Introduction
Peanut (Arachis hypogaea L.) is an important commercial crop throughout the world and is one of the most susceptible crops to Aspergillus flavus invasion (Guo et al., 2009). Aflatoxins are highly toxic and carcinogenic mycotoxins produced as a result of secondary metabolism of four species of Aspergillus: A. flavus, A. parasiticus, A. nomius, and A. tamarii (Payne, 1992). Among these four species, A. flavus and A. parasiticus are the most extensively studied aflatoxigenic fungi, and A. flavus is the most common species involved in pre-harvest aflatoxin contamination of crops (Cary and Ehrlich, 2006). Aflatoxins contaminate peanuts either in the field (pre-harvest) if severe late season drought occurs or during storage (post-harvest) if improper moisture and temperature conditions exist. Pre-harvest aflatoxin contamination is a major economic problem for the peanut industry. Although aflatoxins do not directly cause yield reduction, they are heavily monitored and regulated to ensure crop safety due to their hepatotoxicity and carcinogenicity (van Egmond, 1995). This results in significant financial losses to producers due to substantial price reductions received for their crop and also to buyers, as contaminated peanuts cannot be sold for full value.
Aflatoxin contamination of peanuts mostly occurs through direct invasion of developing peanut pods by A. flavus and eventual contamination of the kernel with aflatoxin (Diener et al., 1987). Drought and high temperatures are conducive to seed infection by A. flavus and contamination with aflatoxins (Guo et al., 2005a). When plants are subjected to late season drought, particularly during the 4 to 6 weeks prior to harvest, aflatoxin contamination can be a severe problem (Sanders et al., 1985). Drought and temperature stress also causes several physiological (decreased photosynthesis, enzyme activity, and production of biocompetitive products such as phytoalexins, phenols, etc.) and compositional changes (decreases in membrane lipids and increases in carbohydrate contents) in plants (Kambiranda et al., 2011).
Simple sugars such as glucose, sucrose and fructose, which are primary peanut soluble sugars, are reported to be excellent carbon sources for aflatoxin biosynthesis under in vitro conditions (Davis et al., 1967; Mateles and Adye, 1965). During periods of drought and temperature stress, carbohydrate content of peanut seed increases (Musingo et al., 1989). In addition, immature seed have higher sugar content than mature seed and aflatoxin contamination occurs more often in these immature seed (Manda et al., 2004). Pickett (1949) was more specific when he reported that total sugar content of Virginia type peanuts ranged from 3.6 to 9.6% dry weight (DW) in immature seed compared to 1.9 to 4.4% DW in mature seed. Pattee et al. (2000) reported total sugar composition of Virginia- and runner-type peanuts as 3.5 and 2.9% DW, respectively. Thus, total sugars in peanuts range from approximately 2 to 10% DW. In all of these studies, sucrose was the predominant sugar found in peanuts, with the reducing sugars glucose and fructose often comprising < 0.5% DW of the peanut. Reported differences in carbohydrate content are important since Manda et al. (2004) found significant positive correlations between peanut aflatoxin levels and sucrose, fructose, and glucose content of peanuts at harvest. Carbohydrate content in developing or drought-stressed peanut seed may play a crucial role in the production of aflatoxin. Therefore, the objectives of the current study were to evaluate the effect of sugar concentrations in peanut seed on the growth of and aflatoxin production by A. flavus under in vitro conditions; and to determine the effects of drought on aflatoxin contamination and soluble sugar composition of peanut seed in greenhouse and field conditions.
Materials and Methods
In vitro studies
Fungal strain and cultural conditions
Strain NRRL 3357 of A. flavus was obtained from Microbial Genomics and Bioprocessing Unit, National Center for Agricultural Utilization Research, USDA, Peoria, IL. The fungus was grown in peptone basal media (peptone 5 g, KH2PO4 1 g, NaCl 30 g, MgSO4·7H2O 0.5 g, distilled water 1 L) containing different concentrations of glucose, fructose, and sucrose (described below). Fungal inoculation to the medium was performed by collecting conidia from a one week old A. flavus culture, adjusting the conidial suspension to 2 × 105 per ml, and adding 1 ml of this conidial suspension to each medium. Flasks of inoculated media were incubated at 28 C with continuous agitation for specified time periods (described below).
Effects of sucrose and reducing sugars (Experiment 1)
Three concentrations (0, 0.1, and 0.3% (w/v)) of reducing sugars (equal parts glucose and fructose), three concentrations of sucrose (0, 2, and 4%), and three incubation periods (7, 10 and 13 days) were evaluated. These treatments were combined in a factorial arrangement for a total of 27 treatments with 2 replications. The amount of media in each flask was 25 ml. Each of the flasks were inoculated with conidia and incubated as described previously. Following removal of mycelial mats (mycelial growth on surface of the liquid broth) after each incubation interval, media in each flask were assayed for aflatoxins. Mycelial mats were dried and weighed for total biomass.
Effects of sucrose and incubation period (Experiment 2)
Six concentrations of sucrose (0, 2.5, 3, 5, 10, and 20% (w/v)) were evaluated after each of three incubation periods (7, 12, and 17 days). Each treatment (sucrose concentration) was replicated 12 times. Similar to experiment 1, each flask with 25 ml media was inoculated with 1 ml spore suspension (105 conidia) and incubated at 28 C with continuous agitation. After each incubation period, four flasks of each sucrose concentration were harvested by removing and weighing dried mycelial mats and analyzing aflatoxins as described below. Total aflatoxins (sum of B1, B2, G1, and G2) and mycelial dry weights were recorded on a per flask basis.
Effects of sucrose and media modification (Experiment 3)
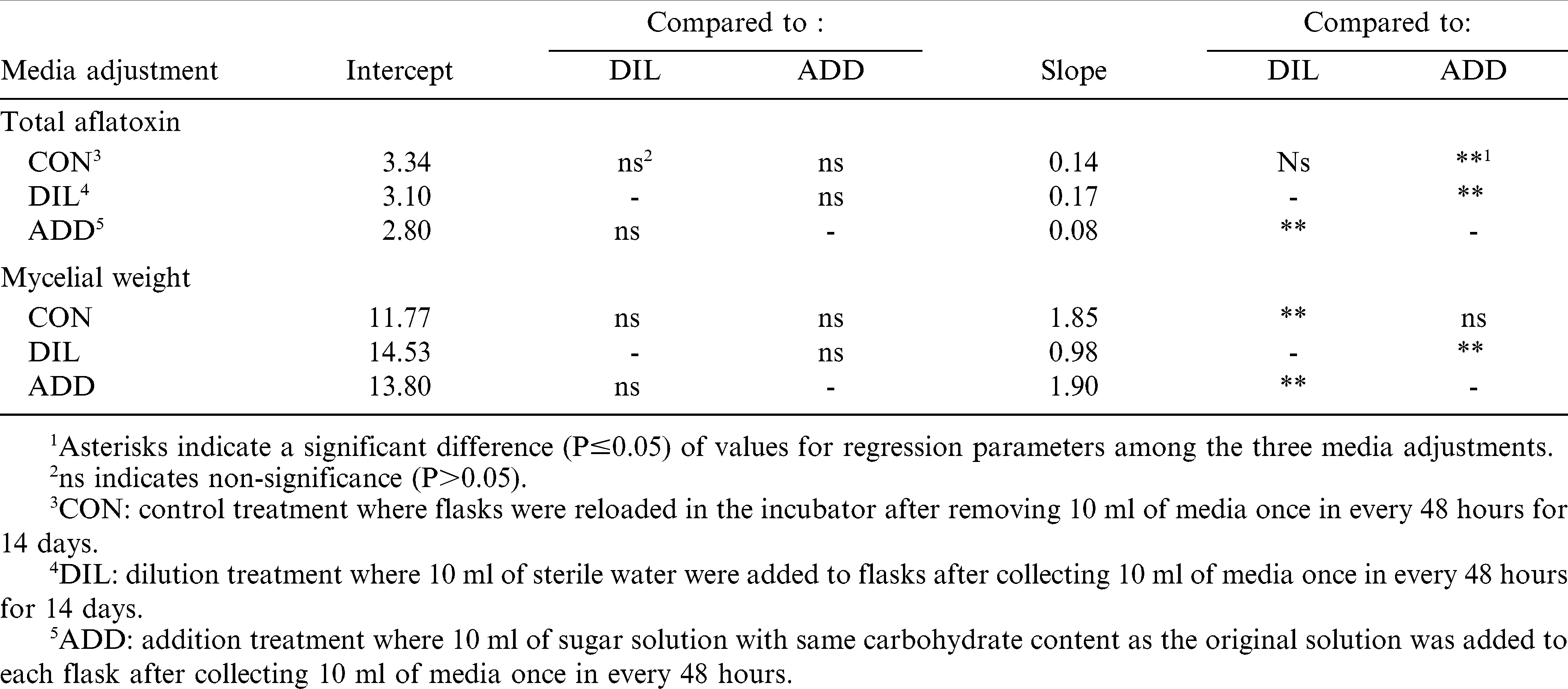
A factorial experiment was implemented using five concentrations of sucrose (0, 2.5, 5, 7.5, and 10% (w/v)) each with 0.4% reducing sugars (equal parts glucose and fructose) and three types of media adjustments/treatments. Media with each concentration of sucrose were distributed into nine flasks (three for each media adjustment) with 100 ml per flask. Flasks, which were inoculated with 4 × 105 conidia and incubated at 28 C, were shaken for one hour each morning and evening. Every 48 hours, 10 ml of media were removed from each flask. Flasks from the control (CON) treatment were returned to the incubator after removing the 10 ml. For the dilution (DIL) media treatment, 10 ml of sterile water were added to flasks after the 10 ml sample was removed. In the addition (ADD) treatment, 10 ml of sugar solution, with same sugar content as the original solution, replaced that which had been removed. The same procedure was repeated for 14 days. Collection of 10 ml from each flask, and addition of water (DIL) and sugar (ADD) were done merely to adjust the sucrose concentrations of media. After 14 days of incubation, media remaining in the flasks were evaluated for aflatoxins and mycelial dry weights were determined. Total aflatoxins (sum of B1, B2, G1, and G2) per 25 ml of the media are reported.
Greenhouse experiment
An experiment with three durations of imposed drought (0, 35, and 60 days before estimated harvest date (DBH)) was conducted at the Plant Science Research Center at Auburn University from 2 Feb. 2010 to 13 July 2010. Each treatment consisted of twenty-five pots. Experimental units (pots) within each drought treatment were randomized. Seven gallon plastic pots, 42 cm wide × 28 cm deep, were each filled with 28 kg of soil. The soil was loamy sand with 82.8% sand, 9.5% silt, and 7.7% clay. Pots were placed on greenhouse benches, where temperatures ranged between 25 to 30 C. Georgia Green cultivar (Branch, 1995) seeds were sown into each pot, and watered as needed until early pod fill.
Sterilized corn grits (500 g) in 1000 ml flasks were inoculated with 50 ml (1 × 105 conidia ml−1) of aqueous conidial suspension from one week old A. flavus strain NRRL 3357. Grits were incubated at room temperature and shaken for 15 min twice a day for three weeks before use. At 75 days after sowing (DAS), plants were inoculated uniformly by sprinkling 10 g of A. flavus-infested corn grits to the soil surface under the canopy in each pot, followed by gentle manual incorporation into the top layer of soil. Plants were irrigated regularly until 90 DAS. After 90 days, plants were subjected to the aforementioned watering schedules to reflect the three drought scenarios. Drought treatments were imposed for 35 and 60 DBH by withholding water; minimal water was provided to individual plants in order to maintain turgidity. Control treatment ( = no drought) plants were copiously watered every other day. Soil moisture was monitored by soil moisture probes (Watchdog Series 1000, Spectrum Technologies Inc., East-Plainfield, IL); greenhouse temperatures were also monitored. Fungicides and insecticides were applied as recommended by Alabama Co-operative Extension System. Upon maturity, peanuts were hand harvested, shelled, and ground into meal which was then assayed for sugar contents and aflatoxins.
Field experiments
One field experiment was conducted at E.V. Smith Research Center (near Tallassee, in east central AL) from 20 May 2010 to 23 Oct. 2010. The experiment consisted of a randomized complete block with drought, rainfed (i.e., no drought), and non-inoculated/rainfed treatments, each replicated four times. Plots consisted of four rows of peanuts on 0.9 m spacing and 5.5 m in length. Soil type at this site was a sandy loam with 76.3% sand, 16.3% silt, 7.5% clay. Five to six ‘Tifguard’ (Holbrook et al., 2008) peanut seeds were sown per 0.3 m. Multiplication of inoculum was performed as previously described and plants were inoculated with 180 g infested corn grits per row at 75 DAS by sprinkling over the foliage then manually dislodging particles from foliage to the soil surface. Rain-out shelters (3.7 × 2.7 m) were installed over the three rows of drought plots at 100 DAS, which was estimated to be 55 DBH for the cultivar Tifguard. Rain-out shelters consisted of plastic sheeting stretched over arched polyvinyl chloride (PVC) pipes.
Another field experiment was conducted at the Wiregrass Research and Extension Center, Headland, in southeastern AL, from 22 May 2010 to 25 October 2010. Experimental plot sizes were 4.6 × 6.4 m, consisting of eight rows at 0.9 m spacing. Peanut cv. ‘Tifguard’ seeds were sown at the rate of five to six per 0.3 m. Only the center four rows were used for sampling. The experiment involved a drought treatment and a control (rainfed), each replicated four times. Soil type was loamy sand with 83.8% sand, 11.3% silt and 5% clay content. Plants were inoculated by uniformly sprinkling 150 g of infested corn grits over each row at 75 DAS. Rain-out shelters were installed over the drought treatment plots at 100 DAS. The dimensions of the rain-out shelters were 3.7 × 2.4 m and covered three rows at 0.9 m spacing.
For both field experiments, fungicides and pesticides were applied as recommended by the Alabama Co-operative Extension System. Weather data (air and soil temperatures) for the period of drought stress, mid- to late-season precipitation data for five years preceding and during the year of study for both field experiments were compiled from the Alabama Mesonet (AWIS, Inc.). Leaf surface temperature under rain-out shelters were monitored occasionally to verify that these structures did not substantially increase heat stress on plants using a handheld infrared thermometer (Fluke Corporation, Everett, WA, USA). At maturity, plants were mechanically dug by tractor and pods were handpicked from turned plants. Where rain-out shelters had been placed, peanuts were collected from the center portion of the middle covered row. Harvested peanuts were dried for three days in ambient laboratory conditions, then shelled and ground for subsequent lab assays.
HPLC analysis of total aflatoxins: a) From media
Aflatoxins were analyzed by HPLC as described by Wilson and Romer (1991) with slight modifications. Aflatoxin extraction from the media was performed with the following procedure: medium was filtered through Whatman No. 1 filter paper (GE Healthcare Inc., UK), the mycelial mat was collected and dried at 55 C for 48 hours, and dry weights were recorded. One ml of the filtrate was added to 2 ml of 90% HPLC grade acetonitrile, and incubated for one hour at room temperature, at which time the solution was purified by passing through a Mycosep Multifunctional Cleanup Column (Romer Labs, Inc., Washington, MO). Two hundred µl of the purified solution was added to 700 µl derivatizing agent (water/trifluoroacetic acid/acetic acid; 70∶20∶10 (v/v/v) and incubated at 55 C for 30 min before injection into HPLC. A commercially available Aflatoxin B and G mixture (Sigma Chemical Co, St. Louis, MO, USA) served as a standard for total aflatoxins quantification (sum of B1, B2, G1, and G2).
b) From peanut meal
Aflatoxins were extracted from ground peanut meal using the following procedure: 50 g of ground peanut meal was mixed with 100 ml of HPLC grade 90% acetonitrile in 250 ml bottles. The peanut meal with acetonitrile was shaken for one hour on a wrist action shaker then filtered through Whatman No. 1 filter paper (GE Healthcare Inc., UK). The filtrate was purified and assayed by HPLC as described in the previous section.
HPLC analysis of peanut soluble sugars
Extraction and analysis of soluble sugars from peanut kernels was performed as described by Basha (1992) with some modifications. Approximately 1 g of peanut meal was ground with 5 ml HPLC grade hexane with a mortar and pestle, and the homogenate was transferred to 15 ml centrifuge tube and covered with aluminum foil. Tubes were placed on a horizontal shaker (Barnstead Lab-Line Model: Max-Q 2508) and left overnight at room temperature with 40% reciprocal shaking. The homogenate was sonicated for 10 min (Branson, model 5510 Branson Ultrasonic Corporation, Danbury, CT) and clarified by centrifugation at 10,000 g for 15 min at 4 C. Clarified supernatant was decanted and the pellet was retained and re-extracted with an additional 5 ml of hexane. The supernatant was recovered and the presence of oil was determined by pipetting an aliquot of supernatant on to Whatman No. 1 filter paper. If the presence of oil was detected, then an additional hexane defatting step was repeated. Alternatively, in the absence of oil, the pellet was air dried and approximately 0.5 g of this pellet was added to 5 ml of 80% HPLC grade ethanol in a Corex™ centrifuge tube (Corning, NY) and sonicated for 30 min followed by a one minute vortex. Centrifuge tubes were shaken on a horizontal shaker for 30 min followed by centrifugation at 10,000 g at 4 C for 15 min. Clarified supernatant was collected and the extraction procedure was repeated with an additional 5 ml of 80% HPLC ethanol. Supernatants were combined and retained and the pellet discarded. Finally, the combined supernatant was filtered through 0.2 µm nylon membrane filter (VWR, Batavia, IL) into chromatography vials (Agilent Technologies Inc., Santa Clara, CA), and analyzed immediately for soluble sugar content. Sucrose, glucose, and fructose composition were determined by HPLC (Shimadzu Scientific Instruments Inc., Columbia, Maryland) with a Bio-Rad Aminex HPX-87P 300 × 7.8 mm pre-packed HPLC carbohydrate analysis column with 9 µm particle size (Hercules, CA); flow rate, 0.6 ml min−1; isocratic de-ionized water mobile phase; 85 C column temperature; and detection with a refractive index (RI) detector.
Data analysis
Transformations were performed when data did not fit a normal distribution. Data analysis was carried out with SAS 9.1.3 (SAS Institute Inc., Cary, NC, USA). Total aflatoxin and mycelial dry weights were analyzed by regression analysis for each experiment. Regression analysis was performed using total aflatoxin and mycelial weight data, as well as aflatoxins per gram of mycelium for the second and third experiments, as dependent variables and sugar concentrations (sucrose and total reducing sugars) as independent variables. For total aflatoxin data in Experiment 2, both simple and quadratic regression models were tested in order to determine the best model fit. Selection of model was based on the values of co-efficient of determination (R2) and distribution of data points. Regression models were considered significant if P≤0.05. In addition, intercepts and slopes of the regression equations in each experiment were tested for differences due to incubation intervals, and for media adjustments in experiment 3, by calculating confidence intervals with P = 0.05. If confidence intervals did not include zero, model parameters (intercepts, slopes) were considered significantly different. If zero was included in the confidence intervals for both parameters, the models were considered similar and data were combined for determination of a single regression equation.
Data on peanut aflatoxins and soluble sugars from greenhouse and field experiments were analyzed by generalized mixed linear model analysis (PROC MIXED; SAS 9.1) for differences due to drought. Mean separations were performed using Fisher's protected least significant difference (LSD) test (P≤0.05). After statistical analysis, means were back-transformed as needed for presentation herein. Relationships between total aflatoxins and peanut sugars were evaluated by correlation analysis. Correlation was considered significant if P≤0.1.
Results and Discussion
Effect of reducing sugars on total aflatoxins and mycelial dry weights
In experiment 1, transformation of data on aflatoxins (ln(1+ppb)) and mycelial weights (ln(mw)) was done to stabilize variance. No differences in total aflatoxins due to total reducing sugars (P = 0.41) nor due to the interaction of sucrose and total reducing sugars (P = 0.33) were noted. Mycelial dry weights also did not differ due to concentrations of reducing sugars (P = 0.53) or the interaction of sucrose and reducing sugars (P = 0.18). Our observation that reducing sugars did not impact aflatoxin levels apparently differs from results noted by others. However, the concentrations of reducing sugars used in our tests were very low (0.1 to 0.4% of equal parts glucose plus fructose) compared to other in vitro studies that used 5% or greater fructose and/or glucose concentrations (Abdollahi and Buchanan, 1981; Davis and Diener, 1968; Davis et al., 1967; Mateles and Adye, 1965). The relatively low concentrations of total reducing sugars selected for the current study were based on concentrations found in peanut seed (Pattee et al., 2000; Pickett, 1949). Since reducing sugars comparable to the levels found in peanut showed no significant effect on aflatoxin and mycelial weights of A. flavus we shifted our focus to the effects of sucrose.
Effect of incubation interval and sucrose on total aflatoxins and mycelial dry weights
In experiment 1, we noted differences in total aflatoxins due to sucrose concentrations of the media at all three incubation periods (P <0.05), however intercepts and slopes of three regression equations did not differ significantly. Thus, data from all incubation intervals and concentrations of reducing sugars were combined. The resultant regression equation shows that aflatoxins increase linearly as sucrose concentrations increase from 0 to 4% (w/v) (ln(1+ppb)) = 2.97 + 0.17(sucrose), R2 = 0.32, P = 0.0004 (data not shown). Mycelial dry weights differed significantly due to sucrose concentrations of the media at all the incubation intervals tested (P<0.0001). Slopes and intercepts of mycelial dry weight regression equations did not differ with incubation interval according to confidence interval tests with P = 0.05. Thus, mycelial dry weight data from all incubation intervals and concentrations of reducing sugars were combined. The combined regression equation shows a linear increase in mycelial dry weight as sucrose concentrations rise from 0 to 4% (w/v) (ln(mw)) = 3.15 + 0.36(sucrose), R2 = 0.85, P = 0.0001 (data not shown). Similar to aflatoxin data, mycelial dry weight differed due to increases in sucrose concentration but not due to different incubation intervals (7, 10, and 13 days). This indicates that mycelia grew significantly up to 7 days, after which little change occurred. While these results were to be expected given previous studies, our investigation differs from published literature in that we did not focus on identity (Abdollahi and Buchanan, 1981; Davis et al., 1967) or concentration (Davis et al., 1966; Shih and Marth, 1974) of sugars that maximize aflatoxin production, instead we evaluated concentrations of sugars which are similar to levels found in field-grown peanuts. It is worthy to note from these results that aflatoxins and mycelial weights increased with increases in sucrose concentration even at those low concentrations.
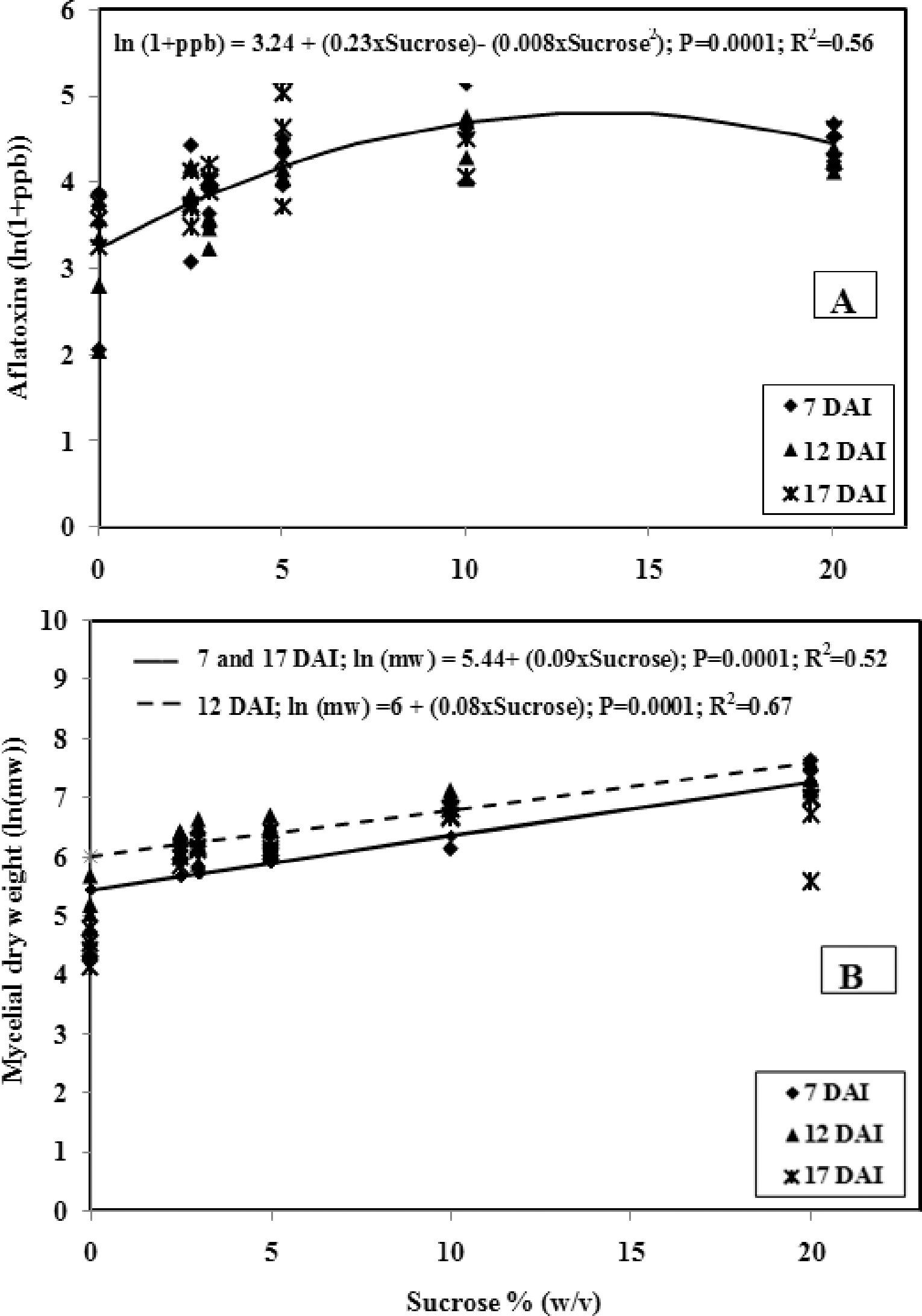
In experiment 2, the quadratic regression models (7 DAI, R2 = 0.61; 12 DAI, R2 = 0.67,); and 17 DAI, R2 = 0.51) with sucrose concentration as an independent variable and ln(1+ppb) as a dependent variable provided better fit to the data than the simple linear regression models (R2 = 0.31, R2 = 0.33 and R2 = 0.38, respectively) for each interval. We observed differences in total aflatoxins due to sucrose at 7 DAI (P<0.0001), 12 DAI (P<0.0001) and 17 DAI (P = 0.002). However, parameters of the regression models from each of the three incubation intervals tested (7, 12, and 17 DAI) did not significantly differ (P>0.05). This result agrees with those of Davis et al. (1966) who found maximal aflatoxin concentration (7.3 mg/L medium) after 5, 7 or 12 DAI, but with longer incubation aflatoxin concentrations declined. Thus, total aflatoxin data from all incubation intervals were combined for a single regression model, for which the quadratic model (R2 = 0.56) with sucrose concentration as an independent variable and ln(1+ppb) as a dependent variable was a better fit than the simple linear regression model (R2 = 0.32). Increasing levels of aflatoxins were observed with increasing sucrose concentrations up to 13.5% then toxin levels declined with further increases of sucrose concentrations (R2 = 0.56, P = 0.0001) (Fig. 1A). These results are similar to Davis et al. (1966) who evaluated increasing concentrations of sucrose (0, 1, 5, 10, 15, 20, 30, and 50%) in 2% yeast extract medium and noted increasing aflatoxin production with increasing sucrose concentrations up to 20% sucrose then decreasing aflatoxins with greater than 20% sucrose.
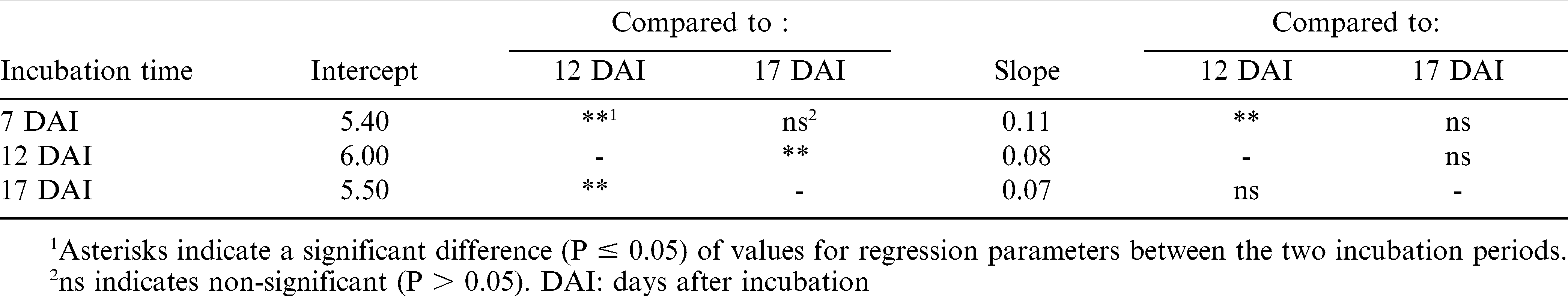
Mycelial dry weight data were also transformed with the natural logarithm to fulfill the normality criterion. Mycelial dry weights differed significantly due to sucrose concentrations of the media at all intervals tested ((7 DAI (P<0.0001); 12 DAI (P<0.0001); and 17 DAI (P = 0.0014)). Slopes and intercepts of regression models for mycelial dry weight between 7 and 17 DAI were similar. However, intercepts for the 7 and 17 DAI models, and the slope for the 7 DAI model, significantly differed (P<0.05) from the 12 DAI regression model (Table 1). The combined 7 and 17 DAI regression model had a lower intercept and higher slope compared to the model from the 12 DAI. Mycelial weights were higher at 12 DAI compared to 7 and 17 DAI indicating a decrease in mycelial weight from 12 to 17 DAI. Davis et al. (1966) also observed decreased mycelial dry weight using yeast extract sucrose medium with 20% sucrose between 12 and 15 DAI. Similar to experiment 1, mycelial dry weights were positively related to sucrose concentrations of the media (Fig. 1B). Davis et al. (1966) reported that mycelial mass didn't increase with > 10% sucrose in the media, which differs from our results as we observed linear increase in mycelial dry weights up to 20% sucrose; these differences could be due to the base medium used in each study.
Regression analysis of data on toxin per gram of mycelium with increasing concentrations of sucrose resulted in models that were not significant at various intervals tested (P>0.11). This indicates that aflatoxin production per gram mycelium was similar across the concentrations of sucrose tested.
Effects of sucrose and media modification on total aflatoxins and mycelial dry weights
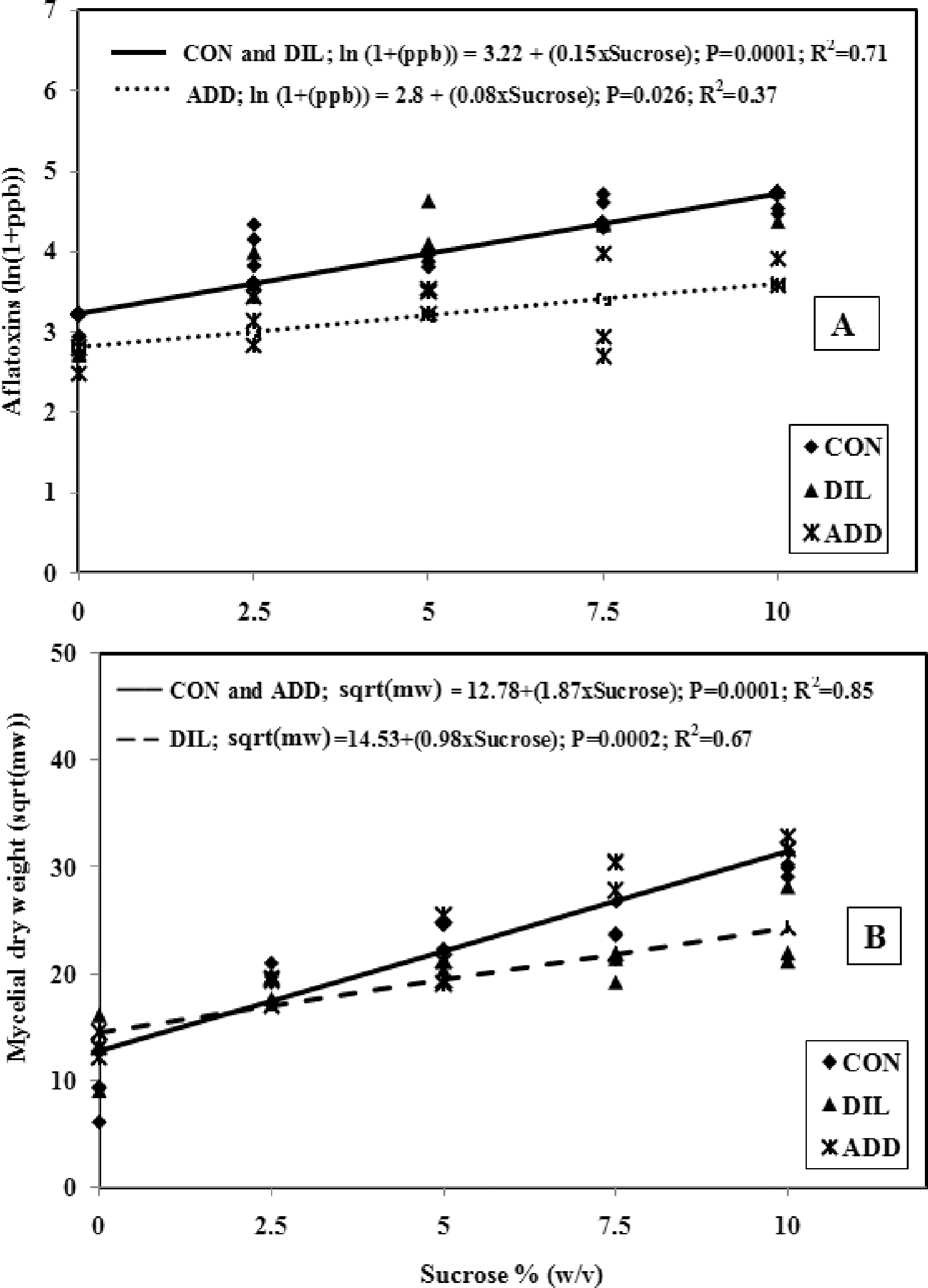
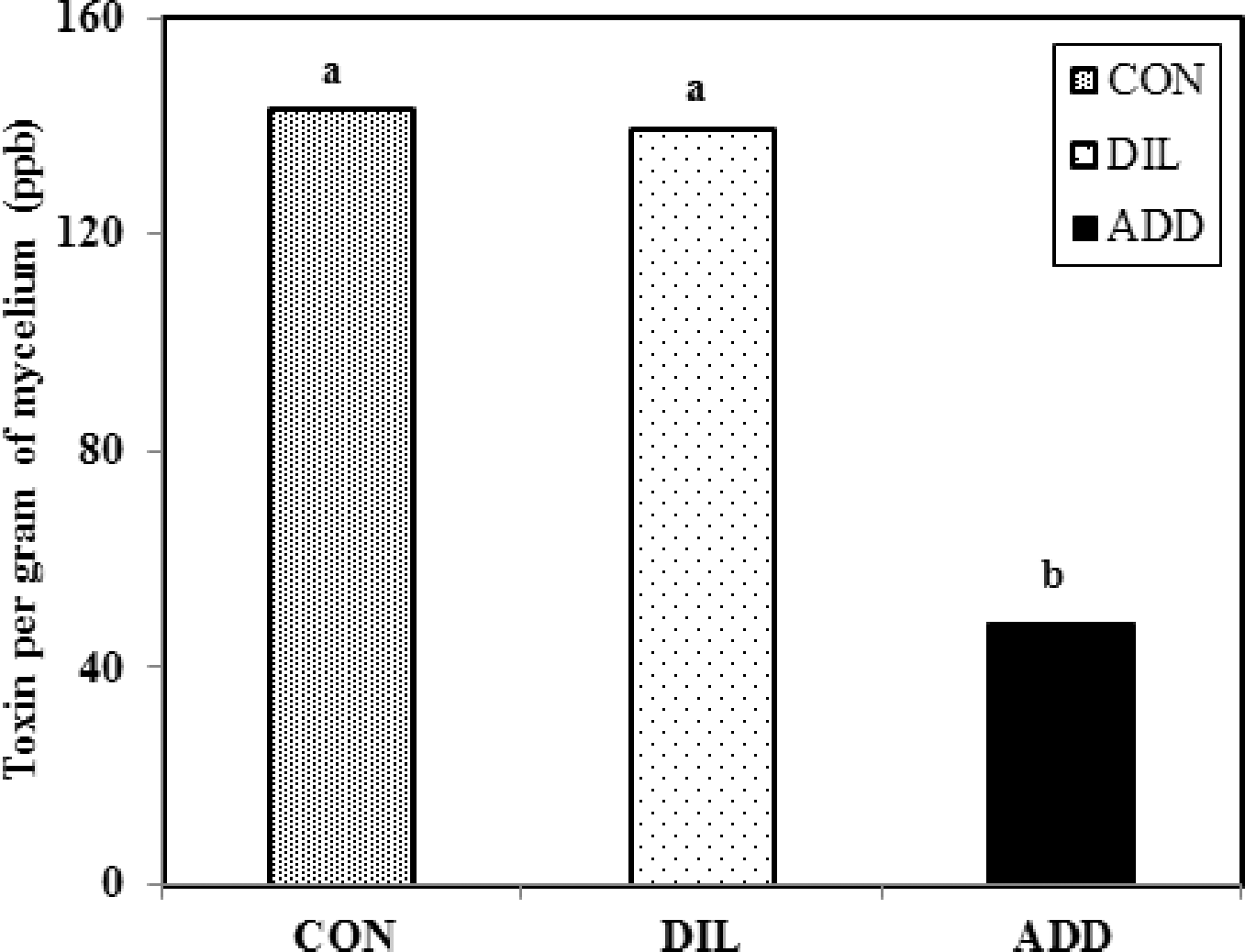
In experiment 3, transformation of data on total aflatoxins (ln(ppb +1)) was done in order to normalize the data. Total aflatoxins increased with increasing sucrose in all media adjustments ((CON (P = 0.0007); DIL (P = 0.0004); and ADD (P = 0.026)). Intercepts of regression models for aflatoxin content did not differ significantly among media adjustments; however, the slope of line for the ADD treatment was significantly (P<0.05) lower than either CON or DIL treatment slopes. There were no significant differences (P>0.05) between slopes for the CON and DIL treatments (Table 2). The regression model of the combined CON and DIL treatments (Fig. 2A) had a higher slope than that of the ADD model. This demonstrates that both the CON and DIL treatments, which over time would both have had declining sucrose concentrations (due to fungal metabolism and removal then dilution, respectively), allowed greater aflatoxin content than the ADD treatment in which sucrose was replenished. Mycelial dry weight data from the media modification experiment was normalized by square root transformation. Similar to the other two experiments, mycelial dry weights increased with increasing sucrose concentrations (Fig. 2B) in all media adjustments ((CON (P = 0.0001); DIL (P = 0.0002); and ADD (P<0.0001)). Intercepts of the three regression models did not differ (P>0.05). There were no differences in CON and ADD treatment slopes (P>0.05), but both of these were significantly (P<0.05) higher than that of the DIL treatment. A higher value for the slope of the combined regression model of CON+ADD indicates a greater rate of mycelial growth than with the DIL treatment as sucrose concentrations increase. Toxins per gram of mycelium data were normalized with natural log transformation; regression analysis of these data on sucrose concentrations resulted in models that were not significant due to various media adjustments (P>0.09). This result indicates that sucrose concentrations had no effect on aflatoxins produced per gram of mycelium weight, therefore, data were combined over sucrose concentrations and analyzed for differences due to media adjustments. Mixed model analysis showed that toxins per gm of mycelial weight after 14 days were significantly different due to various media adjustments and lower in the ADD treatment compared to the CON and DIL treatments (Fig. 3). These results are consistent with Demain's (1986) statement that there is greater aflatoxin production when there is suppression of fungal growth as would happen over time as sugar concentrations in media become depleted (i.e., through metabolism as in our CON treatment). In our in vitro studies, higher mycelial dry weights were observed in media with initially high sucrose concentrations and with replenished sucrose than in media in which sucrose was regularly depleted through removal and dilution or by utilization. Conversely, aflatoxin levels and toxin per gm of mycelia were higher after 14 days in the control media or when sucrose content of the media was regularly removed then diluted. These results indicate that depletion of sucrose over a period of time results in a growth limiting situation and favors secondary metabolite/aflatoxin production by A. flavus. Consistent replenishment of nutrients in the form of sucrose in the ADD treatment led to a high growth rate and greater fungal mass during the test period (14 days), and apparently this is suppressive for secondary metabolite production (Demain, 1986). This could also be seen in results of Experiment 2 in which aflatoxin content appeared to decline while mycelial weight increased with media sucrose content >13.5%. These results are in agreement with those of Shih and Marth (1974) who found that aflatoxin production was maximal in media that was suboptimal for fungal growth based on glucose concentrations.
Effect of sucrose concentrations and media adjustments on total aflatoxin (A) and mycelial dry weights (B) of Aspergillus flavus NRRL 3357 in peptone basal medium after 14 days of incubation at 28 C where CON was a control treatment, DIL was a dilution treatment with 10 ml sterile water added to each flask every 48 hours, and ADD was an addition treatment where 10 ml of sugar solution was added to each flask every 48 hours.
Effect of media adjustments on toxin per gram of mycelium of Aspergillus flavus NRRL 3357 in peptone basal medium after 14 days of incubation at 28 C where CON was a control treatment, DIL was a dilution treatment with 10 ml sterile water added every 48 hours, and ADD was an addition treatment with 10 ml of sugar solution added every 48 hours.
Greenhouse and field studies
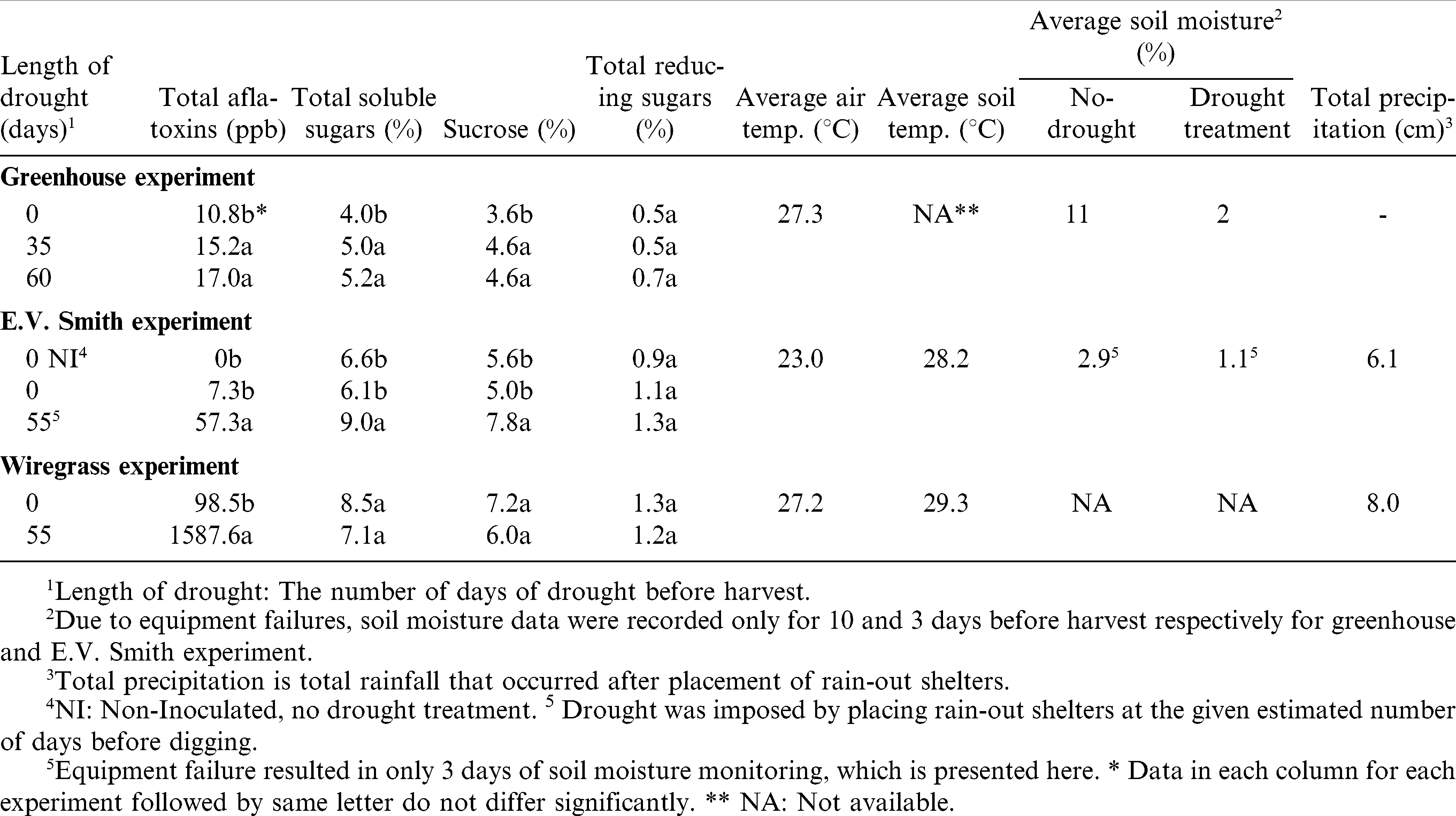
Relatively few kernels were harvested from peanut plants in containers and low levels of aflatoxins (<20 ppb) were detected in these. No data transformations were needed on total aflatoxins (ppb), as the data fit a normal distribution. Significant differences in total peanut aflatoxin levels were observed in seed from control plants versus plants subjected to drought conditions (P = 0.001). When all three treatments were compared, no differences were found between total aflatoxins in the 35 and 60 drought DBH treatments, and aflatoxins with either drought treatment was higher than without drought (Table 3).
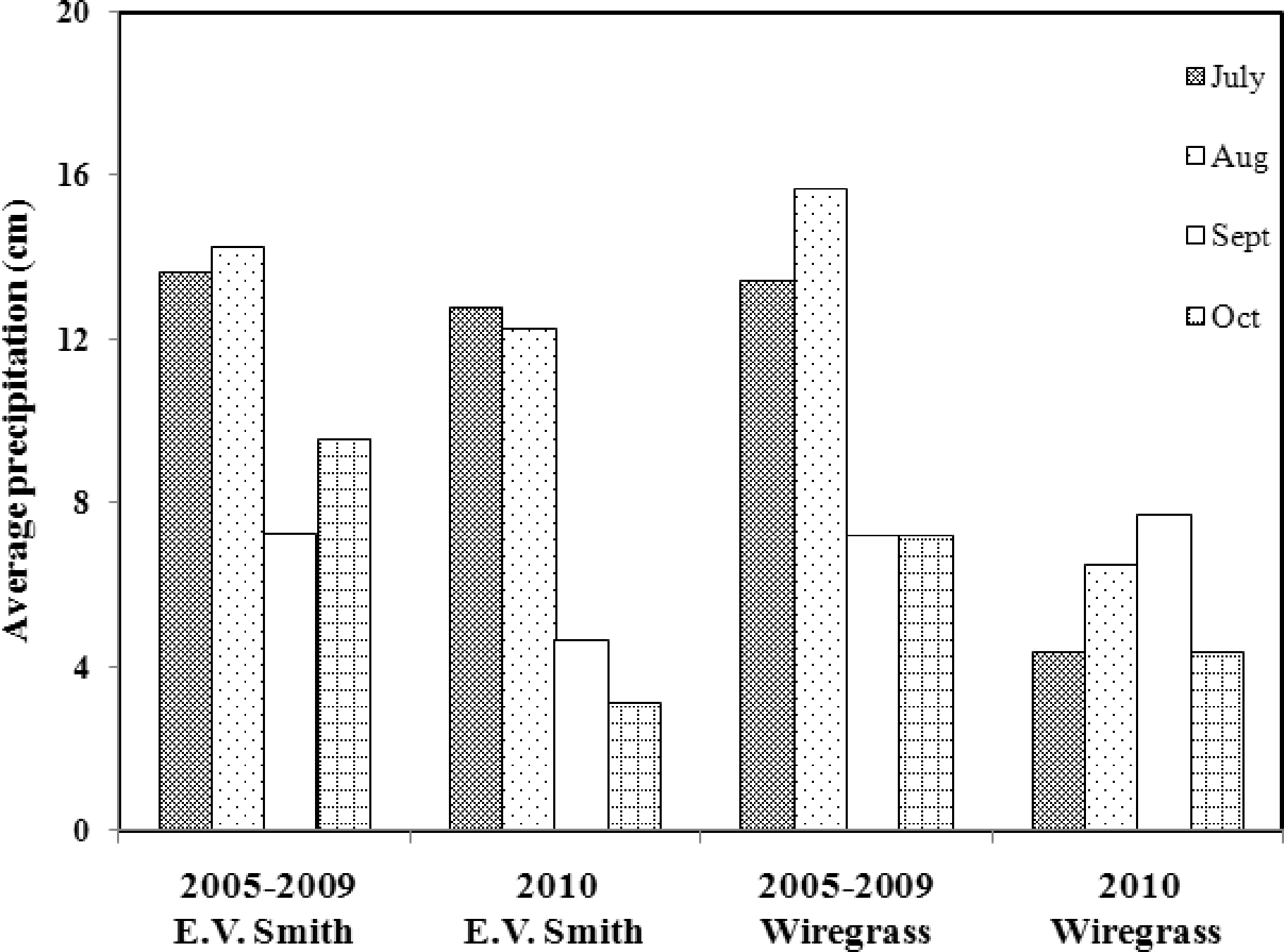
At the E.V. Smith site, the 2010 growing season was exceptionally warm and dry (Table 3; Fig. 4). Monthly rainfall amounts averaged over five years preceding this study show that it is more common to get > 7 cm rain monthly during Aug, Sept, and Oct, but < 5 cm rain was measured during Sept or Oct 2010. Moderate to high concentrations of aflatoxins were detected in harvested peanuts from this site. Normalization of total aflatoxin data was achieved by square root transformation. Mixed linear model analysis showed significant differences in total aflatoxins due to various treatments (P = 0.006). Total aflatoxins were higher in drought-stressed peanuts compared to rainfed peanuts. No aflatoxins were detected in peanuts from the non-inoculated, rainfed treatment (Table 3).
At the Wiregrass Research Center, for five years preceding our experiment, average monthly rainfall during the last four months of peanut growing season was in the range of 7 to 16 cm. Three years preceding our study, peanut seeds from this site had low or non-detectable levels of aflatoxins (Bowen, unpublished data). Since our objective was to evaluate the effects of drought on peanut sugars and, consequently, on aflatoxins, we erected rain-out shelters to assure that some plants would be exposed to drought conditions. Unlike previous years, the 2010 growing season at the Wiregrass Research Center was exceptionally warm and dry (Table 3; Fig. 4), with only 4 to 8 cm average rainfall in the last four months growing season, and high concentrations of aflatoxins were detected in peanuts from both the rainfed and drought-stressed plots. Aflatoxin data were normalized by the natural log transformation. Mixed linear model analysis revealed that aflatoxin levels differed among moisture treatments (P = 0.0002). As expected, peanuts in the drought-stress treatment had higher levels of aflatoxins than rainfed peanuts (Table 3) which is also in agreement with previous reports (Blankenship et al., 1984; Cole et al., 1985; Manda et al., 2004).
Low levels of aflatoxins in kernels from the greenhouse trial, compared to field trials, might be due to the controlled conditions and, therefore, more mild stress conditions than were found in the field. The variation in aflatoxin values between our field experiments might also be explained by weather and soil conditions. At E.V. Smith, where moderate levels of toxins were detected in peanuts, the average soil temperature after installation of rain-out shelters (i.e., during the drought period) was 28.2 C while average soil temperatures at Wiregrass during the drought period averaged 29.3 C. The average soil temperatures over 4, 5, and 6 weeks prior to harvest were 23.8, 25.6, and 27 C, respectively, for E. V. Smith, and 26.3, 27.4, and 28.3 C, respectively, for the Wiregrass site. Higher soil temperatures at Wiregrass would have been more conducive for aflatoxin accumulation than those at E. V. Smith, as similarly observed by Blankenship et al. (1984) who found high levels of aflatoxins in peanuts grown in drought-heated soil with a mean temperature of 30.5 C and somewhat lower aflatoxins concentrations with average soil temperature of 26.35 C. It is interesting to note that even though aflatoxigenic fungi can grow in wide range (12 to 48 C) of temperatures (Klich et al., 1992), the optimal temperature range for aflatoxin production is very narrow. Under controlled conditions the cardinal temperature range for aflatoxin production differs among strains and cultural conditions, with 24–30 C being reported as favorable and higher than 35 C as limiting by many researchers (Joffe and Lisker, 1969; OBrian et al., 2007; Schindler et al., 1967). Under field situations, the cardinal temperature range is reported to be higher than 26 C but less than 31 C (Cole et al., 1985; Blankenship et al., 1984) and is influenced by factors such as damage to kernels, soil moisture content, etc.
Aflatoxin contamination of peanuts can also vary substantially by soil type (Codex Alimentarius Commission, 2004); particularly under dry conditions, sandy soils favor aflatoxigenic fungal growth and aflatoxin production compared to heavier soils with higher water holding capacity (Guo et al., 2005b). The soil at E.V. Smith site is a sandy loam, while the soil at Wiregrass is loamy sand; soil moisture holding capacity of loamy sand is generally less than that of sandy loam (Scherer et al., 1996). Therefore, soil type is likely contributing to lower levels of aflatoxins at E.V. Smith than found in peanuts from the Wiregrass site.
Effect of drought on peanut soluble sugars
Data on total soluble sugar contents in peanuts from the greenhouse experiment were normalized by natural log transformation. Total soluble sugar content of peanut kernels was higher in drought-stressed compared to non-drought-stressed plants (P = 0.008). Highest total soluble sugar content was observed in peanut kernels from the 60 DBH treatment which was similar to the 35 DBH treatment, and lowest total soluble sugars were observed in 0 DBH treatment (Table 3). Sucrose data were normalized by square root transformation. Similar to total sugars, differences in sucrose content of peanut kernels were observed due to drought (P = 0.02). Peanut kernels from both drought treatments had higher sucrose content compared to non-drought-stressed peanuts (Table 3). Mean separation showed no differences in sucrose concentrations between 35 and 60 DBH drought treatments. Data on total reducing sugars (glucose and fructose) were normal hence no transformations were performed. No differences in total reducing sugars were observed due to drought (P = 0.21).
No data transformations were performed on total soluble sugars, sucrose and total reducing sugar data from E.V. Smith experiment. Mixed model analysis showed significant differences in total sugars (P = 0.03) and sucrose contents (P = 0.02) among the three treatments. However, total reducing sugars did not differ significantly (P = 0.45) (Table 3). Total soluble sugars, sucrose and total reducing sugars in peanuts from the non-inoculated, rainfed treatment differed with inoculated-drought treatment but not from the inoculated, rainfed treatment (Table 3). Both sets of results, from our greenhouse trial and the E.V. Smith experiment, are similar to those observed by Musingo et al. (1989) who found higher carbohydrate contents in drought-stressed peanuts compared to irrigated peanuts.
No data transformations were needed on total soluble sugars, sucrose and reducing sugars data from the Wiregrass site experiment. Mixed linear model analysis failed to detect differences in levels of total sugars (P = 0.22), sucrose (P = 0.39), or total reducing sugars (P = 0.66) from drought-stressed compared to rainfed peanut kernels (Table 3). Aflatoxin concentrations were excessive in peanuts in drought treatments at Wiregrass, yet sugar contents of these peanuts were numerically lower than in rainfed peanuts. Thus, these results appear to contradict those from other studies presented herein. However, it may be that the excessively high soil temperatures and dry conditions throughout the season allowed early invasion of peanuts by aflatoxigenic fungi, with subsequent depletion of carbohydrates in those kernels (Basha and Pancholy, 1986). Alternatively, the hot and dry conditions could have resulted in overly mature peanuts by the time of our harvest. Indeed, peanuts from this location had apparently greater incidence of damage and shriveling than from the E.V. Smith site; unfortunately, we did not grade samples from either site.
Concentrations of sugars of peanuts varied among our greenhouse and field experiments. However, sucrose concentrations were consistently higher compared to glucose and fructose and accounted for more than 80% of total sugars. Our results are consistent with previous studies which reported sucrose as predominant sugar in Virginia-type (Oupadissakoon et al., 1980; Pattee et al., 2000; Pickett, 1949) as well as runner-type (Pattee et al., 2000) peanuts.
Relation between peanut aflatoxins and soluble sugars
In the greenhouse experiment, total aflatoxins were weakly but positively correlated with total soluble sugar (r = 0.22; P = 0.08) and sucrose (r = 0.23; P = 0.07) contents of peanut kernels at the 10% level of significance. No significant negative or positive correlations were observed between total aflatoxins and total reducing sugars. In peanuts from the E.V. Smith experiment, total aflatoxins were also positively correlated to sucrose (r = 0.67; P = 0.06) and total sugars (r = 0.61; P = 0.10) at the 10% level of significance. No significant correlations were observed between aflatoxins and total reducing sugars of peanut. Manda et al. (2004) also found a significant positive correlation between aflatoxin and sucrose content of kernels of Virginia type peanuts as well as greater aflatoxin in immature seed compared to mature seed. The current study differs from Manda et al. (2004) in that they also found a positive correlation between the content of reducing sugars (glucose and fructose) and aflatoxin content of kernels, which we did not observe. Manda et al. (2004) reported the results of correlation but not actual levels of sugars in peanut under irrigated and rainfed conditions. In addition, they worked with Virginia-type peanut cultivars, while we used currently available runner-type peanuts. Pattee et al. (2000) consistently found lower reducing sugar contents in runner-types than in Virginia-types, which may partially explain why our results differed from Manda et al. (2004) with respect to reducing sugar and aflatoxin contents of peanut kernels. No significant correlations between sugar concentrations and aflatoxins were observed in peanuts from the Wiregrass site.
In this work, we chose to concentrate on sugars in peanuts and their relationship to aflatoxin accumulation, even though lipids are also an important component (constituting 45 to 48%) of peanut seeds (Sheppard and Rudolf, 1991). As with sugars, lipid content is affected by drought; specifically, drought appears to induce decreases in membrane lipid content and membrane integrity (Apel and Hirt, 2004; Kambiranda et al., 2011; Lauriano et al., 2000). These decreases could affect Aspergillus colonization and subsequent aflatoxin contamination indirectly; however, lipids are not preferred carbon sources for aflatoxin production (Yu et al., 2003). Moreover, peanuts are rich in unsaturated fatty acids (80%) which are reported to be either nonstimulatory (Davis and Diener, 1968) or inhibitory to aflatoxin synthesis (Priyadarshini and Tulpule, 1980) compared to saturated free fatty acids (Fanelli and Fabbri, 1989). When Fanelli et al. (1983) studied the effect of epoxy fatty acids on aflatoxin production by A. flavus and A. parasiticus in synthetic media (Czapek Dox broth), they observed no stimulatory effect on aflatoxin when unsaturated fatty acids were used for the synthesis of epoxy fatty acids.
In controlled studies, we showed that increasing sugar (especially sucrose) content of media allowed greater aflatoxin production by A. flavus, even at low concentrations (< 10%) such as found in peanut seed. Sugar content is greater in immature and drought stressed peanuts than in sound, mature kernels, and this might explain why the former can have greater aflatoxin contamination than the latter. We also showed that replenishment of sugars in the medium resulted in higher mycelial weights but not aflatoxin contents. In contrast, constant nutrient depletion resulted in higher aflatoxin content and toxin production per gram of mycelium. These results support the concept that aflatoxigenic fungi utilize available nutrients during their active growth stage and produce secondary metabolites (aflatoxins) as a result of stress or in conditions that are sub-optimal for fungal growth. The results of our field studies confirmed the significant role of drought stress in increasing the accumulation of sucrose or total soluble sugars in peanut kernels. Positive correlations found between peanut sugar and aflatoxin contents show the role that sugars may have in increasing aflatoxin content in peanuts. Thus, soluble sugars, which have been proven to readily support aflatoxin biosynthesis in vitro, may also have a significant role in aflatoxin contamination of peanuts in vivo. Increases in peanut sugars under drought stress may be a reason for higher aflatoxin contamination in drought years compared to non-drought years.
Acknowledgements
We thank Agricultural Research Service (ARS) – National Center for Agricultural Utilization Research Unit, Peoria, IL for providing the fungal culture (NRRL 3357) for this study. We also thank Dr. Maobing Tu (Assistant Professor, School of Forestry and Wildlife Sciences, Auburn University) for offering HPLC facilities and their excellent research assistance. In addition, appreciation is extended to Dr. S. M. Basha (Florida A&M University) for providing helpful instructions in regard to extraction and analysis of sugars from peanuts. This research was supported by National Peanut Board, Alabama Agricultural Experiment Station (AAES) and Department of Entomology and Plant Pathology, Auburn University, AL.
Literature cited
Abdollahi A and Buchanan R.L 1981 Regulation of aflatoxin biosynthesis: induction by various carbohydrates J. Food Sci. 46 : 633 – 635 .
Apel K and Hirt H 2004 Reactive oxygen species: metabolism, oxidative stress, and signal transduction Annu. Rev. Plant Biol. 55 : 373 – 399 .
Basha S. M 1992 Soluble sugar composition of peanut seed J. Agric. Food Chem. 40 : 780 – 783 .
Basha S.M and Pancholy S.K 1986 Qualitative and quantitative changes in the protein composition of peanut (Arachis hypogaea L.) seed following infestation with Aspergillus spp. differing in aflatoxin production J. Agric. Food. Chem. 34 : 638 – 643 .
Blankenship P.D Cole R.J Sanders T.H and Hill R.A 1984 Effect of geocarposphere temperature on pre-harvest colonization of drought-stressed peanuts by Aspergillus flavus and subsequent aflatoxin contamination Mycopathologia 85 : 69 – 74 .
Branch W.D 1995 Registration of ‘Georgia Green’ peanut Crop Sci. 36 : 806 .
Cary J.W and Ehrlich K.C 2006 Aflatoxigenicity in Aspergillus: molecular genetics, phylogenetic relationships and evolutionary implications Mycopathologia 162 : 167 – 177 .
Codex Alimentarius Commission 2004 Code of practice for the prevention and reduction of aflatoxin contamination in peanuts CAC/RCP 55-2004 .
Cole R.J Sanders T.H Hill T.H and Blankenship P.D 1985 Mean geocarposphere temperatures that induce pre-harvest aflatoxin contamination of peanuts under drought stress Mycopathologia 91 : 41 – 46 .
Davis N.D and Diener U.L 1968 Growth and aflatoxin production by Aspergillus parasiticus from various carbon sources Appl. Microbiol. 16 : 158 – 159 .
Davis N.D Diener U.L and Agnihotri V.P 1967 Production of aflatoxin B1 and G1 in chemically defined medium Mycopathol. 31 : 251 – 256 .
Davis N.D Diener U.L and Eldridge D.W 1966 Production of aflatoxin B1 and G1 by Aspergillus flavus in semi synthetic medium Appl. Microbiol. 14 : 378 – 380 .
Demain A.L 1986 Regulation of secondary metabolism in fungi Pure Appl. Chem. 58 : 219 – 226 .
Diener U.L Cole R.J Sanders T.H Payne G.A Lee L.S and Klich M.A 1987 Epidemiology of aflatoxin formation by Aspergillus flavus Ann. Rev. Phytopathol. 25 : 249 – 270 .
Fanelli C and Fabbri A.A 1989 Relationship between lipids and aflatoxin biosynthesis Mycopathologia 107 : 115 – 120 .
Fanelli C Fabbri A Finotti E and Passi S 1983 Stimulation of aflatoxin biosynthesis by lipophilic epoxides J. Gen. Microbiol. 129 : 1721 – 1723 .
Guo B Widstrom N.W Lee D.R Wilson D.M and Coy A.E 2005b Prevention of pre-harvest aflatoxin contamination: Integration of crop management and genetics in Corn . pp. 437 – 458 In: Abbas H (ed.) Aflatoxin and Food Safety Boca Raton, Florida : CRC press .
Guo B Holbrook C Yu J Lee R.D and Lynch R.E 2005a Application of technology of gene expression in response to drought stress and elimination of pre-harvest aflatoxin contamination . pp. 313 – 331 In: Abbas H (ed.) Aflatoxin and Food Safety Boca Raton, Florida : CRC press .
Guo B Jiujang Y Holbrook C.C Cleveland T.E Nierman W.C and Scully B.T 2009 Strategies in prevention of pre-harvest aflatoxin contamination in peanuts: Aflatoxin biosynthesis, genetics and genomics Peanut Sci. 36 : 11 – 20 .
Holbrook C.C Timper P Culbreath A.K and Kvien C.K 2008 Registration of ‘Tifguard’ peanut J. Plant Registrations. 2 : 92 – 94 .
Joffe A.Z and Lisker N 1969 Effect of light, temperature and pH value on aflatoxin production in vitro Appl. Microbiol. 18 : 517 – 518 .
Kambiranda D.M Vasanthaiah H.K.N Katam R Ananga A Basha S.M and Naik K 2011 Impact of drought stress on peanut (Arachis hypogaea L.) productivity and food safety In Plants and Environment Hemanth K.N (ed.). Available from: http://www.intechopen.com/books/plants-and-environment/impact-of-drought-stress-on-peanut-arachis-hypogaea-l-productivity-and-food-safety. Accessed: 01/01/2013.
Klich M.A Tiffany L.H and Knaphus G 1992 Ecology of the aspergilli of soils and litter . pp. 309 – 354 In: Aspergillus Biology and Industrial Applications Bennett J.W and Klich M.A (eds.) Boston , Butterworth-Heineman, MA .
Lauriano J.A Lidon F.C Carvalho C.A Campos P.S and Matos M.D.C 2000 Drought effects on membrane lipids and photosynthetic activity in different peanut cultivars Photosynthetica 38 : 7 – 12 .
Manda A Naidu B.P Rachaputi N.C Wright G and Fukai S 2004 Aflatoxins and their relationship with sugars in peanut (Arachis hypogaea L.) Proc. 4th Int. Crop Sci Congress , Brisbane, Australia .
Mateles R.I and Adye J.C 1965 Production of aflatoxins in submerged culture Appl. Microbiol. 13 : 208 – 211 .
Musingo M.N Basha S.M Sanders T.H Cole R.J and Blankenship P.D 1989 Effect of drought and temperature stress on peanut (Arachis hypogaea L.) seed composition J. Plant Physiol. 134 : 710 – 715 .
OBrian G.R Georgianna D.R Wilkinson J.R Yu J Abbas H.K Bhatnagar D Cleveland T.E Nierman W and Payne G.A 2007 The effect of elevated temperature on gene transcription and aflatoxin biosynthesis Mycologia. 99 : 232 – 239 .
Oupadissakoon C Young C.T and Mozingo R.W 1980 Evaluation of free amino acid and free sugar contents in five lines of Virginia-type peanuts at four locations Peanut Sci. 7 : 55 – 60 .
Pattee H.E Isleib T.G Giesbrecht F.G and McFeeters R.F 2000 Investigations into genotypic variations of peanut carbohydrates J. Agric. Food. Chem. 48 : 750 – 756 .
Payne G.A 1992 Aflatoxin in maize Crit. Rev. Plant Sci. 10 : 423 – 440 .
Pickett T.A 1949 Composition of developing peanut seed Plant Physiol. 25 : 210 – 224 .
Priyadarshini E and Tulpule P.G 1980 Effect of free fatty acids on aflatoxin production in a synthetic medium Food Cosmet. Toxicol. 18 : 367 – 369 .
Sanders T.H Cole R.J Blankenship P.D and Hill R.A 1985 Relation of environmental stress duration to Aspergillus flavus invasion and aflatoxin production in preharvest peanuts Peanut Sci. 12 : 90 – 93 .
Scherer T.F Seelig B and Franzen D 1996 Soil, water, plant characteristics important to irrigation. EB-66 North Dakota State University Agriculture and University Extension , Fargo, ND .
Schindler A.F Palmer J.G and Eisenberg W.V 1967 Aflatoxin production by Aspergillus flavus as related to various temperatures Appl. Microbiol. 15 : 1006 – 1009 .
Shih C.N and Marth E.H 1974 Some cultural conditions that control biosynthesis of lipid and aflatoxin by Aspergillus parasiticus Appl. Microbiol. 27 : 452 – 456 .
van Egmond H.P 1995 Mycotoxins: regulations, quality assurance and reference materials Food Additives and Contaminants 12 : 321 – 330 .
Wilson D.M and Stansell J.R 1983 Effects of irrigation regimes on aflatoxin contamination of peanut pods Peanut Sci. 10 : 54 – 56 .
Wilson T.J and Romer T.R 1991 Use of the mycosep multifunctional cleanup column for liquid chromatographic determination of aflatoxins in agricultural products J. Assoc. Anal. Chem. 74 : 951 – 956 .
Yu J Mohawed S.M Bhatnagar D and Cleveland T.E 2003 Substrate induced lipase gene expression and aflatoxin production in Aspergillus parasiticus and Aspergillus flavus J. Appl. Microbiol. 95 : 1334 – 1342 .
Notes
- Post-Doctoral Research Scholar, Department Botany and Plant Pathology, 2082 Cordley Hall, Oregon State University, Corvallis, OR 97331
- Professor, Department of Entomology and Plant Pathology, 224 Life Sciences Building, Auburn University, Auburn, AL 36849 * Corresponding authors email: saisreeu@gmail.com
Author Affiliations