Introduction
Peanut has several unique features that contribute to challenging weed management. Peanut cultivars grown in the United States require a fairly long growing season (140 to 160 d), depending on cultivar and geographical region (Henning et al., 1982; Wilcut et al., 1995). Consequently, soil-applied herbicides may not provide season-long control and mid-to-late season weed pressure can occur. Peanut also has a prostrate growth habit, a relatively shallow canopy, and is slow to shade inter-rows allowing weeds to be more competitive (Walker et al., 1989; Wilcut et al., 1995). Additionally, peanut fruit develops underground on pegs originating from branches that grow along the soil surface. This prostrate growth habit and pattern of fruit development restricts cultivation to an early season control option (Brecke and Colvin, 1991; Wilcut et al., 1995). With conventional row spacing (91 to 102 cm), complete ground cover may not be attained until 8 to 10 wk after planting. In some areas of the U.S. peanut growing region, complete canopy closure may never occur.
Weeds compete with peanut for sunlight, moisture, and nutrients and can reduce harvesting efficiency. Weeds are particularly troublesome during digging and inverting procedures (Young et al., 1982). Weed biomass slows field-drying of peanut vines and pods and increases the likelihood of exposure to rainfall, which can increase harvesting losses (Young et al., 1982; Wilcut et al., 1995). The fibrous root system of annual grasses is extremely difficult to separate from peanut (Wilcut et al., 1994c).
The Classic® label (E.I. du Pont, Inc. Crop Protection Division, Wilmington, DE) states that chlorimuron will control several weeds that are a problem in Texas peanut production including several Ipomoea and Amaranthus species, and yellow nutsedge (Cyperus esculentus L.). The Classic® label does note that peanut cultivar tolerance differences may be observed. In particular, the cultivar ‘Southern Runner’ (Gorbet et al., 1987) has shown moderate tolerance to chlorimuron; however, the label does not exclude any cultivar from use (Wehtje and Grey 2004). Southern Runner is no longer commercially grown. Additional research indicated that chlorimuron can result in increased occurrence of spotted wilt of peanut, caused by tomato spotted wilt Tospovirus (TSWV). This increased occurrence has not been linked to a specific application timing (Brown et al. 2003).
The current commercial POST Florida beggarweed [Desmodium tortuosum (Sw.) DC] control program consists of paraquat plus bentazon applied once or twice within 28 d of peanut emergence followed by chlorimuron applied at 60 d after peanut emergence (Webster et al., 1997; Wilcut et al., 1995). However, by 60 d after emergence, Florida beggarweed plants are generally taller than the 25-cm height specified on the chlorimuron label (Anonymous, 2007; Cardina and Brecke, 1991). Wehtje et al. (2000) reported that Florida beggarweed control was 86% with chlorimuron compared to 73% without chlorimuron when averaged across herbicide systems which included PRE, early POST, and mid POST applications.
Chlorimuron cannot be applied to peanut until 60 d after peanut emergence due to crop tolerance (Johnson et al., 1992a,b; Patterson et al. 1989; Wilcut et al., 1989). At 60 d after peanut emergence, peanut absorption of chlorimuron is relatively minimal and what is absorbed is readily metabolized (Wilcut et al., 1989). However, peanut injury can occur even with correct application timing (Wehtje and Grey 2004).
Chlorimuron was implicated in yield suppression in 4 out of 15 trials conducted across the peanut-production region (Brown et al., 1993). It was concluded that the risks of yield loss from Florida beggarweed competition was greater than that from chlorimuron-induced crop injury. The application timing restriction for chlorimuron was based upon field research conducted in the late 1980 s (Patterson et al., 1989). The absorption and metabolism of chlorimuron was evaluated in 3-, 7-, and 10-wk-old peanut plants (Wilcut et al., 1989), and tolerance to chlorimuron was plant age-dependent. This research, conducted with ‘Florunner’ which was then the dominant peanut cultivar, indicated that increased tolerance was attributed to combined effects of reduced absorption and translocation and more extensive metabolism by older plants (Wilcut et al., 1989).
Imazethapyr is used for control of Palmer amaranth (Amaranthus palmeri S. Wats) and morningglory species (Wilcut et al., 1995; Grichar et al., 2004). Imazethapyr at 50 to 70 g ha−1, acifluorfen alone or in combination with bentazon or 2,4,-DB, and lactofen alone, controlled Palmer amaranth greater than 90% in 2 of 3 years of a Texas study (Grichar, 1997b). Imazethapyr control of pitted morningglory was not consistent with PPI applications (Grichar, 1997a). Shaw et al. (1990) reported greater than 90% pitted morningglory control with imazethapyr POST when applied to weeds at the three-leaf stage of development. They noted that imazethapyr applied at the six-and nine-leaf stages provided less than 85% morningglory control. Smallflower morningglory [Jacquemontia tamnifolia (L.) Griseb.] control was at least 88% with PPI imazethapyr applications (Richburg et al., 1995a,b). Rates of imazapic or imazethapyr as low as 36 g ha−1 have controlled smallflower morningglory (Richburg et al., 1995a). No differential response in control of Ipomoea species with imazapic or imazethapyr has been reported (Richburg et al., 1995a; Wilcut, 1991; Wilcut et al., 1994a,b,c).
Chlorothalanil is commonly used to control foliar diseases while 2,4-DB controls broadleaf weed escapes. The combination of 2,4-DB with many POST herbicides improves control of many broadleaf species, particularly if the broadleaf weeds are larger than recommended (Buchanan et al. 1982).
Because of the low growing nature of peanut, weeds that germinate early and are not controlled, “escape” relatively late in the growing season (Buchanan et al., 1982). Covering weeds with soil during cultivation is not practical and creates conditions conducive to foliage, stem, and pod diseases caused by soil-borne fungi (Buchanan et al., 1982; Melouk and Shokes, 1995; Porter et al., 1982). Weed removal is extremely difficult once weeds become established in the peanut row. After peanut and weeds achieve some growth, mechanical removal with tractor-mounted cultivators is impossible. Hand weeding is difficult, costly, and unrealistic under modern day conditions (Grichar et al., 2004). Consequently, peanut growers have readily adopted chemical weed control practices. However, Texas growers have been hesitant to use chlorimuron due to the potential for crop injury.
Since chlorimuron may have potential to control some weeds that are a problem in Texas peanut, the objective of this study was to evaluate peanut response to POST applications of chlorimuron alone, imazethapyr followed by POST applications of chlorimuron, or chlorimuron in combination with 2,4-DB, or chlorothalanil, in two different peanut growing regions of Texas.
Materials and Methods
Field studies were conducted at two locations in the peanut growing regions of Texas in 2005 and 2006 to determine peanut response to chlorimuron alone, following imazethapyr or in combination with 2,4-DB or chlorothalonil. The south Texas study was located at the Texas AgriLife Research Station site near Yoakum, TX on a Tremona loamy fine sand (thermic Aquic arenic Paleustalfs) with less than 1% organic matter and pH 7.0 to 7.2. The southern High Plains location was at the Agricultural Complex for Research and Extension Center (AG-CARES) located near Lamesa, TX on an Amarillo fine sandy loam (fine-loamy, mixed, superactive, thermic Aridic Paleustalf) with 0.4% organic matter and pH 7.8.
Peanut cultivars in south Texas included Tamrun 96 (Smith et al. 1998) in 2005 and Tamrun OL02 (Simpson et al. 2006) in 2006. For the southern High Plains location, FlavorRunner 458 (Mycogen Seeds, Indianapolis, IN) (Beasley and Baldwin, 2009) was planted in 2005 and Tamrun OL02 in 2006. Peanut seeding rate was 112 kg ha−1 at both locations. Each plot in south Texas consisted of two rows spaced 97 cm apart and 7.6 m long while in the southern High Plains plots were two rows spaced 102 cm apart and 9.1 cm long. Sprinkler irrigation was applied on a 2- to 3-wk schedule throughout the growing season as needed.
The experiment was conducted as a randomized complete block design with a factorial arrangement of four herbicide treatments and three application timings with three replications. An untreated check was included for each experiment. One factor was herbicide treatment which included chlorimuron (Classic®, E. I. du Pont, Inc., Crop Protection Division, Wilmington, DE) alone at 9 g ha−1, imazethapyr (Pursuit®, BASF Corporation, P.O. Box 13528, Research Triangle Park, NC 27709) at 70 g ha−1 applied early postemergence (EPOST) approximately three weeks after peanuts were planted followed by (fb) chlorimuron at 9 g ha−1, chlorimuron plus 2,4-DB (Butoxone 200®, S. R. F. A. LLC, One Hallow Lane, Lake Success, NY) at 220 g ha−1, and chlorimuron plus chlorothalonil (Bravo Weather Stik®, Syngenta Crop Protection, P. O. Box 18300, Greensboro, NC) at 1.26 kg ha−1. The other factor was chlorimuron application timings of 67, 81, or 95 DAP in south Texas or 60, 74, or 88 DAP in the southern High Plains. All POST applications included a non-ionic surfactant (Induce®, Helena Chemical Co., 7576 N. Ingram Ave. 101, Fresno, CA 93711) at 0.25% v/v.
Herbicides at the south Texas location were applied with a compressed-air backpack sprayer equipped with Teejet 11002 DG flat fan spray tips (Spraying Systems Company, P.O. Box 7900, North Avenue, Wheaton, IL 60188) which delivered a spray volume of 190 L ha−1 at 180 kPa. At the High Plains location, herbicides were applied with a CO2 pressurized backpack sprayer using Teejet 110015 TT flat fan nozzles calibrated to deliver a spray volume of 140 L ha−1 at 207 kPa. The test areas were maintained weed-free with a preplant incorporated treatment of pendimethalin (Prowl 3.3 EC®, BASF Corporation, P. O. Box 13528, Research Triangle Park, NC 27709) at 1.12 kg ha−1. At the south Texas location, clethodim (Select®, Valent Corp., Walnut Creek, CA 94596) at 180 g ha−1 was applied over the entire test area when annual grasses were at the six- to eight-leaf stage with a tractor-mounted sprayer to control Texas millet [Urochloa texana (Buckl.) R. Webster].
Peanut injury, expressed as stunting, was visually estimated on a scale of 0 to 100 (0 indicating no stunting and 100 indicating complete stunting or plant kill), relative to the untreated check (Frans et al., 1986). Peanut stunting evaluations recorded six wks after last POST chlorimuron treatment are presented. Peanut yields were obtained by digging each plot separately, air-drying in the field for 4 to 7 d, and harvesting peanut pods from each plot with a combine. Weights were recorded after soil and trash were removed from plot samples. Grade samples were determined using screens specified in USDA grading procedures (USDA, 1993).
Peanut injury data were transformed to the arcsine square root prior to analysis of variance, but are expressed in their original form for clarity because the transformation did not alter interpretation. Visual estimates of peanut injury, yield, and grade were subjected to analysis of variance to test effects of POST herbicide and application timing. Means were compared with the appropriate Fisher's Protected LSD test at the 5% probability level. The untreated check was not included in peanut stunting analysis but was included in peanut yield and grade analysis. There was an embedded factorial of chlorimuron herbicide by application timing; therefore, data were subjected to ANOVA unstructured.
Results and Discussion
Analysis of variance indicated that the two-way interactions between the four chlorimuron treatments and three application timings were not significant for all measured variables. Therefore, data for the main effects were combined for presentation. Data is presented by year because a different peanut cultivar was planted each year at both locations. Climatic and weather conditions varied between the two locations; therefore, no attempt was made to combine data over locations.
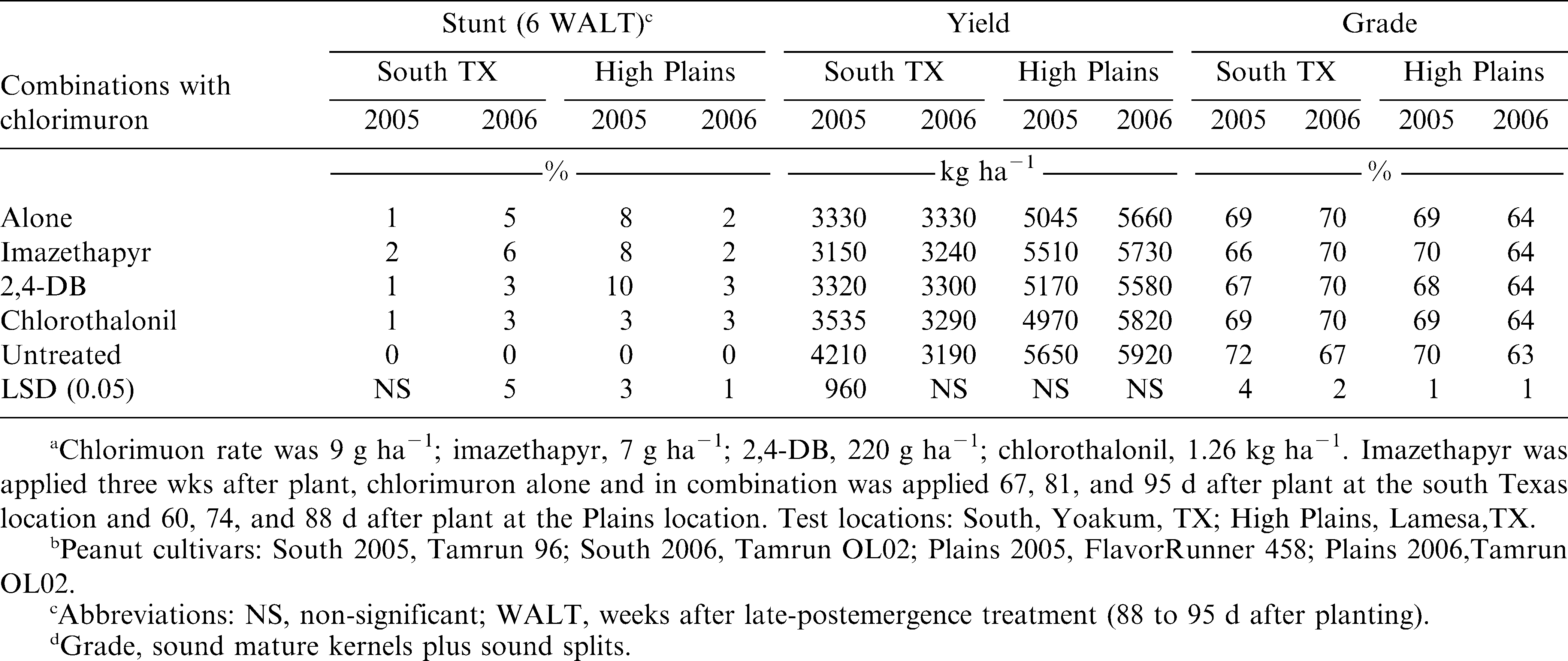
Chlorimuron treatment applications
Peanut stunting observations recorded approximately six weeks after the last chlorimuron POST application indicated that, in 2005 in south Texas, 2% or less stunting was visible. In contrast, in 2006, chlorimuron alone or imazethapyr followed by chlorimuron applications reduced visual peanut growth (5 and 6%, respectively) when compared with the untreated check (Table 1). At the southern High Plains location in 2005, chlorimuron alone, imazethapyr followed by chlorimuron, and 2,4-DB plus chlorimuron applications resulted in greater stunting than chlorimuron plus chlorothalonil combinations while in 2006 chlorimuron plus either chlorothalonil or 2,4-DB caused greater peanut injury than chlorimuron alone or imazethapyr followed by chlorimuron applications (Table 1).
In 2005 at the south Texas location, chlorimuron in combination with imazethapyr resulted in a significant (3150 kg ha−1) reduction in yield as compared to the nontreated control (4210 kg ha−1) (Table 1). No other yield differences between the nontreated control and any chlorimuron treatments were noted at any location. Wehtje and Grey (2004) reported at three of four locations in Alabama and Georgia, chlorimuron did not adversely affect peanut yield when different peanut cultivars were used while Grichar et al. (1997c) report that imazethapyr had no adverse effect on peanut yield.
Peanut grade results were inconsistent at both locations (Table 1). In 2005, at the south Texas location, percentage grade from the untreated check was greater than either imazethapyr followed by chlorimuron or chlorimuron plus 2,4-DB combinations. In 2006, percentage grade was less with the untreated check than all chlorimuron treatments. At the High Plains location in 2005, percentage grade was higher with the untreated check and imazethapyr followed by chlorimuron applications than either chlorimuron alone or chlorimuron applied in combination with either chlorothalonil or 2,4-DB. In 2006, all chlorimuron treatments resulted in higher percentage grade than the untreated check (Table 1). No research could be found that reported an increase in peanut grade when using chlorimuron.
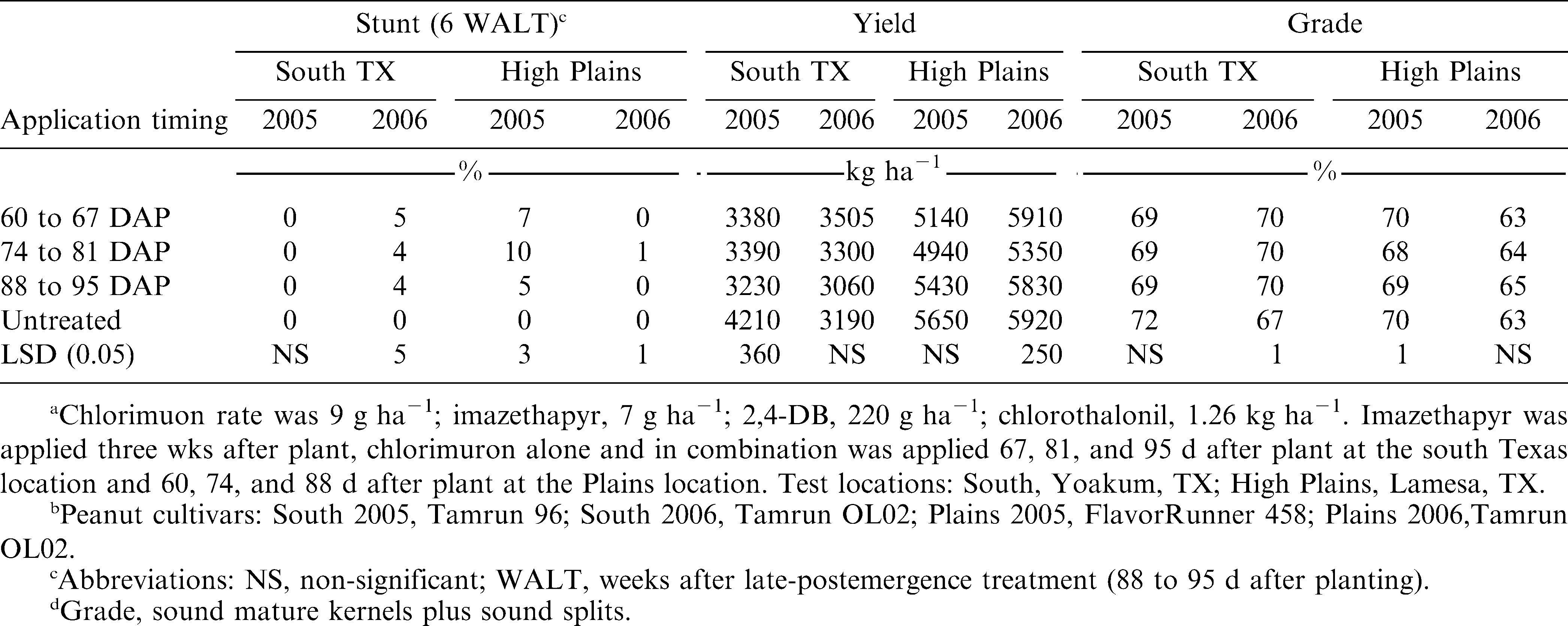
Chlorimuron timing applications
Peanut growth was not affected by chlorimuron timing application at the south Texas location in 2005 (Table 2). In 2006, chlorimuron applications resulted in at least 4% peanut stunting. At the southern High Plains location in 2005 or 2006, chlorimuron timing applications made 81 days after planting (DAP) resulted in greater peanut stunting than applications made 67 or 95 DAP. Grichar et al. (1997c) reported that imazethapyr alone at 70 g ha−1 reduced peanut plant height in one of two years when applied 49 DAP; however, no negative response with imazethapyr was noted when applied 7, 21, 35, or 63 DAP.
Peanut yield was reduced for all chlorimuron timing applications at the south Texas location in 2005 but not 2006 as compared to the nontreated control (Table 2). For the High Plains location, there were no yield differences in 2005. However, in 2006 chlorimuron applied 81 DAP significantly reduced peanut yield as compared to the 67 and 95 DAP treatments, or the nontreated control. Grichar et al. (1996c) reported that imazethapyr did not result in a reduction in peanut yield at any application timing. Wehtje and Grey (2004) reported that chlorimuron applications may be affected by peanut cultivar and application timing of chlorimuron. For that study, chlorimuron had no effect on yield of either AT 201 or Georgia Green. For C99R, Viragard, and Florunner, chlorimuron at 5 weeks after planting (WAP) resulted in a significant yield reduction while the 9- and 11-WAP application timings were equivalent to the nontreated check. Earlier work by Patterson et al. (1989) and Johnson et al. (1992a,b) established the relative safety of later chlorimuron applications and the potential risk of early applications.
The application timing restriction for chlorimuron was based upon field research conducted in the late 1980's (Patterson et al., 1989). The effect of chlorimuron was evaluated in 3-, 7-, and 10-wk-old peanut plants (Wilcut et al. 1989), and tolerance to chlorimuron was plant age-dependent. The increased tolerance of older peanut plants was attributed to combined effects of reduced absorption and translocation, and more extensive metabolism by the older plants.
In summary, the use of chlorimuron for weed control in Texas is a concern due to negative crop response and the weed control spectrum may be limited. Chlorimuron applied 74 to 81 DAP resulted in reduced yield as compared to the nontreated control at south Texas, and for the High Plains experiments in one of two years. Plant stress has been implicated as a factor in peanut yield reduction associated with chlorimuron timing applications (Wehtje and Grey 2004). Plant disease or environmental conditions (rainfall or temperature) were not a factor at either location during the 2005 growing season. Environmental conditions in south Texas were above normal rainfall with cooler than normal temperatures while in the High Plains, minimum and maximum temperatures were near normal (21 and 32° C, respectively) with 1.1 cm of irrigation applied every third day.
Acknowledgements
The Texas Peanut Producers Board provided funds for this research. Kevin Brewer, Dwayne Drozd, Lyndell Gilbert, Bill Klesel and A. J. Jaks provided technical assistance.
Literature Cited
Anonymous 2007 Crop Protection Chemical Reference. 23th ed Chem. Pharmaceutical Publ. Co. and John Wiley & Sons, Inc New York 2485 .
Beasley J. and Baldwin J. 2009 Peanut cultivars and descriptions. http://www.caes.uga.edu/commodities/fieldcrops/peanuts/production/cultivardescription.html. Accessed Feb. 19, 2009.
Brecke B. J. and Colvin D. L. 1991 Weed management in peanuts. In Pimentel D. (ed.) CRC Handbook of Pest Management in Agriculture, Vol. 3, 2nd ed, CRC Press Boca Raton, FL 239 – 251 .
Brown S. M. , Brecke B. J. , Colvin D. L. , Everest J. W. , Gooden D. T. , Grichar W. J. , Johnson W. C. , Swann C. W. , Wehtje G. R. , Wilcut J. W. , and York A. C. 1993 Peanut yield response to Classic (chlorimuron): Results from a beltwide evaluation. Proc. South. Weed Sci. Soc 46 : 41 .
Brown S. , Todd J. , Culbreath A. , Baldwin J. , Beasley J. , Kemerait B. , and Prostko E. 2003 Minimizing spotted wilt of peanut. Univ. of Georgia, College of Agric. and Environ. Bulletin 1165.
Buchanan G. A. , Murray D. S. , and Hauser E. W. 1982 Weeds and their control in peanut. In Pattee H. E. and Young C. T. (eds.) Peanut Science and Technology, Amer. Peanut Res. Educ. Soc., Inc., Yoakum, TX 206 – 249 .
Cardina J. and Brecke B. J. 1991 Florida beggarweed (Desmodium tortuosum) growth and development in peanuts (Arachis hypogaea). Weed Technol 5 : 147 – 153 .
Frans R. , Talbert R. , Marx D. , and Crowley H. 1986 Experimental design and techniques for measuring and analyzing plant responses to weed control practices. In Camper N. D. (ed.) Research Methods in Weed Science. 3rd ed Southern Weed Science Society Champaign, IL 29 – 46 .
Gorbet D. W. , Norden A. J. , Shokes F. M. , and Knauft D. A. 1987 Registration of ‘Southern Runner’ peanut. Crop Sci 27 : 817 .
Grichar W. J. 1997a Influence of herbicides and timing of application on broadleaf weed control in peanut (Arachis hypogaea L.). Weed Technol 11 : 708 – 713 .
Grichar W. J. 1997b Control of Palmer amaranth (Amaranthus palmeri) in peanut (Arachis hypogaea) with postemergence herbicides. Weed Technol 11 : 739 – 743 .
Grichar W. J. 1997c Peanut (Arachis hypogaea L.) response to imazethapyr as affected by timing of application. Peanut Sci 24 : 10 – 12 .
Grichar W. J. , Lemon R. G. , Dotray P. A. , and Besler B. A. 2004 Control of problem weeds and net returns with herbicide programs in peanut (Arachis hypogaea L.). In Inderjit Weed Biology and Management Kluwer Academic Publ Dordrecht, The Netherlands 485 – 515 .
Henning R. J. , Allison A. H. , and Tripp L. D. 1982 Cultural practices. In Pattee H. E. and Young C. T. (eds.) Peanut Science and Technology, Amer. Peanut Res. Educ. Soc., Inc., Yoakum, TX 123 – 138 .
Johnson W. C. , Holbrook C. C. , Mullinix B. G. , and Cardina J. 1992a Response of eight genetically diverse peanut genotypes to chlorimuron. Peanut Sci 19 : 111 – 115 .
Johnson W. C. , Mullinix B. G. , and Brown S. M. 1992b Phytotoxicity of chlorimuron and tank mixtures on peanut. Weed Technol 6 : 404 – 408 .
Melouk H. A. and Shokes F. M. 1995 Peanut health management in peanut production. In Melouk H. A. and Shokes F. M. (eds.) Peanut Health Management, APS Press, St. Paul, MN 1 – 6 .
Patterson K. A. , Hammes G. G. , and Seay R. E. 1989 Timing of chlorimuron for Florida beggarweed control in peanuts. Proc. South. Weed Sci. Soc 42 : 30 .
Porter D. M. , Smith D. H. , and Rodriguez-Kabana R. 1982 Peanut plant diseases. In Pattee H. E. and Stalker H. T. (eds.) Advances in Peanut Science, Amer. Peanut Res. Educ. Soc., Yoakum, TX 326 – 410 .
Richburg J. S. , Wilcut J. W. , and Wiley G. L. 1995a AC 263,222 and imazethapyr rates and mixtures for weed management in peanut (Arachis hypogaea). Weed Technol 9 : 801 – 806 .
Richburg J. S. , Wilcut J. W. , and Eastin E. F. 1995b Weed management in peanut (Arachis hypogaea) with imazethapyr and metolachlor. Weed Technol 9 : 807 – 812 .
Shaw D. R. , Ratnayake S. , and Smith C. A. 1990 Effects of herbicide application timing on johnsongrass (Sorghum halepense) and pitted morningglory (Ipomoea lacunosa) control. Weed Technol 4 : 900 – 903 .
Simpson C. E. , Baring M. R. , Schubert A. M. , Black M. C. , Melouk H. A. , and Lopez Y. 2006 Registration of ‘Tamrun OL 02’ peanut. Crop Sci 46 : 1813 – 1814 .
Smith O. D. , Simpson C. E. , Black M. C. , and Besler B. A. 1998 Registration of ‘Tamrun 96’ peanut. Crop Sci 38 : 1403 .
U.S. Dept. of Agric. (USDA) 1993 Milled Peanuts:Inspection Instructions U. S. Dept. of Agric., Agric. Marketing Serv., Fruit and Vegetable Division Washington, D.C .
Walker R. H. , Wells L. W. , and McGuire J. A. 1989 Bristly starbur (Acanthospermum hispidum) interference in peanuts (Arachis hypogaea). Weed Sci 37 : 196 – 2000 .
Webster T. M. , Wilcut J. W. , and Coble H. D. 1997 Influence of AC 263,222 rate and application method on weed management in peanut (Arachis hypogaea). Weed Technol 11 : 520 – 526 .
Wehtje G. , Padgett D. , and Martin N. R. 2000 Imazapic-based herbicide systems for peanut and factors affecting activity on Florida beggarweed. Peanut Sci 27 : 17 – 22 .
Wehtje G. and Grey T. L. 2004 Response of new cultivars to early postemergence chlorimuron applications. Peanut Sci 31 : 119 – 123 .
Wilcut J. W. , Wehtje G. R. , Patterson M. G. , Cole T. A. , and Hicks T. V. 1989 Absorption, translocation and metabolism of foliar-applied chlorimuron in soybeans, peanuts, and selected weeds. Weed Sci 37 : 175 – 180 .
Wilcut J. W. 1991 Economic yield response to peanut (Arachis hypogaea) to postemergence herbicides. Weed Technol 5 : 416 – 420 .
Wilcut J. W. , Richburg J. S. , Wiley G. , Walls F. R. , Jones S. R. , and Iverson M. J. 1994a Imidazolinone herbicide systems for peanut (Arachis hypogaea L.). Peanut Sci 21 : 23 – 28 .
Wilcut J. W. , Richburg J. S. , Eastin E. F. , Wiley G. R. , Walls F. R. , and Newell S. 1994b Imazethapyr and paraquat systems for weed management in peanut (Arachis hypogaea). Weed Sci 42 : 601 – 607 .
Wilcut J. W. , York A. C. , and Wehtje G. R. 1994c The control and interaction of weeds in peanut (Arachis hypogaea). Rev. Weed Sci 6 : 177 – 205 .
Wilcut J. W. , York A. C. , Grichar W. J. , and Wehtje G. R. 1995 The biology and management of weeds in peanut (Arachis hypogaea). In Pattee H. E. and Stalker H. T. (eds.) Advances in Peanut Science Amer, Peanut Res. Educ. Soc., Stillwater, OK 207 – 244 .
Young J. H. , Person N. K. , Donald J. O. , and Mayfield W. H. 1982 Harvesting, curing, and energy utilization. In Pattee H. E. and Young C. T. (eds.) Peanut Science and Technology, Amer. Peanut Res. Educ. Soc., Yoakum, TX 458 – 487 .
Notes
Author Affiliations
1 Texas AgriLife Research, 3507 Hwy 59E, Beeville, TX 78102.
2 Texas Tech University, Texas AgriLife Research, and Texas AgriLife Extension Service, Lubbock, TX 79403.
*Corresponding author (e-mail: w-grichar@tamu.edu)