Introduction
The cultivated peanut (Arachis hypogaea L.) plant is indeterminate in its flowering and fruiting pattern (Norden, 1980). Consequently, harvest will contain both mature and immature pods and seed. The hull-scrape pod maturity profile method (Williams and Drexler, 1981) quickly became a widely acceptable procedure to determine the optimum digging time among the southeast U.S. peanut farmers.
‘Georgia-02C’ is a recently released runner-type peanut (subsp. hypogaea var. hypogaea) cultivar with high yields, grades, and dollar values from the University of Georgia peanut breeding program (Branch, 2003). It has multiple pest resistance to tomato spotted wilt disease caused by Tomato spotted wilt virus (TSWV), Cylindrocladium black rot disease caused by Cylindrocladium parasiticum Crow, Wingfield, and Alfenas; and moderate resistance to white mold or stem rot disease caused by Sclerotium rolfsi Sacc., and the three cornered alfalfa hopper (3CAH), Spissistilus festinus (Branch and Brenneman, 2003 and 2009; Brown, 2006). It also has the high-oleic and low-linoleic fatty acid seed oil chemistry which significantly increases the shelf-life of peanut products (Norden et al., 1987).
Georgia-02C has a spreading runner growth habit, tan testa color, and medium to medium-late maturity [ca. 145 days after planting (DAP) in south Georgia]. However, its relative maturity range has not been fully established. In the past, the medium maturing ‘Florunner’ peanut cultivar (Norden et al., 1969) was found to have an optimum maturity range of approximately one week or ±3 to 4 days at about 140 DAP in south Georgia. Consequently, Florunner peanuts that were dug too early or too late resulted in yield and dollar value lost compared to the optimum time of digging as reported in a previous 4-yr study (Sanders, 1995). So, the objective of this study was to determine the relative maturity range for the new Georgia-02C runner-type peanut cultivar.
Materials and Methods
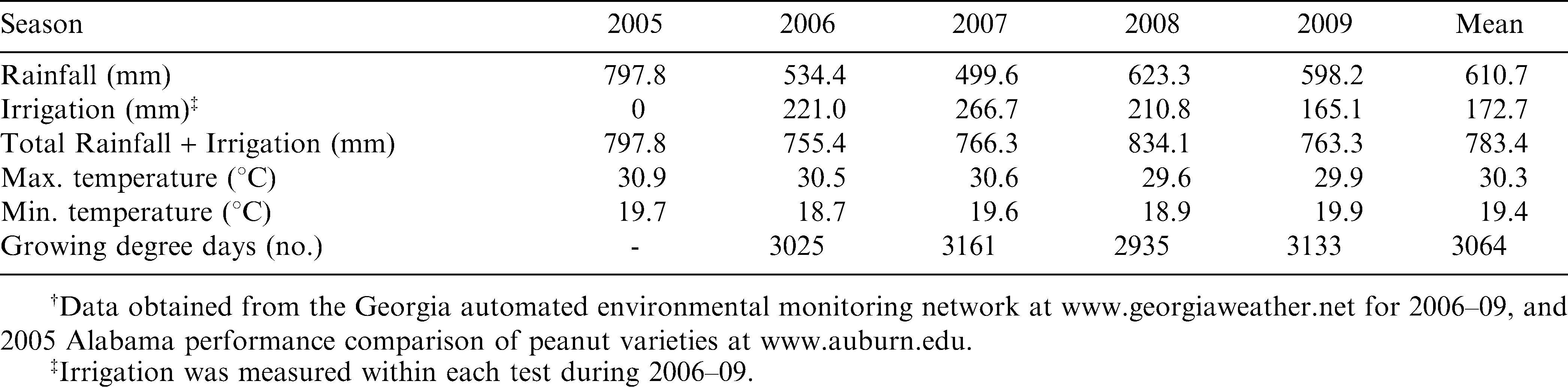
Six-digging dates [127, 134, 141, 148, 155, and 162 days after planting (DAP)] were used to assess the relative maturity range for the Georgia-02C runner-type peanut cultivar. Field experiments were conducted during 2005 at the Wiregrass Research and Extension Center near Headland, Alabama and in 2006, 2007, 2008, and 2009 at the Coastal Plain Experiment Station, Tifton, Georgia. The Georgia-02C cultivar was planted each year in mid-May at six-seed per 30.5 cm of row. The soil type for this study in Alabama was a Dothan fine sandy loam; whereas, the soil type in Georgia was a Tifton loamy sand. Both soils are similar (fine-loamy, kaolinitic, thermic Plinthic Kandiudults). Recommended cultural practices with irrigation were used throughout the growing season. Plots consisted of two rows 6.10m long × 1.83m wide (0.81m within and 1.02m between adjacent plots). A randomized completed block design was used each year and location with four replications per digging date.
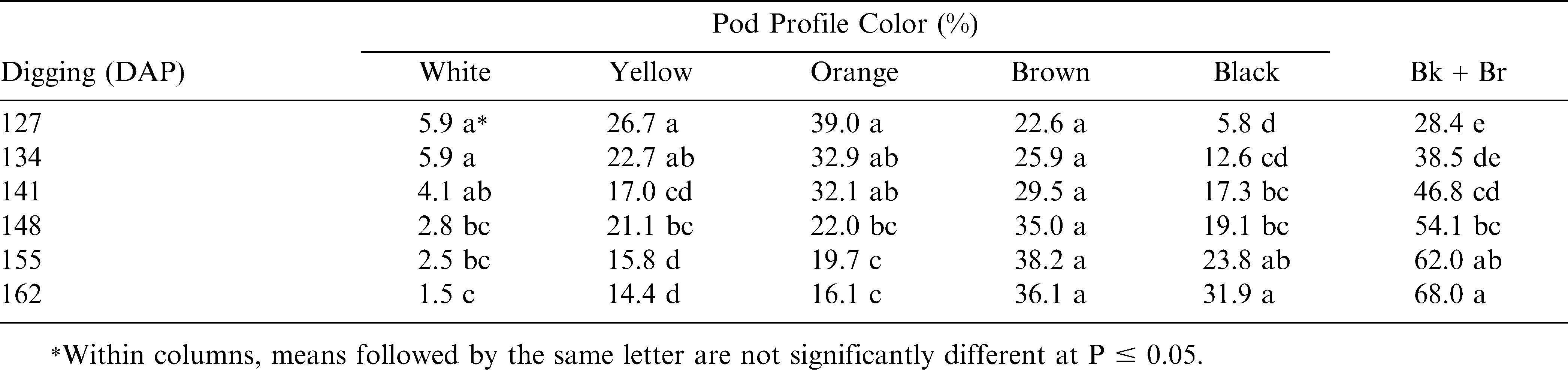
At each of the six-digging dates, border plots were sampled and the hull-scrape maturity method was used to determine the pod profile color percentages (Williams and Drexler, 1981). All pods from several border plants were removed by hand to obtain approximately 180–220 pods per digging date. A high-pressure washer with a spinning or “turbo” type nozzle was used to remove the outer exocarp pod layer and expose the middle mesocarp pod colors (Williams, 2003). Peanut pod mesocarp colors change with maturation from white (most immature) to yellow, orange, brown, and black (most mature). For water blasting, pod samples were placed into a round wire mesh basket set inside a tall bucket with many small drainage holes drilled toward the bottom of the bucket. The pressure washer nozzle was positioned about 15–25 cm above the pod samples and was normally run <1 minute per sample to fully expose the mesocarp pod colors. Each pod was then visually classified into maturity color group before counting the number of pods.
After digging with a two-row peanut digger-shaker-inverter and picking with a small-plot thresher, pods from individual plots were dried inside plastic mesh bags with forced warm air to approximately 6% moisture. Plot pod samples were then hand-cleaned over a screen table before weighing for yield determinations. Grades were also determined according to federal state inspection service official procedures (USDA-AMS, 1998).
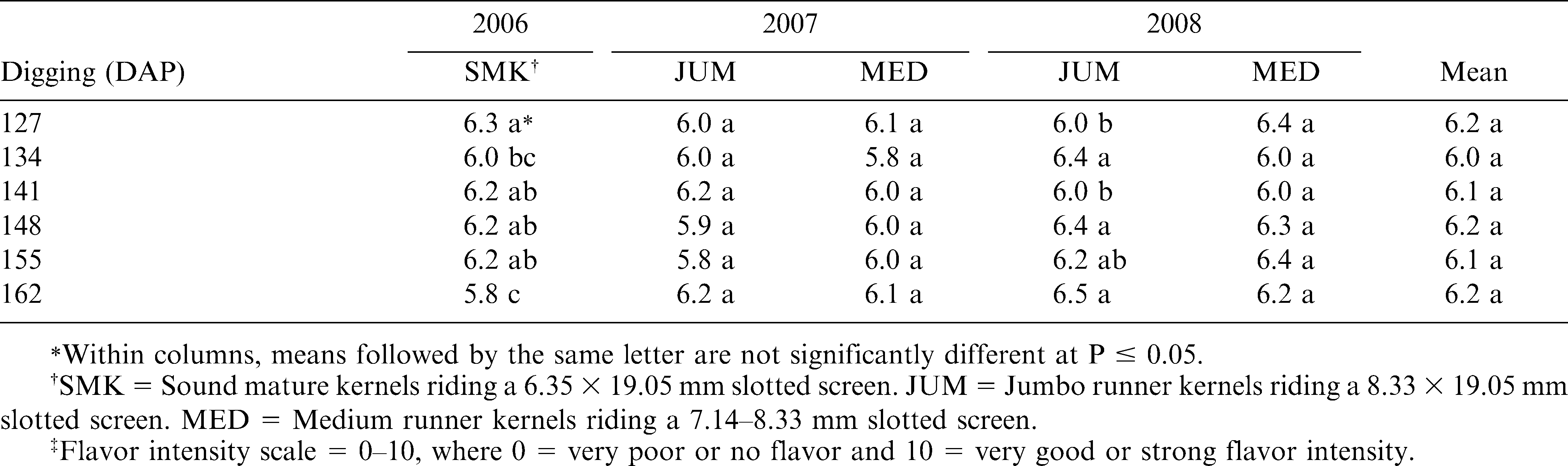
Seed samples from each rep and digging date were then sized into either sound mature kernels (SMK) riding a 6.35 × 19.05 mm slotted screen or jumbo runner kernels (JUM) riding a 8.33 × 19.05 mm slotted screen and medium runner kernels (MED) riding a 7.14 mm but falling through the 8.33 × 19.05 mm slotted screen. These seed samples were subsequently sent to J. Leek and Associates (JLA) Laboratory in Edenton, NC for flavor intensity sensory evaluations using a 0–10 scale where 0 = very poor or no flavor and 10 = very good or strong flavor intensity.
Gross dollar values were calculated from yield and grade based upon USDA – Commodity Credit Corporation (CCC) peanut loan schedules for each year. All data from each test were statistically analyzed by analysis of variance. Waller-Duncan's T-test (k-ratio = 100) was used for mean separations.
Results and Discussion
During 2005–09, the pod profile, yields, and dollar values for the Georgia-02C peanut cultivar resulted in significant differences (P ≤ 0.05) among the six digging dates 127, 134, 141, 148, 155, and 162 DAP (Table 2– 4). The five-year average rainfall and irrigation, maximum and minimum temperatures, and total number of growing degree days were all quite similar across each season during 2005–09 (Table 1). These data would suggest that observed significant differences would most likely be due to the inclusive maturation of the peanut cultivar Georgia-02C, rather than environmental effects in this study. It should be pointed out that recommended cultural practices with irrigation were utilized each year to help minimize any such confounding environmental effects on maturity. The resulting genotypic differences could then be used to determine the relative maturity range for harvest.
As suggested by Rowland et al (2006), since both brown and black pods are considered mature pods, combining these two pod groups provide a good indication of the relative maturity at a particular harvest date. The pod profile percentages resulted in a decrease in the white, yellow, and orange pod color from the first digging date (127 DAP) to the last digging date (162 DAP), and no significant differences were found in the brown pod color percentages across the six-digging dates (Table 2). However, a significant increase occurred in the black pod color percentages with the later digging dates. Consequently, the combined Bk and Br pod color percentages resulted in a progressive increase with each later digging date.
The brown pod color appears to be a transition stage between immature white, yellow, and orange pod color and the mature black pod color (Table 2). The percentage of brown + black pods increased almost linearly from the earliest digging (127 DAP) to the latest (162 DAP). The change in brown and black pod combination also shows a shift in maturity across the six-digging dates with 127–134 DAP having the fewest pods in the brown and black colors and 155–162 DAP having the most brown and black pods.
Based upon these pod profile percentages across the six-digging dates, the most mature pods were found at the later two digging dates 155–162 DAP. However, 155 DAP was not significantly different from 148 DAP in having the largest percentage of mature (Bk + Br) pod colors.
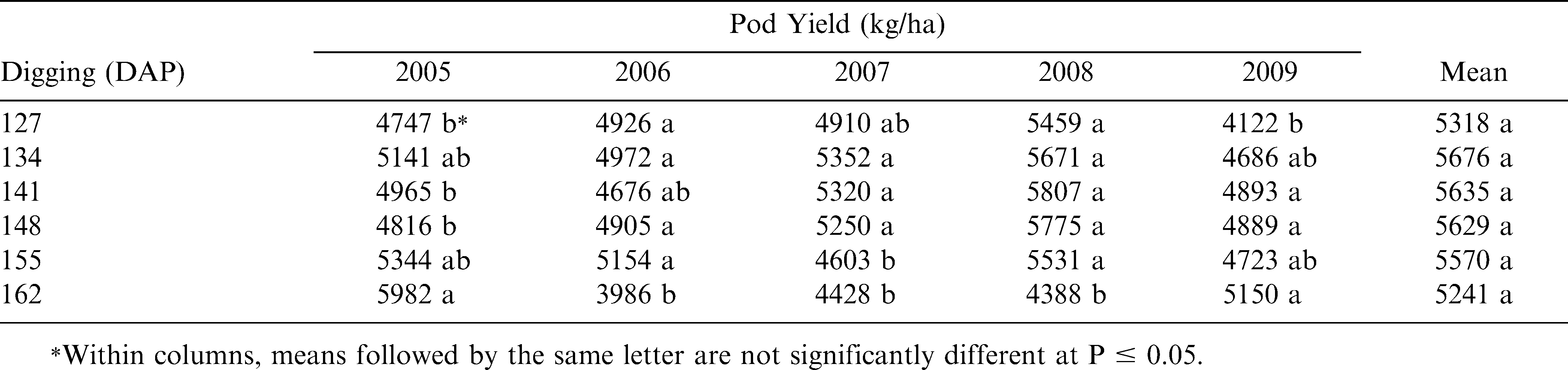
Pod yield results were quite variable and significant across years and locations (Table 3). During 2005 and 2009, the highest yields were obtained at 162 DAP, but were not significantly different from several other earlier digging dates. However in 2006, 2007, and 2008, the lowest yields were obtained at the same 162 DAP. Although the overall mean yields across this 5-yr study showed no significant difference, the 134–155 DAP during each year in general had the highest pod yields for the Georgia-02C peanut cultivar.
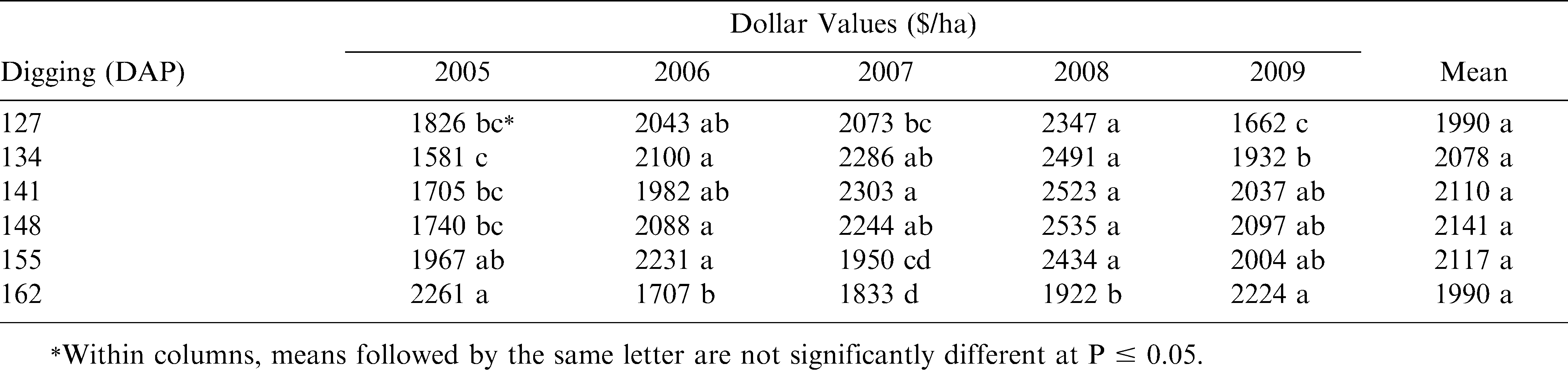
The ultimate determining factor for maturity range is the gross dollar value return per hectare which combines yield and grade. Here again, even though the overall mean dollar values across the 5-yr (2005–09) study showed no significant difference, the greatest dollar values were found most consistently at 141–155 DAP during each of the years (Table 4).
Three-year (2006–08) roasted peanutty flavor results seemed to be fairly uniform across the six-digging dates (Table 5). The roasted peanutty flavor intensity did not vary significantly among the 2007 jumbo runner, 2007 medium runner, and 2008 medium runner seed samples. However, significant (P ≤ 0.05) differences were found among the digging dates within the 2006 sound mature kernels and the 2008 jumbo runner seed samples. Also, there was a trend for more sweet taste intensity with the later-digging dates (data not shown).
Summary
These results would suggest that the Georgia-02C runner-type peanut cultivar has a considerably wider maturity range (14–21d) or harvest window, than compared to other medium-maturity cultivars like Florunner which would allow for more flexibility at digging time. However, pod yield and dollar values did vary from year-to-year. Such yearly variation should be expected even under these near-ideal recommended growing conditions, but Georgia-02C still performed quite uniformly. Similar type of studies would also be beneficial to determine the relative maturity range for other peanut cultivars.
Literature Cited
Branch W. D. 2003 Registration of ‘Georgia-02C’ peanut. Crop Sci 43 : 1883 – 1884 .
Branch W. D. and Brenneman T. B. 2003 Field resistance to Cylindrocladium black rot and tomato spotted wilt virus among advanced runner-type peanut breeding lines. Crop Protection 22 : 729 – 734 .
Branch W. D. and Brenneman T. B. 2009 Field evaluation for the combination of white mould and tomato spotted wilt disease resistance among peanut genotypes. Crop Protection 28 : 595 – 598 .
Brown S. L. 2006 Three-cornered alfalfa hopper damage continues to increase. 69 pp. In Prostko E. P. (ed.) 2006 Peanut Update. Univ. of GA Coop. Ext. Rept. CSS-06-0112.
Norden A. J. 1980 Peanut. pp. 443 – 456 In Fehr W. R. and Hadley H. H. (eds.) Hybridization of Crop Plants Amer. Soc. of Agron. and Crop Sci. Soc. of Am. Madison, WI.
Norden A. J. , Lipscomb R. W. , and Carver W. A. 1969 Registration of Florunner peanuts (Reg. no. 2). Crop Sci 9 : 850 .
Norden A. J. , Gorbet D. W. , Knauft D. A. , and Young C. T. 1987 Variability in oil quality among peanut genotypes in the Florida breeding program. Peanut Sci 14 : 7 – 11 .
Rowland D. L. , Sorensen R. B. , Butts C. L. , and Faircloth W. H. 2006 Determination of maturity and degree day indices and their success in predicting peanut maturity. Peanut Sci 33 : 125 – 136 .
Sanders T. H. 1995 Harvesting, storage, and quality of peanuts. pp. 23 – 31 In Melouk H. A. and Shokes F. M. (eds.) Peanut Health Management Amer. Phytopath. Soc. St. Paul, MN.
USDA-Agricultural Marketing Service 1998 Farmer's stock peanuts inspection instructions U. S. Dept. of Agric. – Agric. Mkt. Ser. Fruit and Veg. Div., USDA-ARS Washington, D. C .
Williams E. J. 2003 A simple, quick, inexpensive peanut blaster. Univ. of GA Coop. Ext. Ser. Misc. Publ. No. ENG 03-004.
Williams E. J. and Drexler J. S. 1981 A non-destructive method for determining peanut pod maturity. Peanut Sci 8 : 134 – 141 .
Notes
- University of Georgia, Dept. of Crop & Soil Sciences, Coastal Plain Experiment Station, Tifton, GA 31793-0748. [^]
- Alabama Crop Improvement Assoc., Wiregrass Research and Extension Center, Headland, AL. 36345-0357. [^]
- University of Georgia, Dept. of Biol. & Agric. Eng., Coastal Plain Experiment Station, Tifton, GA 31793-0748 (retired). [^] *Corresponding author (email: wdbranch@uga.edu)
Author Affiliations