Introduction
Aflatoxins are potent carcinogens produced by the fungi Aspergillus flavus (Link) and A. parasiticus (Speare). In the United States, aflatoxin contamination of peanut (Arachis hypogaea L.) is strictly regulated by the USDA and the peanut industry (Adams and Whitaker, 2004). Peanuts are inspected at the buying point for visible signs of Aspergillus spp. on the kernel. If more than one kernel in the grade sample has signs of the fungi, the farmer's lot is classified as segregation 3 and cannot be used for the edible market. The peanut kernels are also tested for aflatoxins at the shelling plant, where an average of more than 15 ng/g of aflatoxin leads to rejection of the lot. Rejected peanut lots can be reprocessed in an attempt to reduce aflatoxin concentrations or be used for oil.
Aspergillus flavus and A. parasiticus are common in soil and can colonize peanut kernels prior to harvest. After colonization, a combination of high soil temperatures and drought is the primary trigger for aflatoxin production leading to contamination of the kernel. Damaged and small or immature kernels are at a greater risk for aflatoxin contamination than are sound, mature kernels (Cole et al., 1988; Dowell et al., 1990; Wilson et al., 1977). Additionally, insect damage to peanut pods, even when the damage is superficial, increases invasion by Aspergillus spp. and subsequent aflatoxin contamination in plants exposed to drought stress (Bowen and Mack, 1993; Lynch et al., 1990; Lynch and Wilson, 1991).
The peanut root-knot nematode, Meloidogyne arenaria (Neal) Chitwood, is the primary nematode pest affecting peanut in the southeastern United States (Dickson and De Waele, 2005). In peanut, root-knot nematodes not only infect the root, but also the peg and developing pod. Galls produced on the shell are generally superficial; however, heavy pod galling can limit seed develop (Minton, 1984). Moreover, root-knot nematode galls on pods may also create a favorable environment for fungal invasion. In an earlier study to determine whether infection of peanut by M. arenaria increased aflatoxin concentrations in peanut, the authors reported that in treatments where A. flavus inoculum was added, aflatoxin concentrations were high (> 1000 ng/g) and not affected by nematode infection (Timper et al., 2004). However, in treatments without fungal inoculum, aflatoxin concentrations were greater in kernels from plants infected by M. arenaria (1190 ng/g) than in kernels from uninfected plants (79 ng/g). Apparently, adding A. flavus inoculum masked the effects of the nematode. In the first trial (2001) but not the second trial (2002) of this experiment, a high percentage of the pods were galled by M. arenaria, and the authors were able to detect a positive relationship between pod galling and the presence of A. flavus in the kernels. Although it appeared that pod galling increased infection of the kernel by toxigenic species of Aspergillus, it was not clear whether the increased infection of kernels was due to damage to the pod or greater numbers of small or immature pods. The contribution of root galling to aflatoxin production was also unknown. Because nematodes impair the root system, they reduce water-use efficiency subjecting the plant to increased water stress (Wilcox-Lee and Loria, 1987) and possibly greater susceptibility to aflatoxin contamination.
Peanut cultivars resistant to M. arenaria may reduce the risk of aflatoxin contamination in fields infested with the nematode. The first peanut cultivar with resistance to this nematode was COAN (Simpson and Starr, 2001), which was replaced a few years later by the higher yielding cultivar NemaTAM (Simpson et al., 2003). Both COAN and NemaTAM were derived from backcrossing a complex interspecific hybrid (TxAG-6) (Simpson et al., 1993) with the cultivar Florunner (Norden et al., 1969). Although these cultivars have a high level of resistance to M. arenaria, they were unsuitable for planting in the southeastern U.S. due to their extreme susceptibility to Tomato spotted wilt virus (TSWV; genus Tospovirus). The third cultivar with a high level of resistance to M. arenaria was Tifguard, developed from a cross between the cultivars C-99R and COAN. Both Tifguard and its nematode-susceptible sister line TifGP-2 have a high level of resistance to TSWV (Holbrook et al., 2008; Holbrook et al., 2012).
The primary objective of this research was to determine the mechanism by which M. arenaria increases preharvest aflatoxin contamination in peanut. Specifically determine 1) the role of nematode infection of roots vs. pods in increased aflatoxin contamination and 2) whether increased aflatoxin production in nematode-infected peanut is due to a greater percentage of small or immature kernels. An additional objective was to determine whether a peanut cultivar with resistance to M. arenaria would reduce the risk of preharvest aflatoxin contamination in soil infested with the nematode.
Materials and Methods
Pods vs. Roots Experiment
Pots were constructed to physically separate peanut pod set from root growth (Fig. 1). This was achieved by inserting a PVC tube (5 cm dia. × 10 cm) through the center of a plastic pan (40.6 cm dia. × 10.2 cm deep) where a hole had been previously cut to fit the tube. Silicone caulk was used to seal the seam between the tube and the pan. The pan with the attached tube was placed over a clay pot (21 cm dia. × 21.5 cm deep) containing soil. The tube extended 1 cm below the pan and into the soil. The soil was loamy sand (83% sand, 9% silt, 7% clay, 1% organic matter) that had been steam heated to 100 C for 6 hr to kill plant pathogens. The soil was also used to fill the tubes and the pans within 2 cm of the rim. To irrigate the root zone, drip emitters were attached to 6.4 mm dia. plastic tubing and inserted under the pans and near the location of the tube. A pre-germinated seed of the cultivar Georgia Green (Branch, 1996) was planted in the tube. To promote nodulation on the peanut roots, 0.6 cm3 of granular Bradyrhizobium inoculum was added to the planting hole. Gypsum (20 g/pan) was sprinkled on the soil surface to improve pod development. As the plants developed, the pod set was restricted to the soil-filled pans while the roots grew underneath the pan into the pot. A similar method of separating pod set from root growth was used by Anderson et al. (1996) to induce drought stress in the pod zone while maintaining adequate moisture for plant growth.
Planting system to physically separate pod set from root growth. Pods were formed in the soil-filled pans and roots growth extended into the soil-filled clay pots under the pan. Inset shows PVC tube which was filled with soil and extended from above the soil surface in the pan into the clay pot containing soil. The peanut plants received water by drip irrigation of the root zone under the pans.
The experiment was a 2 × 2 factorial design: root zone with and without M. arenaria, and pod zone with and without M. arenaria. The nematode was originally isolated from peanut in Tifton, GA and was cultured in a greenhouse on eggplant (Solanum melongena L.).Eggs of M. arenaria used for inoculum were extracted from the roots with 0.6% NaOCl (Hussey and Barker, 1973). Thirty days after planting (DAP), 3000 eggs of M. arenaria were added to the tubes (root zone). In the first two trials of the experiment, M. arenaria eggs were added to the pod zone at 60 and 80 DAP by evenly distributing a suspension of 20,000 eggs in 50 mL of water onto the soil surface and under the peanut foliage. Eggs were applied only to the areas of the pan where the peanut plant was pegging. All pans were lightly watered with an overhead sprinkler after the eggs were applied. In Trials 3–6, nematodes were applied using the same methods outlined above, except that 8000 eggs were added weekly to the pans starting 60 DAP and ending 102 DAP (six pod-zone applications). In each trial, there were 10–15 replicates per treatment combination. Inoculum of A. flavus NRRL 3357 and A. parasiticus NRRL 2999 was grown on cracked corn according to the methods of Will et al. (1994) and 2.5 mL of the inoculum (1 × 105 of each fungus/ml) was sprinkled under the peanut foliage of each pan at mid bloom (ca. 70 DAP). The plants were maintained in a greenhouse where temperatures ranged between 21 and 35 C. The root zones were watered by drip irrigation at the first sign of wilting and the pans were watered from above when the soil surface was dry. At 102 DAP, drought stress was initiated by ceasing irrigation of the pod zone and irrigating the root zone only when plants showed signs of severe wilt. In all trials, the peanut seed was planted in April or May and harvested in August or September. The experiment was conducted yearly from 2004 to 2009.
The peanut pods were harvested from the pans between 135 and 140 DAP. Pods that developed inside the tube were discarded. The pods from each pan were examined for galls caused by M. arenaria and rated on a 0–10 scale based on percentage of the pod surface with galls (0 = no galls, 1 = 1–10%, 2 = 11–20%, etc). The root systems of each plant were also rated on a 0–10 scale based on the percentage of the root system with galls. After air drying to ≤ 8% moisture, the pods were shelled and the kernels from each plant were ground and weighed. Aflatoxin concentrations were determined by the immunoaffinity column-fluorometer method using Vicam P columns (Trucksess et al., 1991). The fluorometer was calibrated from 0 to 400 ng/g aflatoxin range. Samples exceeding 400 ng/g were diluted 10-fold and then re-analyzed. Additional dilutions were performed up to a maximum detection level for aflatoxins of 40,000 ng/g.
Rainout Shelter Experiment
To determine the effect of M. arenaria infection on peanut size and also whether resistant cultivars reduce the risk of alfatoxin contamination, a 2 ×2 factorial experiment was conducted with two peanut genotypes (Tifguard and TifGP-2) and two nematode treatments (with and without M. arenaria). The experiment was arranged in a randomized block design with six replicates and was conducted in 24 field microplots equipped with a rainout shelter that closed automatically whenever rain fell. The experiment was conducted five times from 2006 to 2010 and fumigated with metam sodium (598 L/ha a.i.) between each experiment to kill M. arenaria and Aspergillus spp. The microplots were pre-cast concrete containers 1.7 × 1.4 m and 91-cm deep with 8-cm thick walls and filled with a sandy soil (92% sand, 5% silt, 3% clay, and < 1% OM).
The two peanut genotypes were planted in separate microplots with 12 plots per genotype. Both peanut genotypes have the same level of resistance to TSWV; however, Tifguard is highly resistant to M. arenaria and TifGP-2 is susceptible. The peanuts were planted in late May or early June in two rows 91 cm apart with a final plant density of 20 plants/m row. A 5-cm3 quantity of grain infested with A. flavus and A. parasiticus was broadcast over each plot at mid bloom (Holbrook et al., 1994). Eggs of M. arenaria, harvested from a greenhouse culture, were applied to six replicates of each cultivar approximately 23 d after planting (DAP), and again approximately 72 DAP and 86 DAP to maximize pod galling. The other six replicates of each cultivar served as no-nematode controls. At 23 DAP, 30,000 M. arenaria eggs were applied to a 2.5 cm deep trench on both sides of each row of peanuts (60,000 eggs/row). At 72 and 86 DAP, 100,000 eggs were applied under the canopy on each side of the row (200,000 eggs/row). After each application of nematode eggs, the plots were irrigated with 7.6 mm of water. Applications of fertilizer, insecticides and herbicides followed University of Georgia Extension Service recommendations and were the same for all plots. The plants were irrigated as needed until 100 DAP and then the shelters were turned on to induce drought stress.
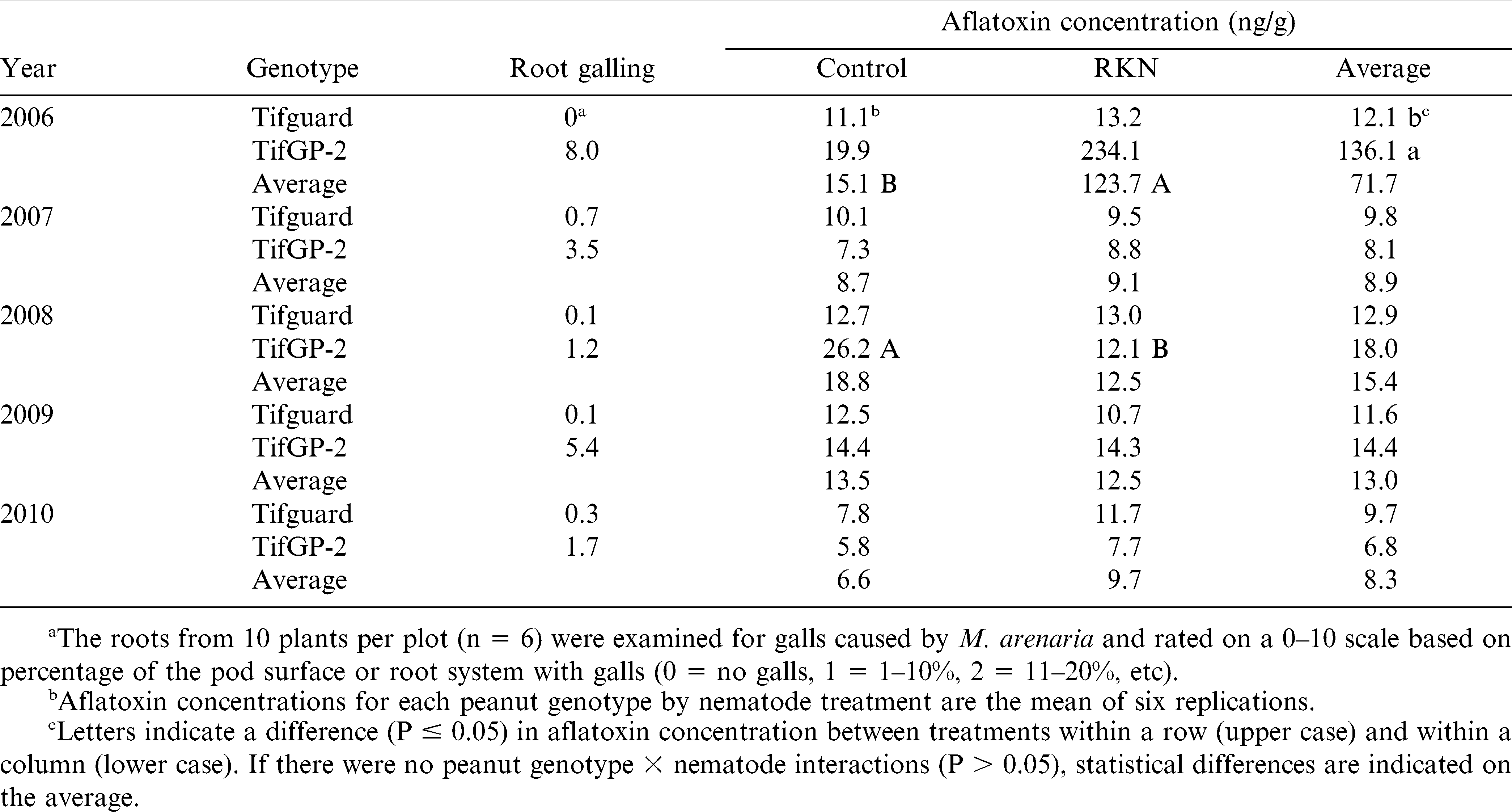
The peanuts were harvested between 135 and 140 DAP, and the roots of 10 plants per plot were rated for galling on a 0–10 scale. The pods were hand-picked, dried to ≤ 8% moisture, rated for pod galling on a 0–10 scale, and weighed. Harvested pods from each plot were shelled and graded according to the following standard methods for shelled peanuts in the U.S. Seed that passed through a 16/64 × ¾ inch screen (16S) were classified as immature kernels (IMK) and those retained on the screen were classified as sound mature kernels (SMK). Any split seed that were undamaged were classified as sound splits (SS) and added to the SMK for the total SMK (TSMK) prior to aflatoxin analysis. Kernels that showed signs of insect damage, sprouting, or discoloration were classified as damaged kernels (DK). Aflatoxin concentrations were determined for each grade (IMK, TSMK, and DK) from 100-g or less subsamples of ground kernels by the immunoaffintiy column fluorometer method. All other methods were the same as those described for the first experiment. The total aflatoxin concentration (ng/g) from kernels harvested (DK + IMK + TSMK) was calculated by adding the amount (ng) per grade and dividing by the total weight of kernels from each plot.
Statistical analysis
Aflatoxin concentrations were natural log-transformed
prior to statistical analysis. In the pod vs. root experiment, analysis of variance (ANOVA; PROC GLM, V. 9.2, SAS Institute, Cary, NC) was used to determine the effect of trial, root inoculation, pod inoculation, and all two- and three-way interactions on aflatoxin concentrations. Regression analysis (PROC REG) was used to determine if there was a relationship between aflatoxin concentration and the level of galling on either peanut roots or pods. For the rainout shelter experiment, ANOVA was used to determine the effect of peanut grade, year, and their interaction on aflatoxin concentrations, and the effect of nematodes, peanut genotype, year, and all two- and three-way interactions on aflatoxin concentrations. Additionally, the effect of M. arenaria inoculation on the proportion (arcsine transformed) of peanuts in each grade class was determined by ANOVA using only data from TifGP-2. When there was more than two levels of a factor (e.g., peanut grade), Fisher's Protected LSD was used to separate means (P ≤ 0.05).
Results and Discussion
Pods vs. Roots Experiment
Root galling by M. arenaria was relatively high (4.5 to 8.2) for all trials except Trial 6 (Table 1). Pod galling was more variable, ranging from 0.2 to 5.7. The aflatoxin concentrations were also quite variable among trials. Trial 4 had the highest aflatoxin concentrations with an average across all treatments of 4358 ng/g of kernel. In the full ANOVA model, which included all trials, there was no effect of root or pod inoculation with M. arenaria on afltoxin concentration. However, there was an interaction between trial and root inoculation (P = 0.007). There were no interactions between root and pod inoculation and no additional two or three-way interactions with trial. When the trials were analyzed separately, aflatoxin concentrations in the kernels were greater in Trial 1 (P = 0.02) and Trial 2 (P = 0.0002) when roots were inoculated with M. arenaria than in non-inoculated roots, and were greater in Trial 3 (P = 0.02) when pods were inoculated with the nematode (Table 1). In an earlier study, research determined very high concentrations of alfatoxins masked the effect of nematodes (Timper et al., 2004). When Trial 4 was eliminated with uniformly high aflatoxin concentrations from the full ANOVA model, root inoculation with M. arenaria increased (P = 0.003) aflatoxin concentrations; however, there were interactions between trial and both root (P = 0.04) and pod (P = 0.03) inoculations indicating that the effect of nematode inoculation was inconsistent among trials. Within a trial, there was no relationship between the level of pod galling and aflatoxin concentration using regression analysis; however, there was a positive relationship between the level of root galling and aflatoxin concentration in Trial 1 (P = 0.008, r = 0.40), Trial 2 (P = 0.005; r = 0.40), and Trial 5 (P = 0.02; r = 0.31).
Rainout Shelter Experiment
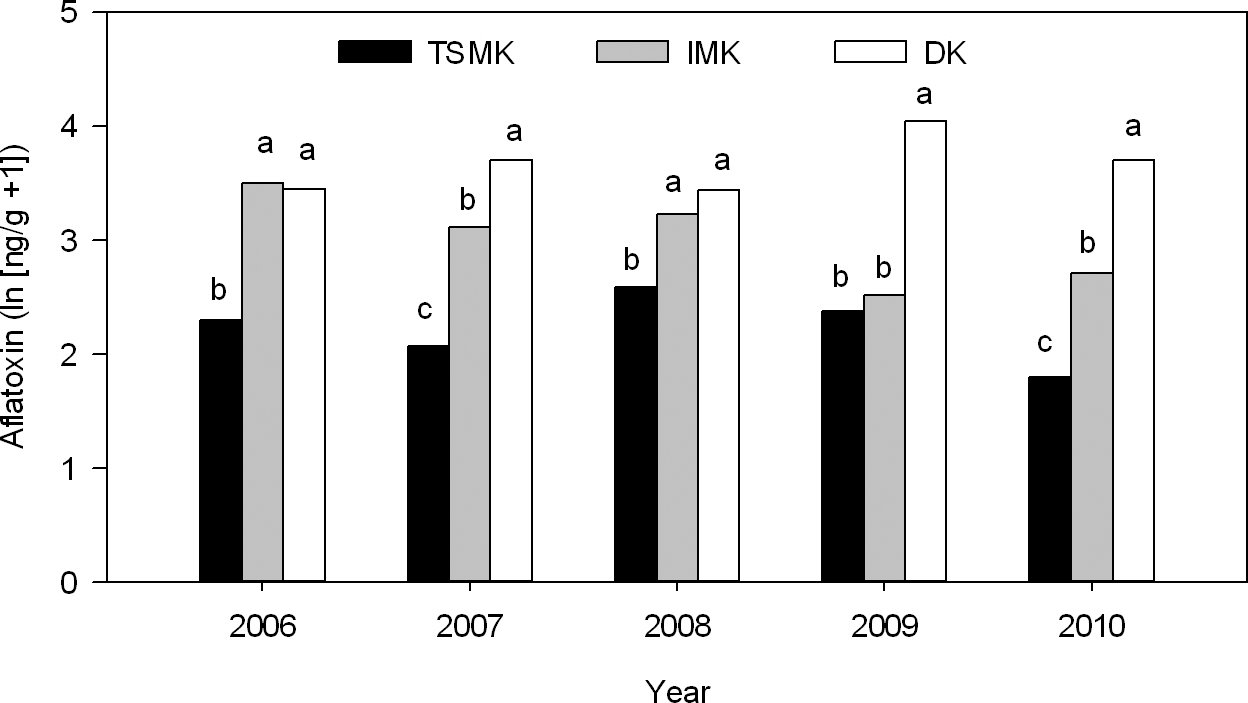
Aflatoxin concentrations differed among peanut grades (P < 0.0001), but there was an interaction between grade and year (P = 0.004). In all years, aflatoxin concentrations were greater in DK than in TSMK (Fig. 2). Aflatoxin concentrations in the IMK varied among years; depending on the year, concentrations were similar to the DK, the TSMK or intermediate between the DK and TSMK. Across all treatments, the percentages of IMK were 4.9, 7.4, 4.0, 16.7, and 4.1 in 2006, 2007, 2008, 2009, and 2010, respectively. The percentages of DK were 2.9, 11.8, 2.2, 2.0, and 0.8 in 2006, 2007, 2008, 2009, and 2010, respectively. To determine the effect of M. arenaria infection on grade, only data from TifGP-2 were used because Tifguard is resistant to the nematode. In years when there was moderate to high root galling (2006, 2007, and 2009), root-knot nematodes did not influence the percentage of IMK or TSMK. Nematodes also did not influence the percentage of DK, except in 2007, when plots inoculated with M. arenaria had a greater percentage of DK than control plots (11.9 vs. 8.5%).
Aflatoxin concentrations in the total sound mature kernels (TSMK), immature kernels (IMK) and damaged kernels (DK) in different years of the rainout shelter experiment. Aflatoxin concentrations are presented as ln (ng/g + 1). Letters above bars indicate differences (P ≤ 0.05) among grades in a given year.
The total aflatoxin concentration of the kernels harvested from each plot was not affected by peanut genotype (Tifguard and TifGP-2) or nematode inoculation; however, there was a year × genotype (P = 0.019) and a year × nematode (P = 0.013) interaction. When the data were analyzed by year, inoculation with M. arenaria increased (P = 0.033) aflatoxin concentrations compared to the control only in 2006 (Table 2). Also in 2006, TifGP-2 had greater (P = 0.042) levels of aflatoxin compared to Tifguard, though there was no interaction between peanut genotype and nematode inoculation (P = 0.060) indicating that the effect of the nematodes on aflatoxin levels was similar between genotypes. In 2008, there was an interaction between peanut genotype and nematode inoculation (P = 0.010) due to greater aflatoxin concentrations in the control compared to the M. arenaria treatment in TifGP-2 only (Table 2). In all other years except 2006, alfatoxin concentrations were relatively low.
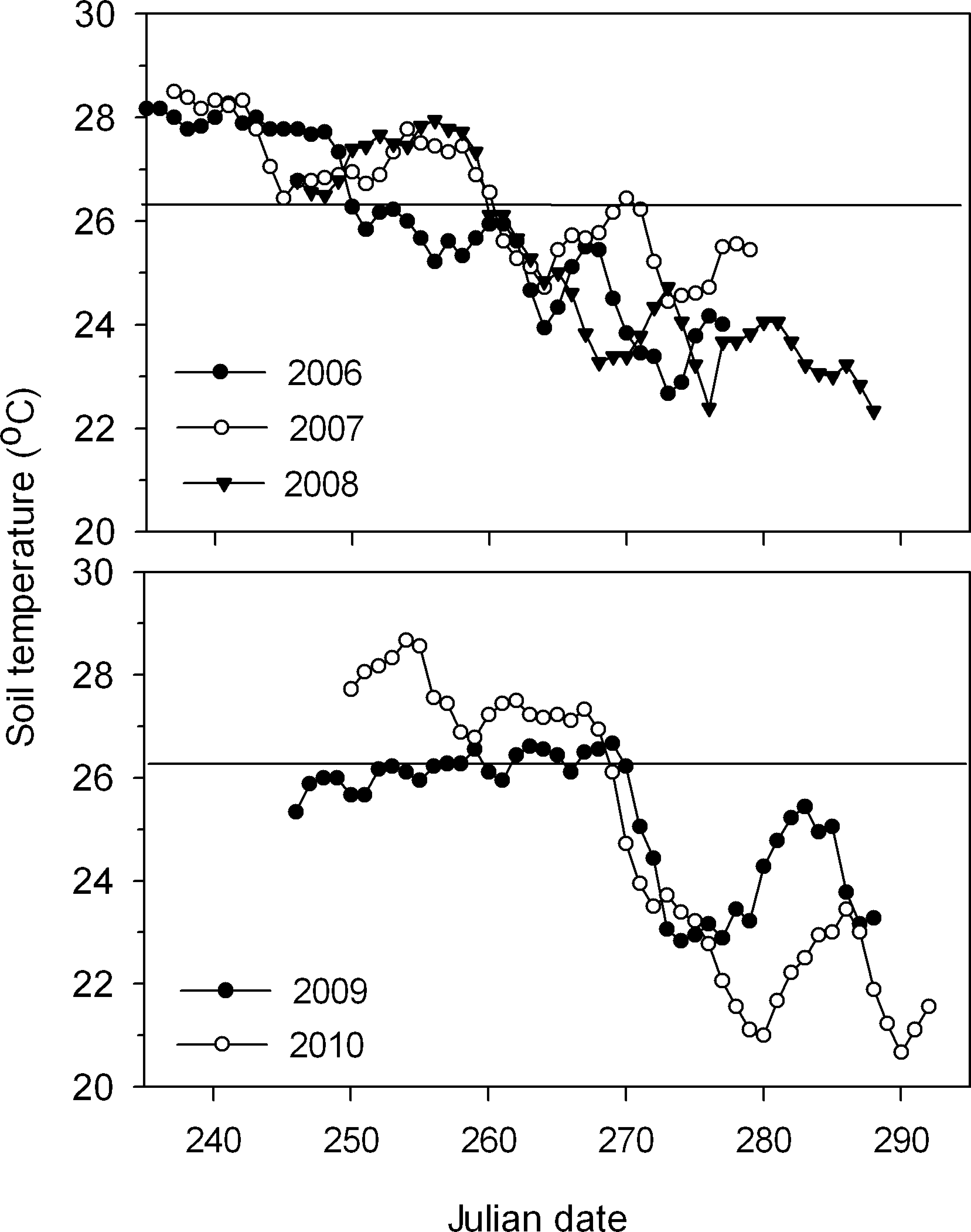
Soil temperatures at 10 cm recorded at a nearby (1.8 km) weather station during the last 6 wk before optimal peanut maturity were above the critical threshold of 26.3 C for aflatoxin production (Cole et al., 1985) until the end of September (260–270 JD) in most years (Fig. 3). The exceptions were in 2006, when temperatures dropped below the threshold in early September (250 JD), and in 2009, when soil temperatures were low during the entire 6-wk period. Soil temperatures never reached 29 C during the last 6 wk in any year; however, temperatures were at or above 28 C for 6 d in 2006 and 2007, and for 5 d in 2010 (Fig. 3).
Soil temperatures at 10 cm recorded at a nearby (1.8 km) weather station during the last 6 weeks before optimal maturity of the peanuts from 2006 to 2010. The line indicates the lower temperature for aflatoxin production. The years are presented in separate graphs to reduce dense clusters of data points.
These data confirmed that M. arenaria infection of peanut sometimes increases preharvest aflatoxin contamination. Aflatoxin concentrations were greater in kernels from nematode-infected plants than from control plants in three trials of the pods vs. roots experiment and in one trial of the rainout shelter experiment. However, M. arenaria infection did not consistently lead to increased aflatoxin levels. In three to four trials of each experiment, we failed to observe an increase in aflatoxin concentrations in nematode-infected plants. Moreover, in 2008, there were greater aflatoxin concentrations in the control than in the nematode-treated plots for TifGP-2. Other environmental factors likely mediate the interactions among the nematode, plant, and fungus leading to increased aflatoxin production. The primary conditions that favor aflatoxin production are a combination of water stress and high soil temperatures (> 26.3 C) 4 to 6 wk before harvest; neither water stress nor high temperatures alone lead to aflatoxin contamination in SMK (Cole et al., 1985; Hill et al., 1983; Wilson and Stansell, 1983). With increasing temperatures, there is an increase in aflatoxin concentrations until an upper limit near 30 C is reached (Cole et al. 1985). We induced water stress starting 100 DAP, but we could not control soil temperature in either the pods vs. roots experiment or the rainout shelter experiment. Aflatoxin concentrations were typically greater in the pods vs. roots experiment than in the rainout shelter experiment, perhaps because the greenhouse air temperature was 2–3 C warmer than outside. In the rainout shelter experiment, soil temperatures alone cannot explain the variation in aflatoxin production among years; for example, 2007 and 2010 had the most sustained soil temperatures above the critical threshold (26.3 C) among the years, yet had relatively low aflatoxin concentrations compared to 2006. The level of root galling was greater in 2006 than in other years. It is possible that extensive root galling can increase aflatoxin production when environmental conditions, such as soil temperature, are marginal for toxin production. In 2009, there was substantial root galling; however, soil temperatures never exceeded 27 C during the last 6 wk before harvest.
Because galling of the pods may provide a portal for Aspergillus spp. to gain access to the peanut kernel, it is hypothesized that pod infection by M. arenaria would lead to greater aflatoxin contamination than root infection. In one trial of this study, aflatoxin concentrations were increased by adding M. arenaria to the pod zone. The authors reported in an earlier study a positive relationship between the level of pod galling and the presence of Aspergillus spp. in the kernels (Timper et al., 2004). However, data indicated that root infection by the nematode was more consistently associated with elevated aflatoxin concentrations than pod infection. In Trials 1 and 2 of the pods vs. roots experiment, inoculation of the root zone led to an increase in aflatoxin concentrations in the peanut kernels. Moreover, in Trial 5, there was a weak, but positive correlation between the amount of root galling and aflatoxin concentration, even though ANOVA showed no effect of root inoculation on toxin production. The mechanism by which nematode infection of roots leads to an increase in aflatoxin contamination of the peanut kernel is unknown. A reasonable assumption is that the nematode damage to the peanut root system inhibits water uptake and puts the plant under greater water stress. However, Sanders et al. (1993) showed that drought stress around the pods contributed to preharvest aflatoxin production, whereas drought stress in the root zone did not. Nematode infection of roots may also cause physiological changes in the plant which may increase its susceptibility to infection by toxigenic Aspergillus spp. Indeed, root-knot nematodes are known to increase several fungal diseases of plants (Abawi and Chen, 1998). In some cases, the wound caused by nematode feeding serves as an entry point for the fungus; however, in other cases, nematode infection increases fungal infection in a separate part of the root system. We did not determine colonization of the kernel in this study, but in an earlier study, application of M. arenaria did not increase the percentage of kernels colonized by Aspergillus spp. (Timper et al., 2004). Infection of the roots by M. arenaria may also cause physiological changes in the plant that increase aflatoxin production by Aspergillus spp. For example, nematode infection can create oxidative stress in plants which could trigger aflatoxin production by the fungus (Guida et al., 1992; Reverberi et al., 2010).
Our results confirm those of other studies showing greater aflatoxin concentrations in damaged kernels and intermediate concentrations in immature kernels compared to sound mature kernels (Cole et al., 1988; Whitaker et al., 2005; Whitaker et al., 1998; Wilson et al., 1977). Heavy nematode galling on the peanut pod can reduce kernel size. Baldwin et al. (2003) reported that the percentage of sound mature kernels increased and the percentage of small kernels decreased when nematicides were used to suppress populations of M. arenaria. In our study, however, infection of the peanut plant by M. arenaria did not lead to greater percentages of small kernels. In only one year (2007), nematode infestation appeared to be associated with an increase in the percentage of damaged kernels, though the level of root galling that year was lower compared to 2006 and 2009. Aflatoxin concentrations were also not affected by nematodes in 2007 indicating that the increase in damaged kernels did not lead to greater aflatoxin levels in the combined sample.
It was hypothesized that peanut cultivars with resistance to M. arenaria would have a lower risk of aflatoxin contamination compared to susceptible cultivars in the presence of the nematode. In the rainout shelter experiment, 2006 was the only year where nematode infection of peanut increased aflatoxin concentrations. In that year, there were lower aflatoxin levels in the resistant cultivar Tifguard than the susceptible germplasm TifGP-2, thus supporting our hypothesis. However, with only one year of supporting data, the protective effect of resistant cultivars on enhanced aflatoxin contamination caused by M. arenaria are only suggestive and not conclusive.
Conclusions
By physically separating root growth from pod set, we showed for the first time that root infection of the peanut plant could lead to increased aflatoxin levels in the kernel. The results of the current study, as well as our earlier study (Timper et al., 2004) indicate that pod galling may also lead to an increase in aflatoxin concentrations in the kernel. Furthermore, we conclude that M. arenaria is not causing greater aflatoxin contamination in peanut by increasing the percentage of damaged or small kernels. The nematode-resistant cultivar Tifguard appeared to reduce the risk of aflatoxin contamination in soil infested with M. arenaria, but the cultivar is not likely to reduce the risk when conditions are optimal for aflatoxin production.
Acknowledgements
This work was supported, in part, by the Multicrop Aflatoxin Working Group. The authors wish to thank David Clements, William Wilson, Bonnie Evans, Betty Tyler, Shannon Atkinson, and Jason Golden for technical assistance. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Literature Cited
Abawi G.S and Chen J 1998 . Concomitant pathogen and pest interactions, pp. 135 – 158 . In Barker K.R Pederson G.A and Windham G.L (eds.) . Plant and Nematode Interactions . American Society of Agronomy , Madison, WI .
Adams J.G and Whitaker T.B 2004 . Peanuts, aflatoxin, and the U.S. origin certification program, pp. 183 – 196 . In Barug D van Egmond H Lopez-Garcia R van Osenbruggen T and Visconti A (eds.) . Meeting the Mycotoxin Menace . Wageningen Academic Publishers , The Hague .
Anderson W.F Holbrook C.C and Wilson D.M 1996 . Development of greenhouse screening for resistance to Aspergillus parasiticus infection and preharvest aflatoxin contamination in peanut . Mycopathologia 135 : 115 – 118 .
Baldwin J.A Padgett G.B and Johnson A.W 2003 . Bahiagrass and other crops in a rotational study to reduce nematodes and other pests affecting peanut yield and quality, pp. 173 – 177 . In Rhoads F.M (ed.) . Proceedings of Sod Based Cropping Systems Conference . North Florida Research and Education Center-Quincy, University of Florida . http://nfrec.ifas.ufl.edu/programs/sod_rotation_conf.shtml
Bowen K.L and Mack T.P 1993 . Relationship of damage from the lesser cornstalk borer to Aspergillus flavus contamination in peanuts . J. Entomol. Sci. 28 : 29 – 42 .
Branch W.D 1996 . Registration of ‘Georgia Green’ peanut . Crop Sci. 36 : 806 .
Cole R.J Dorner J.W Kirksey J.W and Dowell F.E 1988 . Comparison of visual, enzyme-linked immunosorbent assay screening, and HPLC methods in detecting aflatoxin in farmers stock peanut grade samples . Peanut Sci. 15 : 61 – 63 .
Cole R.J Sanders T.H Hill R.A and Blankenship P.D 1985 . Mean geocarposphere temperatures that induce preharvest aflatoxin contamination of peanuts under drought stress . Mycopathologia 91 : 41 – 46 .
Dickson D.W and De Waele D 2005 . Nematode parasites of peanut, pp. 393 – 436 . In Luc M Sikora R.A and Bridge J (eds.) . Plant Parasitic Nematodes in Subtropical and Tropical Agriculture . CABI Publishing , Wallingford, UK .
Dowell F.E Dorner J.W Cole R.J and Davidson J.I 1990 . Aflatoxin reduction by screening farmers stock peanuts . Peanut Sci. 17 : 6 – 8 .
Guida G Zacheo G and Bleve-Zacheo T 1992 . Activation of detoxifying enzymes in tomato roots following paraquat treatment and nematode infection . Nematol. Mediterr. 20 : 203 – 209 .
Hill R.A Blankenship P.D Cole R.J and Sanders T.H 1983 . Effects of soil moisture and temperature on preharvest invasion of peanuts by the Aspergillus flavus group and subsequent aflatoxin development . Appl. Eviron. Microbiol. 45 : 628 – 633 .
Holbrook C.C Matheron M.E Wilson D.M Anderson W.F Will M.E and Norden A.J 1994 . Development of a large-scale field system for screening peanut for resistance to preharvest aflatoxin contamination . Peanut Sci. 21 : 20 – 22 .
Holbrook C.C Dong W.B Timper P Culbreath A.K and Kvien C.K 2012 . Registration of peanut germplasm line TifGP-2, a nematode-susceptible sister line of ‘Tifguard’ . J. Plant Registr. 6 : 208 – 211 .
Holbrook C.C Timper P Culbreath A.K and Kvien C.K 2008 . Registration of ‘Tifguard’ peanut . J. Plant Registr. 2 : 92 – 94 .
Hussey R.S and Barker K.R 1973 . A comparison of methods of collecting inocula for Meloidogyne spp., including a new technique . Plant Dis. Rep. 57 : 1025 – 1028 .
Lynch R.E and Wilson D.M 1991 . Enhanced infection of peanut, Arachis hypogaea L., seeds with Aspergillus flavus group fungi due to external scarification of peanut pods by lesser cornstalk borer, Elasmopalpus lignosellus (Zeller) . Peanut Sci. 18 : 110 – 116 .
Lynch R.E Wilson D.M and Maw B.W 1990 . Enhanced aflatoxin contamination of peanut as a result of insect damage to pods . Proc. Amer. Peanut Res. Educ. Soc. 22 : 78 .
Minton N.A 1984 . Nematode parasites of peanuts, pp. 373 – 394 . In Nickle W.R (ed.) . Plant and Insect Nematodes . Marcel Dekker, Inc. , New York .
Norden A.J Lipscomb R.W and Carver W.A 1969 . Registration of Florunner peanuts 1 (Reg . No. 2). Crops Sci. 9 : 850 .
Reverberi M Ricelli A Zjalic S Fabbri A.A and Fanelli C 2010 . Natural functions of mycotoxins and control of their biosynthesis in fungi . Appl. Microbiol. Biotechnol. 87 : 899 – 911 .
Sanders T.H Cole R.J Blankenship P.D and Dorner J.W 1993 . Aflatoxin contamination of peanuts from plants drought stressed in pod and root zone . Peanut Sci. 20 : 5 – 8 .
Simpson C.E Nelson S.C Starr J.L Woodward K.E and Smith O.D 1993 . Registration of TxAG-6 and TxAG-7 peanut germplasm lines . Crop Sci. 33 : 1418 .
Simpson C.E and Starr J.L 2001 . Registration of ‘COAN’ peanut . Crop Sci. 41 : 918 .
Simpson C.E Starr J.L Church G.T Burow M.D and Paterson A.H 2003 . Registration of ‘NemaTAM’ peanut . Crop Sci. 43 : 1561 .
Timper P Wilson D.M Holbrook C.C and Maw B.W 2004 . Relationship between Meloidogyne arenaria and aflatoxin contamination in peanut . J. Nematol. 36 : 167 – 170 .
Trucksess M.W Stack M.E Nesheim S Page S.W Albert R.H Hansen T.J and Donahue K.F 1991 . Immunoaffinity column coupled with solution fluorometry or liquid-chromatography postcolumn derivatization for determination of aflatoxins in corn, peanuts, and peanut butter: Collaborative study . J. Assoc. Off. Anal. Chem. 74 : 81 – 88 .
Whitaker T.B Dorner J.W Lamb M and Slate A.B 2005 . The effect of sorting farmers' stock peanuts by size and color on partitioning aflatoxin into various shelled peanut grade sizes . Peanut Sci. 32 : 103 – 118 .
Whitaker T.B Hagler W.M Giesbrecht F.G Dorner J.W Dowell F.E and Cole R.J 1998 . Estimating aflatoxin in farmers' stock peanut lots by measuring aflatoxin in various peanut-grade components . J. AOAC Int. 81 : 61 – 67 .
Wilcox-Lee D and Loria R 1987 . Effects of nematode parasitism on plant-water relations, pp. 260 – 266 . In Veech J.A and Dickson D.W (eds.) . Vistas on Nematology: A Commemoration of the Twenty-fifth Anniversary of the Society of Nematologists . Society of Nematologists, Inc. , Hyattsville, MD .
Will M.E Holbrook C.C and Wilson D.M 1994 . Evaluation of field inoculation techniques for screening peanut genotypes for reaction to preharvest A. flavus group infection and aflatoxin contamination . Peanut Sci. 21 : 122 – 125 .
Wilson D.M Mixon A.C and Troeger J.M 1977 . Aflatoxin contamination of peanuts resistant to seed invasion by Aspergillus flavus . Phytopathology 67 : 922 – 924 .
Wilson D.M and Stansell J.R 1983 . Effect of irrigation regimes on aflatoxin contamination of peanut pods . Peanut Sci. 10 : 54 – 56 .
Notes
- USDA ARS, Crop Protection and Management Research Unit, P.O. Box 748, Tifton, GA 31793
- Department of Plant Pathology, University of Georgia, Tifton, GA 31794
- USDA ARS, Crop Genetics and Breeding Research Unit, P.O. Box 748, Tifton, GA 31793 * Corresponding author, email: patricia.timper@ars.usda.gov
Author Affiliations