Introduction
The indeterminate phenology of peanut makes digging decisions a challenge because of the wide range of pod maturity associated with each plant (Sanders et al., 1982). Virginia market type peanut in North Carolina require between 142 and 160 d from emergence to optimum pod maturity (Jordan, 2008a). Negative ramifications associated with abiotic or biotic stress can occur in some years (Wynne and Coffelt, 1980). Cooler temperatures in September and October can prevent pods from reaching full maturity (Jordan, 2008a). The balance between digging prior to optimum pod maturity but before pod shed due to inclement weather or disease is often difficult to realize (Jordan, 2008a). Timing of digging to obtain optimum yield and quality varies annually due to differences in environmental and edaphic conditions, cultivar selection, and plant health (Jordan, 2008a; Roberson, 2008; Shew, 2008; Young et al., 1982). Delaying digging often improves market grade characteristics even though pod yield is compromised due to pod shed (Jordan, 2008a). Increasing pod retention would be advantageous when environmental conditions require digging past the optimum timing (Beam et al., 2002).
The plant growth hormone ethylene plays a key role in the maturation process of peanut and is involved in several metabolic processes in most plant tissues (Tiaz and Zieger, 2006) at every stage of plant development (Tso, 1990). Ethylene is involved in physiological processes associated with senescence and ripening of leaves and fruit (Mattoo and Aharoni, 1988). Ethylene increases the sensitivity of the signaling pathway promoting senescence and ripening that is initiated by other age-related factors (Buchanan-Wollaston, 1997). Plants often respond to external stresses caused by biotic and abiotic agents by shedding non-essential vegetative and/or reproductive structures such as peanut pods (Buchanan-Wollaston, 1997; Mattoo and Aharoni, 1998).
Toole et al. (1964) reported that Virginia market type peanut seeds displaying dormancy were induced to germinate by ethylene. Seeds of other crops displaying non-dormancy exhibited better germination and growth when subjected to ethylene (Balls and Hale, 1940; Haber, 1926) where ethylene is responsible for the mobilization and utilization of stored carbohydrates and other constituent reserves (Ketring and Morgan, 1969). Gynophore development of peanut is accompanied by ethylene production (Ketring et al., 1982) with increased concentrations during initial stages of peg growth (Lee et al., 1972). Ethylene also affects gynophores by determining size and growth of peanut pods (Shlamovitz et al., 1995). Foliar applications of ethylene converted prostrate growth habit of peanut to a more upright growth habit (Ziv et al., 1976) with physical stunting noted at concentrations exceeding 1.0% (Ketring, 1977). Ketring and Schubert (1980) found that applications of ethylene to peanut resulted in either no effect or a decrease in yield and quality, with increased peg formation at the expense of pod formation. However, Azu (1979) reported that ethylene had no effect on peanut pod yield.
Several plant growth regulators have been previously evaluated in peanut. Prohexadione calcium (Beam et al., 2002), the auxin transport inhibitor cyclanilide (Jordan et al., 2004), the growth regulators Early Harvest® (Beasley et al., 2004) and PGR-IV® (York et al., 1996), the nitrophenolic plant growth regulator Chaperone® (Faircloth et al., 2007), and the harpin protein product Messenger® (Jordan et al., 2005). Most growth regulating materials have had minimal effects on pod yield or market grade characteristics.
The ethylene binding inhibition of 1-MCP was discovered in the late 1980's (Prange and Delong, 2003), and by 1999 was approved by the EPA for use in ornamental crops (Watkins, 2006). When binding between 1-MCP and the ethylene binding receptor occurs, signal transduction by ethylene is inactivated and physiological effects of endogenous and exogenous ethylene are prevented (Blankenship and Dole, 2003; Serek et al., 2006). The proposed limiting factor of 1-MCP efficacy is restricted absorption through leaf and fruit material where tissue structure and cuticle resistance limit gas diffusion of 1-MCP (Nanthachai, 2007).
Previous research has documented response of several vegetables, fruits, and flowers to 1-MCP (Zhang et al., 2007; Saftner et al., 2007; Jones et al., 2001; Chope et al., 2007; Sisler and Serek, 1997). Effects of 1-MCP on peanut and other agronomic row crops have not been evaluated or published in the scientific literature. Theoretically, 1-MCP may reduce the senescence and abscission of mature pods by inhibiting the ethylene cascade. Reducing senescence and abscission may provide greater harvest flexibility in peanut. Therefore, one objective of this research was to determine the effects of 1-MCP on pod yield and market grade characteristics when applied at 7 or 14 d before projected optimum digging date when peanut was dug on this date or when digging was delayed. A second objective was to determine the effects of 1-MCP on pod shed when 1-MCP was applied 10 d before projected optimum digging date when peanut was dug on this date or when digging was delayed.
Methods and Materials
Three separate experiments were conducted in North Carolina to evaluate the effects of 1-MCP on pod yield, market grade characteristics, and pod retention of peanut. In the first experiment, the cultivar NC-V 11 (Wynne et al., 1991) was evaluated during 2005 at the Peanut Belt Research Station near Lewiston-Woodville, NC on a Goldsboro sandy loam soil (fine-loamy, siliceous subactive, thermic, Aquic Paleudults) and in 2007 at the Upper Coastal Plain Research Station near Rocky Mount, NC on a Goldsboro sandy loam soil (fine-loamy, mixed, semiactive, thermic Typic Hapludults). In the second experiment, the cultivar Phillips (Isleib et al., 2006) was evaluated during 2006 and 2007 at the Upper Coastal Plain Research Station on a Goldsboro sandy loam soil (fine-loamy, mixed, semiactive, thermic Typic Hapludults). The final experiment was conducted during 2008 in four separate fields at the Peanut Belt Research Station with the cultivar Perry (Isleib et al., 2003). Soil in three fields was a Rains sandy loam (fine-loamy siliceous, semiactive, thermic Typic Paleaquults) while soil in the fourth field was a Pantego loam (fine-loamy, siliceous, semiactive, thermic Umbric Paleaquults). Experiments were established in conventional tillage systems on raised seed beds with a plot size of 2 rows (91-cm spacing) by 9-m long. Peanut was seeded at a rate to obtain a final in-row population of 13 plants/m.
Treatments consisted of 1-MCP at 26 g/ha applied 7 and 14 d prior to the projected optimum pod maturity as determined by the hull scrape method (Williams and Drexler, 1981) in experiments with NC-V 11 and Phillips. In the experiment with Perry, 1-MCP was applied at 26 g/ha at 10 d prior to the projected optimum pod yield as determined by the hull scrape method (Williams and Drexeler, 1981). Peanut was dug at projected optimum pod maturity and 7 d after projected optimum pod maturity in experiments with NC-V 11 and Phillips. Experiments with the cultivar Perry were dug at projected optimum pod maturity and 20 d after projected optimum pod maturity. A no-1-MCP control was included for all digging dates in all experiments. Crop oil concentrate at 1.0% (v/v) (Agri-Dex®, Helena Chemical Co., Memphis TN) was included immediately after mixing 1-MCP in distilled water. Treatments were applied with a CO2-pressurized backpack sprayer calibrated to deliver 281 L/ha using regular flat fan nozzles (Spraying System Co., Wheaton, IL).
The treatment structure consisted of either a factorial arrangement of three 1-MCP treatments (experiments with NC-V 11 and Phillips) or two 1-MCP treatments (experiment with Perry) and two digging dates. Agronomic and pest management practices other than applications of 1-MCP and digging dates were held constant over the entire experiment and were based on Cooperative Extension Service recommendations specific to each location (Brandenburg, 2008; Jordan, 2008a, 2008b; Shew, 2008).
Peanut was allowed to air dry for 5 to 8 d after digging prior to threshing and final pod yield was adjusted to 8% moisture. A 500-g sample of pods from each plot was removed and used to determine percentages of sound mature kernels (%SMK), sound splits (%SS), total sound mature kernels (%TSMK), extra large kernels (%ELK), other kernels (%OK), and fancy pods (%FP) in experiments with Phillips (2006 and 2007) and Perry (2008). Pod yield only was determined in experiments with NC-V 11 (2005 and 2007); market grades were not recorded for this cultivar. The effect of 1-MCP on pod retention, measured by counting the number of pods from five randomly selected plants in each plot within 5 d after digging was determined in the experiment with the cultivar Perry (2008). It is postulated that a higher number of pods remaining on plants reflects pod retention, assuming pod set occurred prior to 1-MCP application.
The experimental design for all experiments was a randomized complete block with treatments replicated four times. Data for pod yield, %SMK, %SS, %TSMK, %ELK, %OK, %FP, and pod number and mass from five plants were subjected to analysis of variance appropriate for the factorial arrangement of treatments for individual experiments. Means for significant main effects and interactions were separated using Fisher's protected LSD test at P ≤ 0.05.
Results and Discussion
Experiments with the cultivars NC-V 11 and Phillips
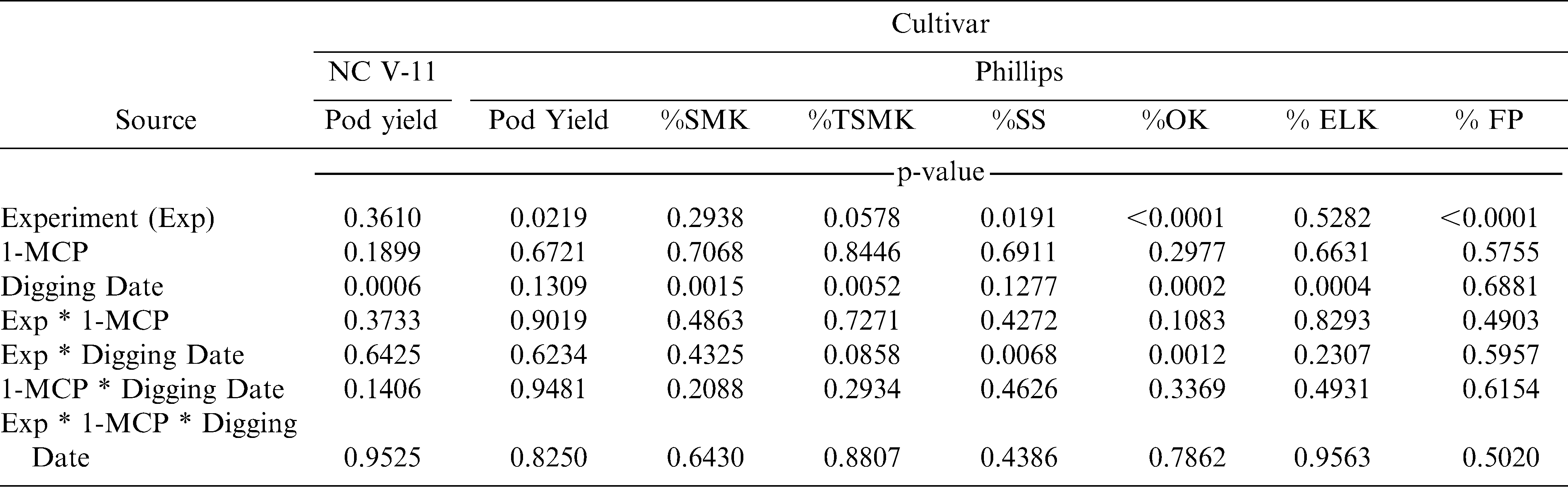
In the experiment with NC-V 11, the interaction of 1-MCP by digging date (p = 0.1406) and the main effect of 1-MCP (p = 0.1899) were not significant for pod yield (Table 1). However, the main effect of digging date was significant (p = 0.0006) (Table 1). When pooled over years and 1-MCP treatment, pod yield increased from 4930 kg/ha to 5800 kg/ha when digging was delayed (data not shown).
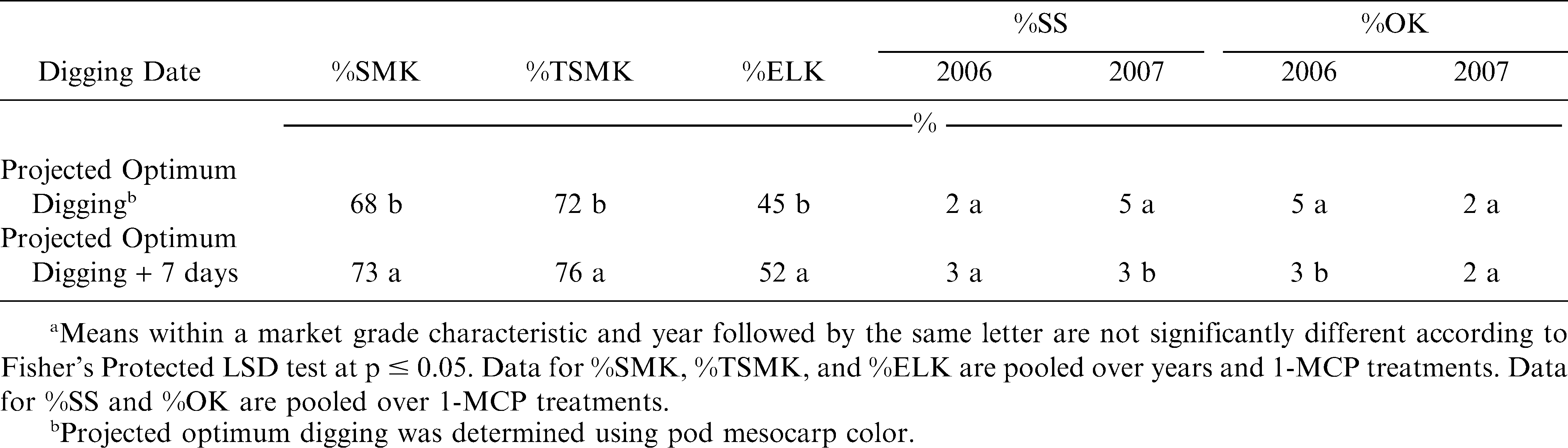
In the experiment with the cultivar Phillips, main effects and interactions were not significant for pod yield (Table 1). However, either the main effect of digging date or the interaction of experiment by digging date were significant for %SMK, %TSMK, %SS, %OK, and %ELK (Table 1). Delaying digging increased %SMK, %TSMK, and %ELK in all experiments but decreased %SS and %OK in one of two experiments (Table 2).
Experiment with the cultivar Perry
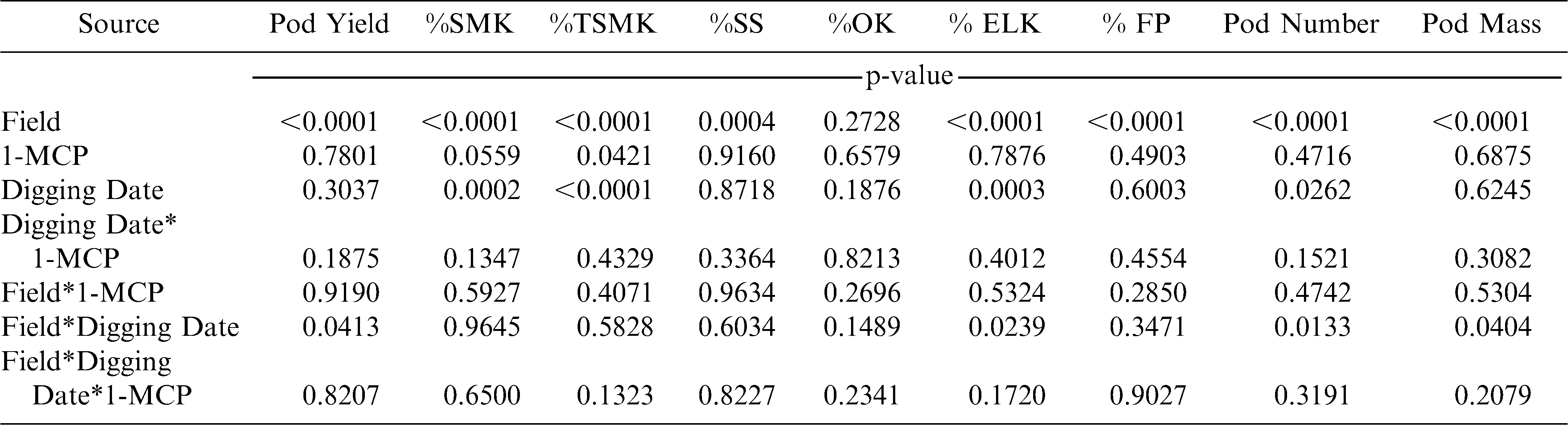
Pod yield, %SMK, %TSMK, and %ELK were affected by digging date or field by digging date (Table 3). As was noted for the cultivars NC-V 11 and Phillips, 1-MCP had no effect on pod yield of the cultivar Perry (Table 3). With the exception of %SMK and %TSMK, 1-MCP had no affect on market grade characteristics (Table 3).
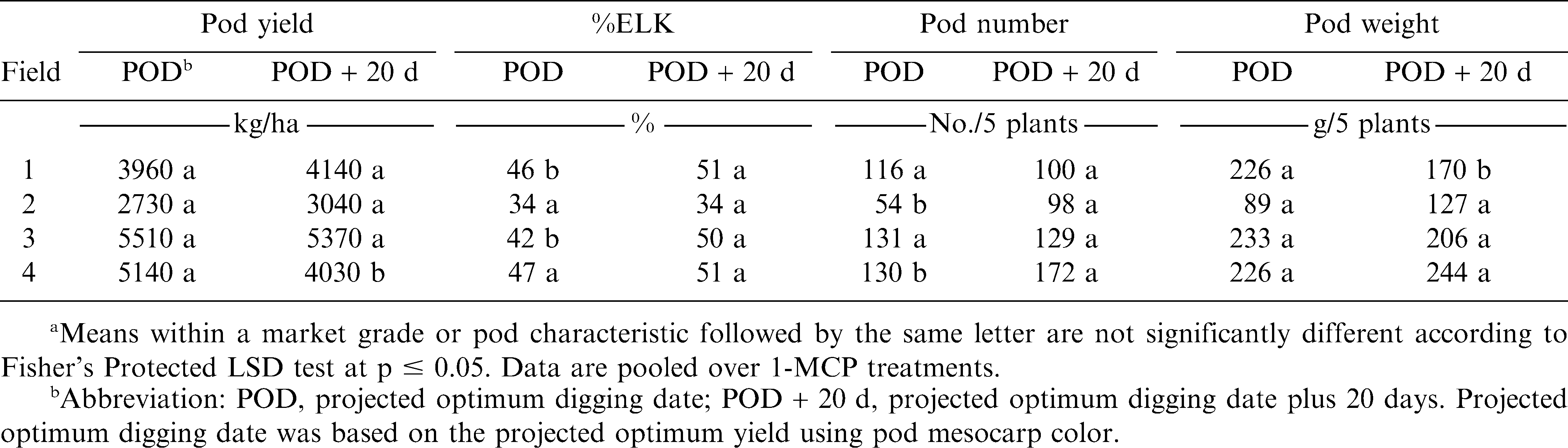
In three of four fields pod yield did not differ when digging was delayed by 20 d (Table 4). However, in one field pod yield decreased when digging was delayed past the projected optimum (Table 4). Additionally, %ELK increased in two fields when digging was delayed by 20 d (Table 4). When pooled over the four fields and 1-MCP treatments, both %SMK and %TSMK increased from 62 to 69% and 66 to 69%, respectively, when digging was delayed past the optimum digging date (data not shown). In contrast to results with the cultivar Phillips, %SMK and %TSMK increased from 64 to 65% and 67 to 69%, respectively, when 1-MCP was applied when compared with non-treated peanut (data not shown). Previous research (Mozingo et al., 1991) indicated that delaying digging past optimum maturity can result in lower yields but an increase in %ELK.
The number of pods and the mass of pods was affected by the interaction of field and digging date (Table 3). In two of four fields, pod number increased when digging was delayed while pod mass decreased when digging was delayed in one field (Table 4).
Collectively, results for these experiments suggest that 1-MCP applied at 7, 10, or 14 d prior to the projected optimum digging date most likely will not affect pod yield, the majority of most market grade characteristics, and pod retention of peanut. Several factors may have influenced peanut response to 1-MCP. The most limiting factor of 1-MCP may be absorption through leaf material (Nanthachai, 2007). Effectiveness of 1-MCP is also based on concentration, application timing, plant maturity, and plant species (Blankenship and Dole, 2003; Watkins, 2006). Concentrations of 1-MCP may have been too low or applied after endogenous ethylene had already acquired a position in the ethylene binding site of peanut. Inactivity of 1-MCP could also be due to volatilization of the compound before or during application. Additionally, peanut pods are underground and ability of 1-MCP to reach critical sites of ethylene binding associated with pods may have been difficult. Although these experiments suggest that 1-MCP may not affect peanut, additional research with higher rates, different application timings, and scenarios with extremes in environmental and edaphic conditions are needed to definitively determine potential of 1-MCP in peanut.
Acknowledgements
Funding for these studies was provided by the North Carolina Peanut Growers Association. Appreciation is expressed to the staff at the Peanut Belt Research and Upper Coastal Plain Experiment Stations and to Dewayne Johnson and Carl Murphey for assisting with these experiments.
Literature Cited
Azu J. N. 1979 Growth and development of Spanish peanuts (Arachis hypogaea L.) in southern Ontario. Ph.D. Diss., Univ. of Guelph, Ontario, Canada, Diss. Abstr. Intl. 40 : 524B .
Balls A. K. and Hale W. S. 1940 The effect of ethylene on freshly harvested wheat. Cereal Chem 17 : 490 – 494 .
Beam J. B. , Jordan D. L. , York A. C. , Isleib T. G. , Bailey J. E. , McKemie T. E. , Spears J. F. , and Johnson P. D. 2002 Influence of prohexadione calcium on pod yield and pod loss of peanut. Agron. J 94 : 331 – 336 .
Beasley J. P. , Grichar W. J. , Jordan D. L. , Lemon R. G. , Besler B. A. , Brewer K. D. , Beam J. B. , Johnson P. D. , and Paulk J. E. 2004 Peanut (Arachis hypogaea L.) response to the hormonal plant growth regulator early harvest. Peanut Sci 31 : 70 – 73 .
Blankenship S. M. and Dole J. M. 2003 1-Methylcyclopropene: a review. Postharvest Bio. and Tech 28 : 1 – 25 .
Brandenburg R. L. 2008 Peanut insect and mite management. 73 – 92 In Peanut Information 2008. Pub. Ag-331, North Carolina Coop. Ext. Serv, Raleigh, NC 143 .
Buchanan-Wollaston V. 1997 The molecular biology of leaf senescence. Experimental Botany 48 : 181 – 199 .
Chope G. A. , Terry L. A. , and White P. J. 2007 The effects of 1-methylcyclopropene (1-MCP) on the physical and biochemical characteristics of onion cv. SS1 bulbs during storage. Postharvest Bio. and Tech 44 : 131 – 140 .
Faircloth J. C. , Jordan D. L. , Coker D. L. , Johnson P. D. , and White G. U. 2007 Virginia market type peanut (Arachis hypogaea L.) response to the nitrophenolic plant growth regulator Chaperone®. Peanut Sci 34 : 105 – 108 .
Haber E. S. 1926 A preliminary report on the stimulation of growth of bulbs and seeds with ethylene. Am. Soc. Hort. Sci 23 : 201 – 203 .
Isleib T. G. , Rice P. W. , Mozingo R. W. , Bailey J. E. , Mozingo R. W. , and Pattee H. E. 2003 Registration of “Perry” peanut. Crop Sci 43 : 739 – 740 .
Isleib T. G. , Rice P. W. , Mozingo R. W. , Copeland S. C. , Graeber J. B. , Pattee H. E. , Sanders T. H. , Mozingo R. W. , and Coker D. L. 2006 Registration of “Phillips” peanut. Crop Sci 46 : 2308 – 2309 .
Jones M. L. , Kim E. S. , and Newman S. E. 2001 Role of ethylene and 1-MCP in flower development and petal abscission in zonal geraniums. Hort. Sci 36 : 1305 – 1309 .
Jordan D. L. 2008a Peanut production practices. 21 – 44 In Peanut Information 2008. Pub. Ag-331, North Carolina Coop. Ext. Serv, Raleigh, NC 134 .
Jordan D. L. 2008b Weed management in peanuts. 45 – 48 In Peanut Information 2008. Pub. Ag-331, North Carolina Coop. Ext. Serv, Raleigh, NC 134 .
Jordan D. L. , Beam J. B. , Lanier J. E. , Lancaster S. H. , and Johnson P. D. 2004 Peanut (Arachis hypogaea L.) response to cyclanilide and prohexadione calcium. Peanut Sci 31 : 33 – 36 .
Jordan D. L. , Faircloth J. C. , Lancaster S. H. , Lanier J. E. , Johnson P. D. , and White G. U. 2005 Peanut (Arachis hypogaea L.) response to the harpin protein product Messenger®. Peanut Sci 32 : 144 – 147 .
Ketring D. L. 1977 Physiology of oil seeds. Part VI. A means to break dormancy of peanut (Arachis hypogaea L.) seeds in the field. Peanut Sci 4 : 42 – 45 .
Ketring D. L. and Schubert A. M. 1980 Growth, flowering and fruiting responses of peanut (Arachis hypogaea L.) plants to ethrel. Crop Sci 20 : 327 – 329 .
Ketring D. L. and Morgan P. W. 1969 Ethylene as a component of the emanations from germinating peanut seeds and its effect of dormant Virginia-type seeds. Plant Physiol 44 : 326 – 330 .
Ketring D. L.
,
Brown R. H.
,
Sullivan G. A.
, and
Johnson B. B.
1982
Growth Physiology.
In
Pattee H. E.
and
Young C. T.
(eds.)
Peanut Science and Technology,
American Peanut Research and Education Society, Inc.,
Yoakum, TX
.
Lee T. A. , Ketring D. L. , and Powell R. D. 1972 Flowering and growth response of peanut plants (Arachis hypogaea L. var. Starr) at two levels of relative humidity. Plant Physio 49 : 190 – 193 .
Mattoo A. K. and Aharoni N. 1988 Ethylene and Plant Senescence. In Nooden L. D. and Leopold A. C. (eds.) Senescence and Aging in Plants, Academic Press, New York, USA .
Mozingo R. W. , Coffelt T. A. , and Wright F. S. 1991 The influence of planting and digging dates on yield, value, and grade of four Virginia type peanut cultivars. Peanut Sci 18 : 5 – 62.
Nanthachai N. , Ratanachinakorn B. , Kosittrakun M. , and Beaudry R. M. 2007 Absorption of 1-MCP by fresh produce. Postharvest Bio. and Tech 43 : 291 – 297 .
Prange R. K. and Delong J. M. 2003 1-Methylcyclopropene: The “magic bullet” for horticultural products? Chronica Horticulturae 42 (1) : 11 – 14 .
Roberson G. T. 2008 Planting, harvesting and curing peanuts. 121 – 134 In Peanut Information 2008. Pub. Ag-331, North Carolina Coop. Ext. Serv, Raleigh, NC 134 .
Saftner R. , Luo Y. , McEvoy J. , Abbott J. A. , and Vinyard B. 2007 Quality characteristics of fresh-cut watermelon slices from non-treated and 1-methylcyclopropene and/or ethylene-treated whole fruit. Postharvest Bio. and Tech 44 : 71 – 79 .
Sanders T. H. , Schubert A. M. , and Pattee H. E. 1982 Maturity methodology and Postharvest Physiology. In Pattee H. E. and Young C. T. (eds.) Peanut Science and Technology, American Peanut Research and Education Society, Inc, Yoakum, TX 77995 .
Serek M. , Woltering E. J. , Sisler E. C. , Frello S. , and Sriskandarajah S. 2006 Controlling ethylene response in flowers at the receptor level. Biotech. Adv 24 : 368 – 381 .
Shew B. B. 2008 Peanut disease management. 93 – 105 In Peanut Information 2008. Pub. Ag-331, North Carolina Coop. Ext. Serv, Raleigh, NC 134 .
Shlamovitz N. , Ziv M. , and Zamski E. 1995 Light, dark, and growth regulator involvement in groundnut (Arachis hypogaea L.) pod development. Plant Growth Regul 16 : 37 – 42 .
Sisler E. C. and Serek M. 1997 Inhibitors of ethylene responses in plants at the receptor level: recent developments. Physiol. Plant 100 : 577 – 582 .
Tiaz L. and Zeiger E. 2006 Plant Physiology 4th Edition Sinauer Associates, Inc Sunderland, MA, USA .
Toole V. K. , Bailey W. K. , and Toole E. H. 1964 Factors influencing dormancy of peanut seeds. Plant Physiol 39 : 822 – 832 .
Tso T. C. 1990 Production, physiology, and biochemistry of tobacco plant IDEALS, Inc Beltsville, MA .
Watkins C. B. 2006 The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotech. Adv 24 : 389 – 409 .
Williams E. J. and Drexler J. S. 1981 A non-destructive method for determining peanut pod maturity. Peanut Sci 8 : 134 – 141 .
Wynne J. C. and Coffelt T. A. 1980 The effect of genotype x environment interactions on varietal development in North Carolina and Virginia. Proc. Amer. Peanut Res. and Educ. Soc 12 : 75 .
Wynne J. C. , Coffelt T. A. , Mozingo R. W. , and Anderson W. F. 1991 Registration of “NC-V 11” peanut. Crop Sci 31 : 484 – 485 .
York A. C. , Batts R. B. , and Culpepper A. S. 1996 Response of peanut to PGR-IVTM growth regulator. Peanut Sci 12 : 54 – 57 .
Young J. H. , Person N. K. , Donald J. O. , and Mayfield W. D. 1982 Harvesting curing and energy utilization. In Pattee H. E. and Young C. T. (eds.) Peanut Science and Technology, American Peanut Research and Education Society, Inc, Yoakum, TX 77995 .
Zhang M. , Jiang Y. , Jiang W. , and Liu X. 2006 Regulation of ethylene synthesis of harvested banana fruit by 1-methylcyclopropene. Food Tech. Biotech 44 : 111 – 115 .
Ziv M. , Koller D. , and Halevy A. H. 1976 Ethylene and the geotropic response of lateral branches in peanuts. Plant and Cell Physiology 17 : 333 – 339 .
Notes
Author Affiliations
1 Res. Spec., Assist. Prof., and Prof., Dept. Crop Sci., North Carolina State Univ., Box 7620, Raleigh, NC 27695.
*Corresponding author e-mail: david_jordan@ncsu.edu