those harvested on October 6, regardless of planting date. In contrast, 2003 weather conditions between the first and second harvests were cool with only trace amounts of rainfall. Standard germination of peanuts harvested on October 21 did not differ from seed harvested on October 7, regardless of planting date. Seed vigor of peanuts for both planting dates, as measured by CG and EC actually, increased when harvest date was delayed. However, CG and EC tests for the two years revealed that across genotype, high-oleate lines had lower vigor than normal peanut lines in each planting date and harvest date environment. Peanut growers planting high-oleic cultivars should increase the seeding density than over normal peanut cultivars.
Influence of Temperature Regime on Seed Quality in the Greenhouse
As expected, peanut growing under the cooler temperature regime required more days to reach optimum maturity based on pod mesocarp color than warmer regimes. When pooled over runs of the experiment, approximately 175, 160, and 130 d from emergence to optimum harvest maturity were noted for the 22/18 C, 26/22 C, and 30/26 C temperature regimes, respectively (data not shown in tables).
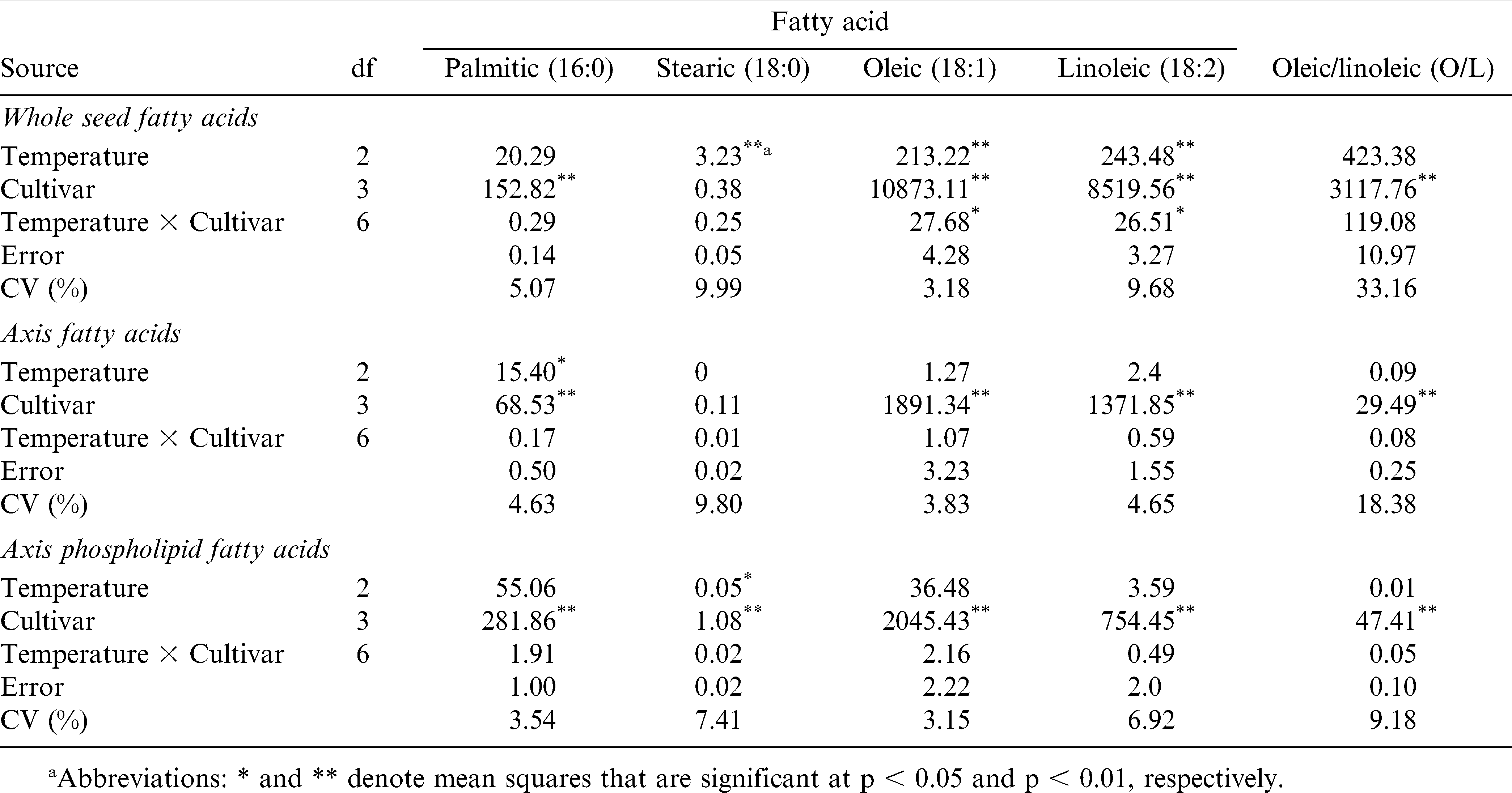
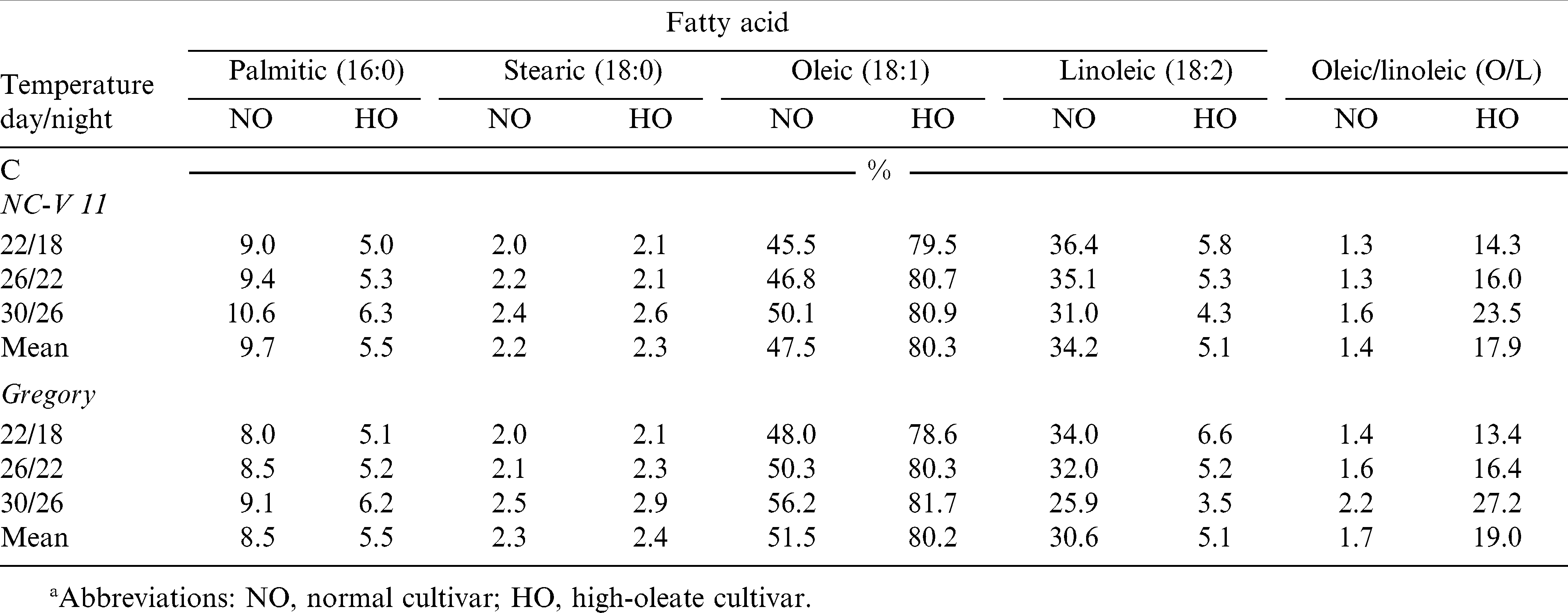
There was no significant temperature or temperature by cultivar interaction effect on whole seed levels of palmitic acid (16∶0) (Table 5). When averaged across the three temperatures, palmitic acid levels of NC-V 11, NC-V 11 HO, Gregory, and Gregory HO, were 9.7, 5.5, 8.5, and 5.5%, respectively. Cultivar effect for palmitic acid was significant. The high-oleate lines had lower 16∶0 content than their paired normal lines in all environments (Table 6).
There was a significant temperature effect on stearic acid (18∶0) content (p < 0.01), but no cultivar or temperature by cultivar interaction (Table 5). Stearic acid content increased slightly but significantly with increasing growth temperature (Table 6). Average stearic acid levels across the four cultivars for three temperature regimes, 22/18 C, 26/22 C, and 30/26 C were 2.1, 2.3, and 2.6%, respectively.
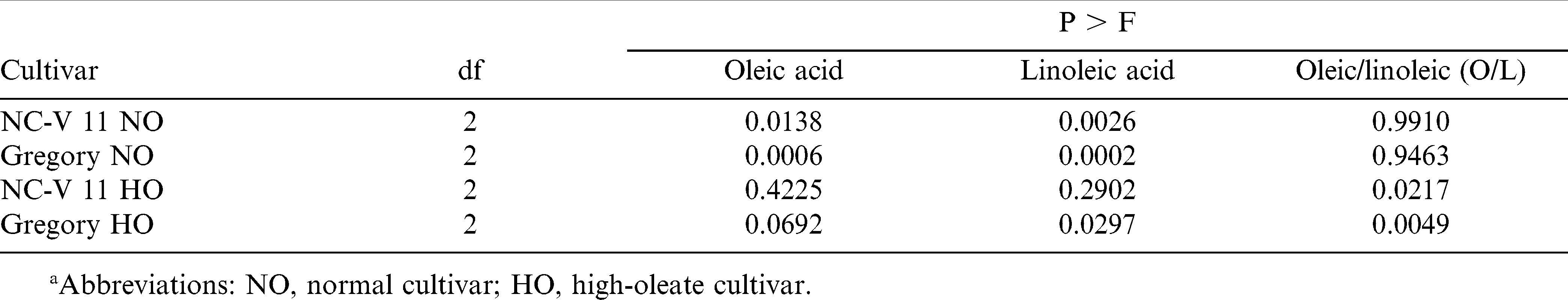
Interaction of temperature by cultivar was significant for whole seed oleic acid and linoleic acid levels (Table 5). Therefore, temperature effect was sliced by cultivar (Table 7). The oleic acid level of all four cultivars increased when the temperature increased from 22/18 C to 30/26 C for both cultivars. For their corresponding high-oleate lines however, increases were not significant (Table 8). Linoleic acid levels of the four cultivars were reduced when the temperature increased from 22/18 to 30/26 C. The reduction was significant for NC-V 11, Gregory, and Gregory HO but not for NC-V 11 HO (Tables 6 and 7). The low growth temperature resulting in decreased oleic content and increased linoleic levels were also observed in soybean [Glycine max (L.) Merr.] (Wilson, 2004), rapeseed (Brassica mapus L.) oil (Marie et al., 1999), and sunflower (Helianthus annus L.) (Harris et al., 1978). The increase in linoleic content for seeds grown in low temperature may be due to increased activity of desaturase enzymes involved in the conversion of oleic to linoleic acid (Harris et al., 1978). Earlier studies indicated that the increase in desaturase activity at lower growth temperature could be associated with the increased availability of O2 in the tissue as a result of its greater solubility at low temperature (Harries and James, 1969).
Temperature effects on oleic/linoleic ratio varied depending on cultivar (Table 6). The O/L ratio for all cultivars increased with the increased growth temperature, however, only the increases for the high-oleate lines, NC-V 11 HO and Gregory HO were significant. The average O/L ratio of these two normal cultivars grown in 22/18 C, 26/22 C, and 30/26 C, measured 1.3, 1.5, and 1.9, respectively. The O/L ratio for their high-oleate pairs increased from 13.8 when grown in 22/18 C to 16.2 when grown in 26/22 C and 25.4 when grown in 30/26 C (Tables 5– 7).
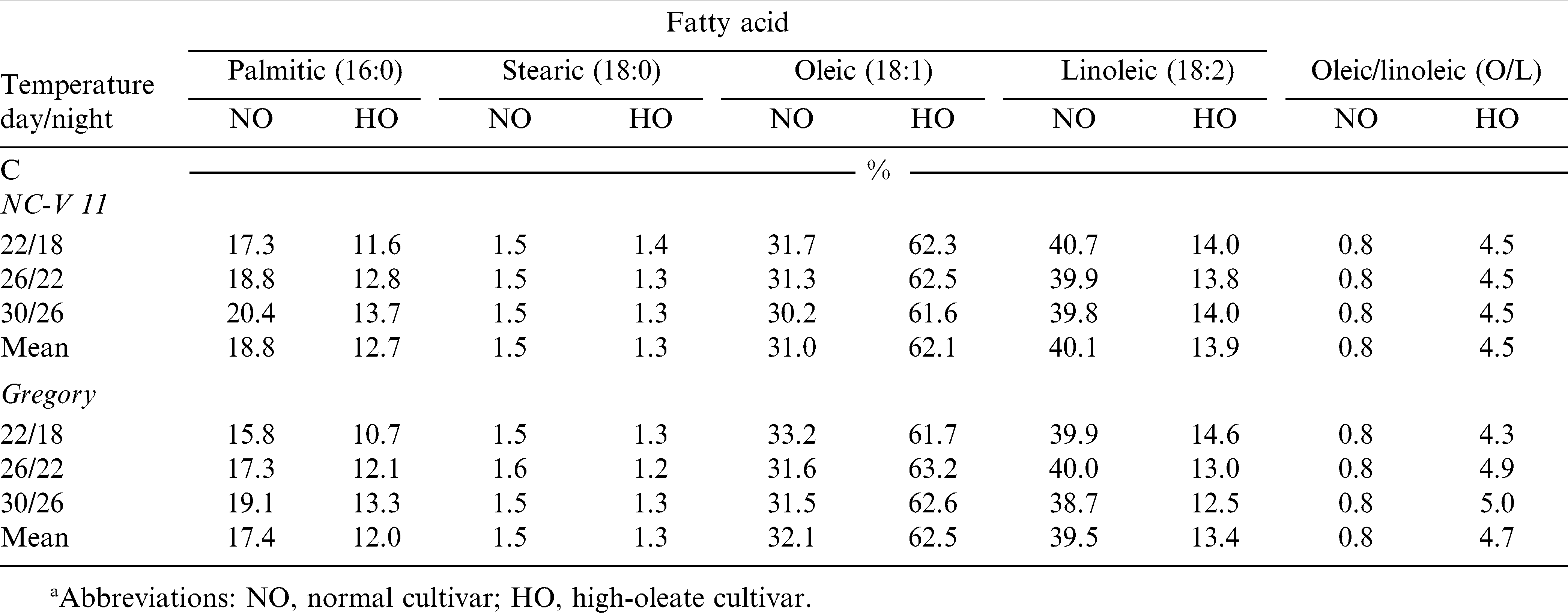
Main effect of temperature and the interaction of temperature by cultivar were not significant for axis lipid fatty acid composition or O/L ratio, except for the temperature effect on palmitic acid. When averaged across the three temperature regimes for NC-V 11, NC-V 11 HO, Gregory, and Gregory HO, palmitic levels were 18.8, 12.7, 17.4, and 12.0%, respectively. The average levels of palmitic acid across the four cultivars were 13.9, 15.3, 16.6% in three temperature regimes, 22/18, 26/22, and 30/26 C. Thus, seed produced in the low growth temperature had significantly lower amounts of palmitic acid in their axis (Tables 5 and 8).
The high-oleate trait expressed in the oils of the whole seed (primarily storage oils) was also found in the total lipid fraction of embryonic axis. The average oleic acid content for whole seed of NC-V 11 and Gregory was 47.5 and 51.5% (Table 6) and for their axis, these values were 31.0 and 32.1% (Table 8). Similarly, whole seed values of oleic for NC-V 11 HO and Gregory HO were 80.3 and 80.2%, while axis values were 62.1 and 62.5%. Likewise, the lower linoleic acid levels in the high-oleate whole seed lipids were also expressed in the axis total lipid fraction. The respective changes in axis oleic and linoleic acid content are reflected in the O/L ratio. While the whole seed O/L ratio of the high-oleate cultivars was much greater than that of the axis, these data show that axis O/L ratio is much higher for the high-oleate lines than for their paired normal lines.
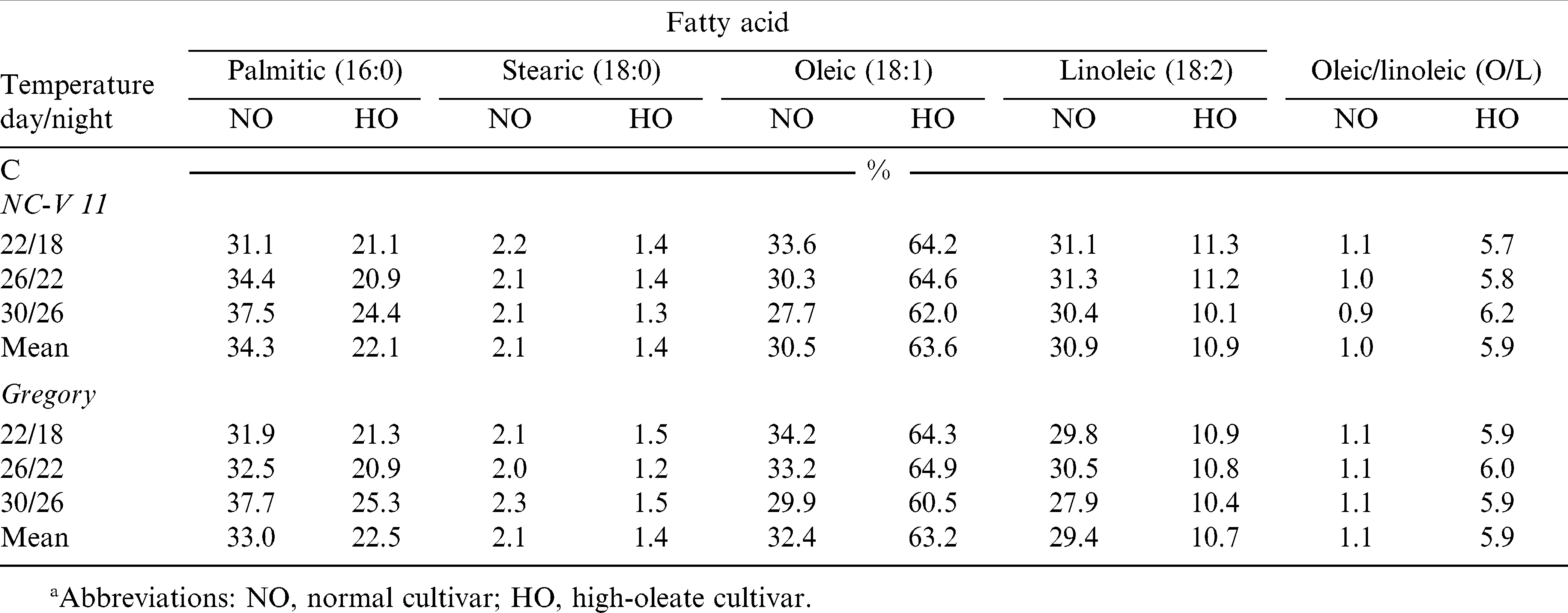
The effects of temperature on axis phospholipid fatty acids were similar to the effects on axis total lipid composition. There were no significant interactions between temperature and cultivar on any axis phospholipid fatty acid components, nor on O/L ratio. Except for stearic acid, temperature had no significant effect on axis phospholipids fatty composition or O/L ratio (Tables 5 and 9). Like the axis total lipid fraction, NC-V 11 HO and Gregory HO axis phospholipids had much higher levels of oleic acid (63.6 and 63.2%) compared to their normal paired cultivars (30.5 and 32.4%). The comparable reduction in linoleic acid content was also seen and reflected in the O/L ratio.
Phospholipids are primarily located in membranes, and membrane function and fluidity are greatly influenced by fatty acid composition. Membranes with high levels of unsaturated fatty acids (18∶2) are more fluid and more functional (Somerville, 1992). This research shows that the high-oleate trait is expressed, not only in the storage lipids of the cotyledons and axis, but also the membranes of the axis. The embryonic axis is the seed component where seedling growth begins. Therefore, it is possible that changing axis membrane fatty acid composition and function will impact seedling growth rate and the ability of the seedling to tolerate less than optimum growth temperatures. Low seed supply in this study prevented accurate evaluation of seed quality. NC-V 11 and NC-V 11 HO seed from the 2004 study were analyzed for electrical conductivity of seed soak water (data not shown). These data indicate that the high-oleate line had higher conductivity (lower seed vigor) than the normal cultivar at all production temperature regimes and that conductivity increased five-fold for seeds grown in the 22/18 C environment compared to those grown in 30/26 C. Sun et al. (2005) noted that four out of six high-oleate peanut lines were lower in vigor than their paired normal cultivar in field experiments conducted in 2003 and 2004.
Conclusions
This research indicates that low growth temperature affected whole seed fatty acid composition, but had little or no effect on axis total or phospholipid fatty acids. Seed grown in low temperatures had decreased whole seed oleic and increased linoleic content, resulting in a decrease in the O/L ratio for seeds grown in cooler temperatures. For this research, data supports that there was significant temperature by cultivar interactions for the O/L ratio. The whole seed O/L ratio of high-oleate cultivars decreased significantly with decreasing growth temperature, but the corresponding decrease in the normal cultivars was not significant. The high oleate trait seen in the storage lipids of the whole seed was also expressed in the embryonic axis. Data indicated a significant increase in the O/L ratio of NC-V 11 HO and Gregory HO axis total lipids and axis phospholipid when compared to their normal paired cultivars. Further studies are needed to determine if the change in axis phospholipids O/L ratio seen in the high-oleate lines is responsible for any change in membrane function and peanut seed vigor.
Acknowledgements
This research was supported financially by the North Carolina Peanut Growers Association.
Literature Cited
Ahmed, E.M. and Young, C.T. 1982 Composition, quality, and flavor of peanuts, pp 655 – 688 In: Pattee, H.E. and Young, C.T. (eds.) , Peanut Science and Technology Am. Peanut Res. Educ. Soc., Inc. , Yoakum, TX .
[AOSA] Association of Official Seed Analysts 2002 Seed vigor testing handbook. No. 32 Assoc. Official Seed Analysts , Las Cruces, NM .
Braddock, J.C. Sims, C.A. and O'Keefe, S.F. 1995 Flavor and oxidative stability of high- and normal-oleic acid peanuts J. Food Sci., 60 : 489 – 493 .
Branch, W.D. Takayama, T. and Chinnan, M.S. 1990 Fatty acid variation among U.S. runner-type peanut cultivars J. Am. Oil Chem. Soc. 67 : 591 – 593 .
Copeland, L.O. and McDonald, M.B. 2001 Seed vigor and vigor testing, pp. 165 – 191 In: Principle of seed science and technology. 4th edition Kluwer Academic .
Craufurd, P.Q. Vara Prasad, P.V. and Simmerfield, R.J. 2002 Dry matter production and rate of change of harvest index at high temperature in peanut Crop Sci. 42 : 145 – 151 .
Downs, R.J. and Thomas, J.F. 1991 Phytotron procedural manual for controlled-environment research at the Southeastern Plant Environment labotory. Technical Bulletin, 224 (revised) North Carolina State University , Raleigh .
Dwivedi, S.L. Nigam, S.N. Jambunathan, R. Sahrawat, K.L. Nagabhushanam, G.V.S. and Raghunath, K. 1993 Effect of genotypes and environments on oil content and oil quality parameters and their association in peanut (Arachis hypogaea L.) Peanut Sci. 20 : 84 – 89 .
Egli, D.B. TeKrony, D.M. Heitholt, J.J. and Rupe, J. 2005 Air temperature during seed filling and soybean seed germination and vigor Crop Sci. 45 : 1329 – 1335 .
Guerke, W.R. 2005 Evaluating peanut seed vigor Seed Technol. 27 : 121 – 126 .
Harris, P. and James, A.T. 1969 Effect of low temperature on fatty acid biosynthesis in seeds Biochem. Biophys. Acta 187 : 13 – 18 .
Harris, H.C. McWilliam, J.R. and Mason, W.K. 1978 Influence of temperature on oil content and composition of sunflower seed Aust. J. Agric. Res. 29 : 1203 – 12 .
Isleib, T.G. 1997 Registration of ‘NC 12C’ peanut Crop Sci. 37 : 1976 .
Isleib, T.G. Rice, P.W. Mozingo, R.W. Mozingo, R.W. and Pattee, H.E. 1999 Registration of ‘Gregory’ peanut Crop Sci. 39 : 1526 .
[ISTA] International Seed Testing Association 1995 H.A. van de Venter (ed.). Seed Vigour Testing Seminar, Copenhagen. 7 June 1995 ISTA , Zurich .
James, S.L.H. and Young, C.T. 1983 Comparison of fatty acid content of imported peanuts J. Am. Oil Chem. Soc. 60 : 945 – 94 .
Keigley, P.J. and Mullen, R.E. 1986 Changes in soybean seed quality from high temperature during seed fill and maturation Crop Sci. 26 : 1212 – 1216 .
Ketring, D.L. 1984 Temperature effects on vegetative and reproductive development of peanut Crop Sci. 24 : 877 – 882 .
Knauft, D.A. Moore, K.M. and Gorbet, D.W. 1993 Further studies the inheritance of fatty acid composition in peanut Peanut Sci. 20 : 74 – 76 .
Leong, S.E. and Ong, C.K. 1983 The influence of temperature and soil water deficit on the development and morphology of groundnut (Arachis hypogaea L.) J. Exp. Bot. 34 : 1551 – 1561 .
Marie, A. Blondel, T. and Renard, M. 1999 Effect of temperature and water stress on fatty acid composition of rapeseed oil Proc. 10th International Rapeseed Congress , Canberra, Australia.
Matthews, S. and Powell, A.A. 1987 Electrical conductivity test, pp. 49 – 57 In: Perry, D.A. (ed.) Handbook of Vigor Test Methods, 2nd ed ISTA , Zurich, Switzerland .
McDonald, M.B. 1994 The history of seed vigor testing J. Seed Tech. 17 : 93 – 100 .
Mugendi, J.B. Sims, C.A. Gorbet, D.W. and O'Keefe, S.F. 1998 Flavor stability of high-oleic peanuts stored at low humidity J. Am. Oil Chem. Soc. 75 : 21 – 25 .
Norden, A.J. Gorbet, D.W. Knauft, D.A. and Young, C.T. 1987 Variability in oil quality among peanut genotypes in the Florida breeding program Peanut Sci. 14 : 7 – 11 .
Norden, A.J. Smith, O.D. and Gorbet, D.W. 1982 Breeding of the cultivated peanut, pp. 95 – 122 In: Pattee, H.E. and Young, C.T. (eds.) Peanut Science and Technology Am. Peanut Res. Educ. Soc. , Yoakum, TX .
O'Keefe, S.F. Wiley, V.A. and Knauft, D.A. 1993 Comparison of oxidative stability of high- and normal-oleic peanut oils J. Am. Oil Chem. Soc. 70 : 489 – 492 .
Savage, G.P. and Keenan, J.I. 1994 The composition and nutritive value of groundnut kernels . pp. 173 – 213 In: Smart, J. (ed.) The Groundnut Crop: A Scientific Basis for Improvement Chapman and Hall , London .
Somerville, C. 1992 Direct tests of the role of membrane lipid composition in low-temperature-induced photo inhibition and chilling sensitivity in plants and cyanobacteria . pp. 6215 – 6218 In: Proc. Nat. Acad. Sci. USA.
Spears, J.F. TeKrony, D.M. and Egli, D.B. 1997 Temperature during seed filling and soybean seed germination and vigour Seed Sci. Technol. 25 : 233 – 244 .
Spears, J.F. Jordan, D.L. and Bailey, J.E. 2002 Peanut seed production - a guide for producers of Virginia-type peanut seed N.C. Coop. Ext. Serv. Bull. No. 622 .
Sun, M.H. 2005 Effect of production environment on seed quality of normal and high-oleate large seeded Virginia-type peanut (Arachis hypogaea L.) In: Seed Quality Issues Associated with High-Oleic Peanut NCSU .
TeKrony, D.M. Egli, D.B. Balles, J. Tomes, L. and Stuckey, R.E. 1984 Effect of date of harvest maturity on soybean seed quality and Phomopsis sp. seed infection Crop Sci. 24 : 189 – 193 .
Williams, E.J. and Drexler, J.S. 1981 A non-destructive method for determining peanut pod maturity Peanut Sc. 8 : 134 – 141 .
Wilson, R.F. 2004 Seed composition, pp. 621 – 629 In: Soybeans: Improvement, Production, and Uses. 3rd edition Boerma, H.R. and Specht, J.E. (eds.) Madison, Wisconsin, USA .
Wynne, J.C. Beute, M.K. Bailey, J. and Mozingo, R.W. 1991 Registration of ‘NC 10C’ peanut Crop Sci. 31 : 484 .
Wynne, J.C. Coffelt, T.A. Mozingo, R.W. and Anderson, W.F. 1991 Registration of ‘NC-V 11’ peanut Crop Sci. 31 : 484 – 485 .
Wynne, J.C. Mozingo, R.W. and Emery, D.A. 1979 Registration of ‘NC 7’ peanut (Reg. No. 22) Crop Sci. 19 : 563 .
Wynne, J.C. Mozingo, R.W. and Emery, D.A. 1986 Registration of ‘NC 9’ peanut Crop Sci. 26 : 197 .
Zeile, W.L. Knauft, D.A. and Kelly, C.B. 1993 A rapid non-destructive technique for fatty acid determination in individual peanut seed Peanut Sci. 20 : 9 – 11 .
Notes
Author Affiliations
* Former Graduate Research Assistant, Professor, Professor, Professor, Research Technician, Research Technician, and Research Technician, Department of Crop Science, Box 7620, North Carolina State University, Raleigh, NC 27695. Corresponding author's e-mail: david_jordan@ncsu.edu.