Introduction
The use of a working collection or a core collection enables researchers to more economically evaluate attributes of genetically diverse resources such as the U.S. peanut (Arachis hypogaea L.) germplasm collection. This collection contains 7432 accessions and is considered to have a great amount of genetic diversity (Holbrook et al., 1993). To this end, this collection was reduced first to a core collection of 831 samples and then further reduced to a “mini” core or core of the core collection which consists of 112 accessions (Holbrook and Dong, 2005). This reduction was based on country of origin, available morphological data, and cluster analysis for eight above ground and eight below ground characteristics. The groups were assumed to be genetically similar and a random selection of a 10% sample from each group resulted in 112 accessions being identified as the Core of the Core collection.
The nutritional properties of peanuts have been demonstrated in chemical and epidemiological studies to be favorable and these characteristics are considered as a major marketing tool. The relatively high lipid content of peanuts is offset by the fact that the fatty acid profile is generally favorable, with the monounsaturated fatty acid, oleic acid, composing at least 40% of the total fatty acids. Mature peanuts contain approximately 25% protein which is of relatively high quality with a protein efficiency ratio (PER) score of 1.7 (compared to casein at 2.5), although peanuts are know to be deficient in the essential amino acids, lysine, methionine and threonine (Sarwar et al., 1989). The significant folate content of peanuts positions them favorably among other plant food sources to be marketed as an important source of naturally occurring folates. Knowledge of the actual levels of these parameters in the accessions in the peanut germplasm collection would allow for selection to enhance them in commercial varieties.
This study was designed to evaluate the amino acid, fatty acid, tocopherol, and folic acid contents of the accession that make up the core of the core of the U.S. peanut germplasm collection at a single time point to give an initial evaluation of these lines. Studies of this type have been previously published and provide information that can be used to increase the variability of cultured lines (Grosso and Gutman, 1995, Grimm et al., 1996). Of the 112 samples that make up the mini core, 108 were available for use in this study.
Materials and Methods
Samples of 108 of the accessions that make up the Core of the Core were grown in Tifton, GA in crop year 2005. These samples were harvested on 3 different dates to obtain optimal estimated maturity, dried, shelled and shipped to the Market Quality and Handling Research Unit in Raleigh, NC in plastic food storage bags at room temperature. After weighing, the samples were stored at 4 C in similar bags. The weights of samples received ranged from 5 g to 1544 g.
Reagents and Chemicals
All reagents and chemicals used were purchased from the Fisher Chemical Corporation (Fairlawn, NJ) unless otherwise stated.
Total Fat and Moisture Measurements
The total fat and the moisture of each sample was measured in duplicate using a Maran Ultra pulsed nuclear magnetic resonance analyzer (Resonance Instruments, Ltd., Whitney, Oxfordshire, UK). Approximately five grams of whole seed were loaded into the instrument tubes and used in the measurement. The instrument was operated at 11 MHz.
Amino Acid Analysis
The samples were ground in a Braun coffee mill (Gillette Inc., Boston, MA) to a fine meal and removal of the oil was initiated using a Carver hydraulic press (Carver, Inc., Wabash, IN). The partially defatted meal was then loaded into cellulose thimbles and the meal was completely defatted by continuous extraction with hexane in Soxhlet tubes for 6 hr. The oil free samples were then hydrolyzed for amino acid analysis using the method of Hagen and coauthors (1989) with a modification of the derivatizing agent. Each sample was hydrolyzed in duplicate in a heating block for 18 hr at 110 C using 6 N HCl containing 0.1% phenol. Prior to hydrolysis, a separate set of samples was oxidized with performic acid to protect the sulfur groups of methionine and cysteine as described in the reference. These samples were then hydrolyzed using 6 N HCl containing 0.1% phenol for 18 hr at 110 C. An aliquot of the hydrolysate was then derivatized using AccQ•Fluor™ reagent (Waters Corp., Milford, MA) as outlined in the manual (WAT052874, Rev 0). The derivatives were analyzed using a Summit Model HPLC (Dionex Corp., Sunnyvale, CA). The column used was a Waters AccQ•Tag (C18, 4 µ, 150 mm × 3.9 mm). An aqueous phosphate buffer solution containing triethylamine was purchased from Waters, diluted with water as per package directions, and used as received as Eluant A. Acetonitrile mixed with water (60∶40, v∶v) was used as Eluant B. The gradient for the analysis was 0% B to start, increased to 2.5% in 0.5 min, to 7% in 15 min, to 10% in 19 min, to 33% in 32 min and held for 1 min. Eluant B was then increased in 1 min to 100%, held for 3 min, and then decreased to 0% in 3 min. The column was then re-equilibrated at 0% B for 13 min. The total run time was 50 min. The column temperature was 37 C and the flow rate was 1.0 mL per min. The injection volume was 20 µL. Detection was by fluorescence with the excitation wavelength set to 250 and the emission wavelength set to 395. The samples were spiked with α-aminobutyric acid (Sigma Chemical Corp., St. Louis, MO) as an internal standard. A mixed standard containing all of the amino acids except tryptophan was purchased from Pierce (Pierce Biotechnology, Inc., Rockford, IL) and aliquots were derivatized as per the samples and run daily with each sample set to construct a five point curve over a range of 0.2 to 1.0 µ/mL. As a control, a standard reference sample of peanut butter, SRM 2387 (NIST, Washington, DC) was treated as a sample and hydrolyzed and derivatized with every sample set.
Since tryptophan is destroyed by acid hydrolysis, additional samples were digested in 4.2 M NaOH for 18 hr at 110 C and analyzed by HPLC without any additional derivatization. Each sample was analyzed in duplicate. The analysis was done using a Summit HPLC (Dionex, Sunnyvale, CA) with UV detection at 254 nm. The column was a LiChrospher 100 RP-18 (250 mm × 4.6 mm, 5 µ) (Alltech Corp., Deerfield, IL). The mobile phase was 90% 0.02 M phosphate buffer, pH 3.3, 10% Acetonitrile. The runs were isocratic at 1.0 mL/min. The column oven temperature was 30 C. A standard curve was prepared from 1 to 100 ppm using an authentic standard of L-tryptophan (Pierce Biotechnology Inc., Rockford, IL) in water.
Fatty Acid Analysis
Fatty acid analyses were conducted on the oils initially pressed from the ground seeds as described above. The oils were directly converted to fatty acid methyl esters according to AOCS Ce 2-66 (Firestone, 2004) and analysed using gas chromatography (GC). In brief, 0.02 to 0.03 g of oil were weighed in triplicate into glass screw capped tubes. One mL of 0.5 N NaOH in methanol was added to each and the tubes were heated for 10 min at 85 C in a water bath. After cooling to ambient temperature, 1 mL of 14% Boron trifluoride in methanol (Sigma Chemical Corp., St. Louis, MO) was added to each tube. The tubes were recapped, vortexed and returned to the water bath for 10 min. After cooling again to ambient temperature, 1 mL of water, followed by 1 mL of hexane was added to each tube. The tubes were vortexed at top speed for 30 sec, then after phase separation, the top layer (organic) containing the fatty acid methyl esters, was removed and dried over sodium sulfate. The fatty acid methyl esters were analyzed using a Perkin Elmer Autosystem XL GC (Sheldon, CT) fitted with a capillary BPX-070 column (SGE Inc., Austin, TX). The column length was 30 m with an internal diameter of 0.25 mm and a film thickness of 0.25 µm. The temperature gradient was 60 C with a 2 min hold time, increased at 4 C per min to 180 C and then increased at 10 C to a final temperature of 235 C. The run time was 27.7 min. The carrier gas used was helium at a flow rate of 40 psi. The injection was split at 150 mL/min. The results were reported as percent of the total fatty acids based on peak areas according to the official method (AOCS Ce1f-96) (Firestone, 2004). A standard mixture of fatty acid methyl esters (GLC-21A, NuChek Prep, Elysian, MN) was run with each sample set to confirm identification of the fatty acids based on retention times and for determination of recoveries. Iodine values (IV) were calculated from the fatty acid content according to AOCS method Cd 1c-85.
Tocopherol Analysis
The oil from the initial pressing of the ground meal was analyzed for tocopherols in triplicate using HPLC according to the method described by Hashim and coworkers (1993). Briefly, 0.2 to 0.4 g of oil were diluted to 1 mL with hexane in an HPLC vial. After vortexing, the samples were injected on a Luna Silica column (250 mm × 4.6 mm, 5 µ, Phenomenex, Torrance, CA). The mobile phase was 1% isopropanol in hexane. The column temperature was 30 C and the flow rate was 1.2 mL/min. The HPLC consisted of a Varian Model 9010 pump (Varian Corp., Palo Alto, CA) connected to a Waters Model 2487 UV/Vis detector Waters Corp., Milford, MA). The wavelength for detection was 294 nm. Standard curves were prepared using authentic standards of α, β, and γ-tocopherols obtained from Sigma (Sigma Chemical Corp., St. Louis, MO) over a range of 1 to 1,000 µL/mL in hexane.
Folic Acid Analysis
The samples were analyzed for folic acid by Medallion Laboratories of Minneapolis, MN using AOAC method 960.46 (Helrich, 1990). This is the official method for this analyte and all forms of folate are reported as folic acid. This method measures the growth of Streptococcus faecalis (faecium) when inoculated with sample extracts after a series of enzymatic digestions. Due to the cost of the analysis, each sample was only analyzed one time.
Results and Discussion
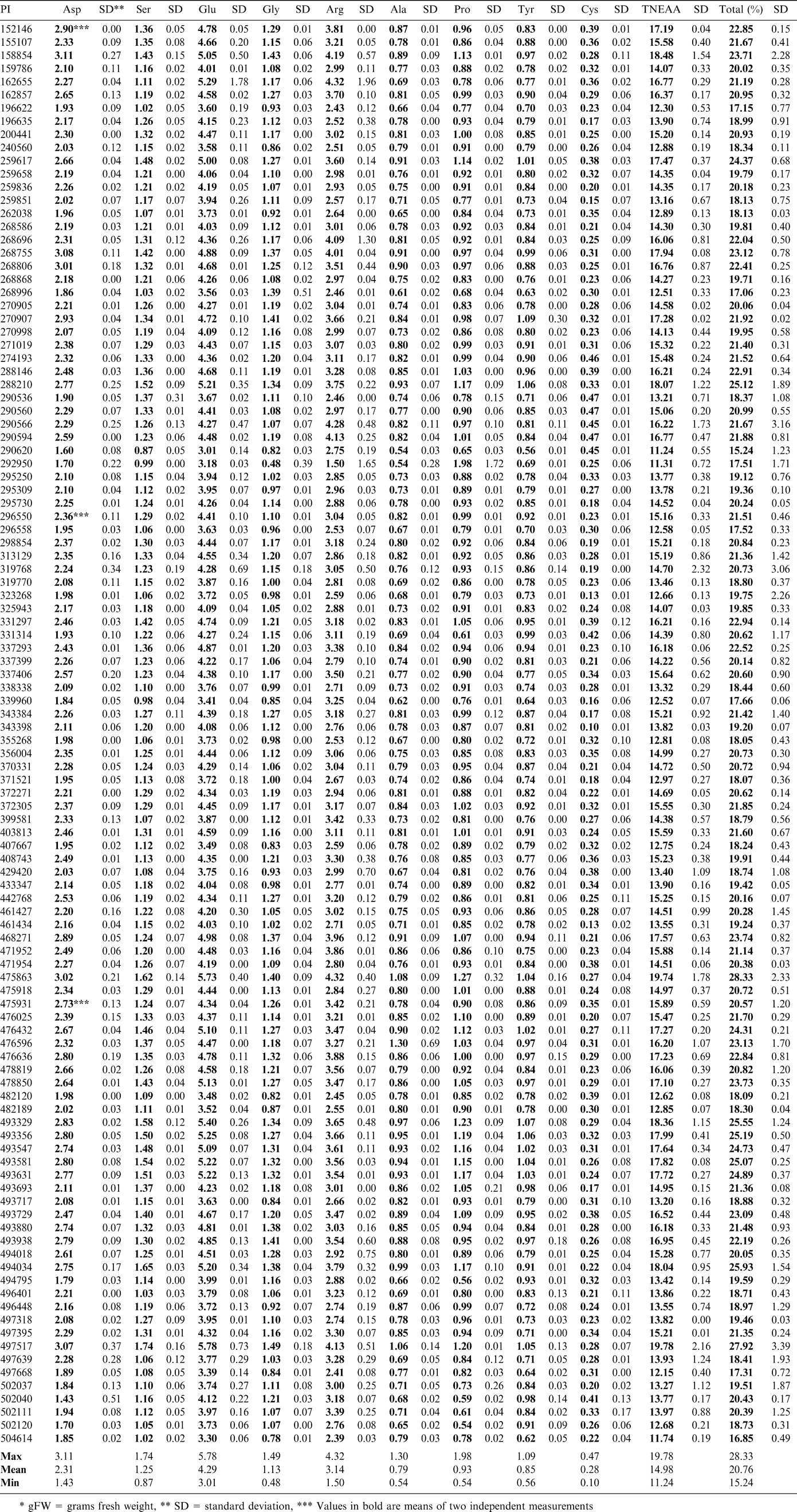
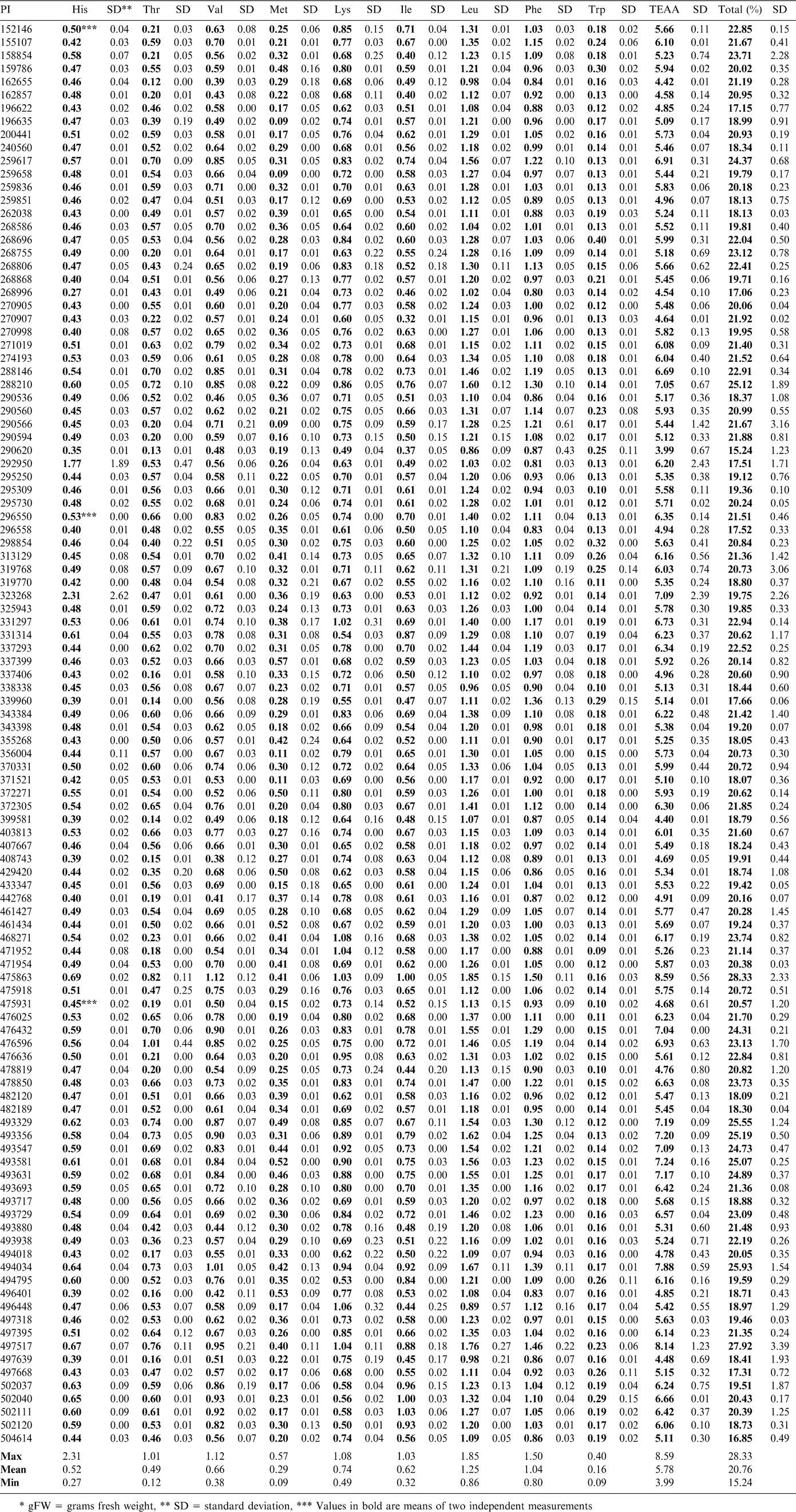
The lipid and moisture values measured for the samples using NMR were used to correct the amino acid results measured on the defatted meal samples back to the fresh seed weight. The amino acid profiles of the samples showed a range of differences. The results for the essential amino acids are reported in Table 1 and those for the nonessential in Table 2. The limiting amino acids in peanuts are known to be lysine, methionine and threonine. The range for lysine was a low of 0.49 g/100 g (PI 290620) and a high of 1.08 g/100 g (PI 468271), which represented a difference of over 100%. Methionine ranged from a low of 0.09 g/100 g (PI 290566, PI 196635, and PI 259658) to a high of 0.57 g/100 g (PI 337399). Threonine ranged from 0.12 g/100 g (PI 162655) to 1.01 g/100 g (PI 476596). The purpose of this study was to compare the lines within the confines of this selection, rather than with commercial lines, or other lines in the entire germplasm collection. Other research has shown that in some cases, amino acids such as methionine are moderately heritable (Kelley and Bliss, 1975). The analyses suggest there are some varieties that have potential for breeding increases in these amino acids that limit the nutritional potential of peanuts. However, in soybeans, it has been found that in many cases, improvements to protein content through breeding have resulted in reductions in oil concentration (Panthee et al., 2005). It may be questioned that increases in methionine, a sulfur containing amino acid, might give rise to flavor changes.
Arginine is a nonessential amino acid that is of interest due to its positive associations with vascular health (Gornik and Creager, 2004 and Moriguti et al., 2005). Peanuts are known to be a source of arginine (Anderson, 1998). This selection of samples was found to contain arginine over a range of 1.50 g/100 g (PI 292950) to a high of 4.32 g/100 g (PI 162655 and PI 475863). The SRM is reported to contain 2.65 g/100 g of arginine. As this value is representative of commercial varieties, the higher samples in this sample set would be of interest for increases to present lines. Free arginine has been used as an indicator of crop maturity in peanuts and may be influenced by more than genetic factors (Young, 1972). This study shows the total arginine values, both free and bound, and should be more useful in selection of lines with more potential for increases (or decreases) of some amino acids.
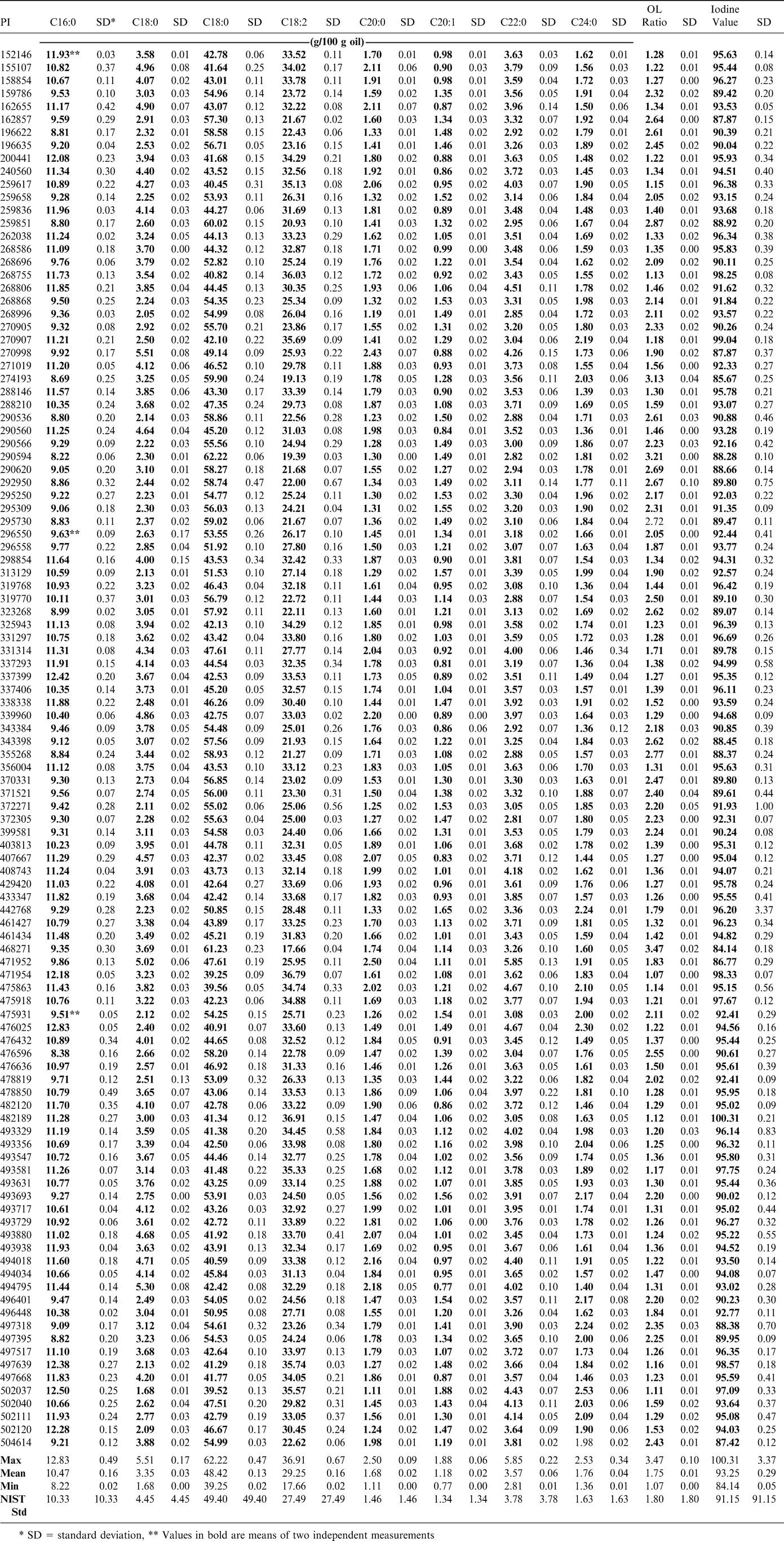
The fatty acid profiles were not highly different for the samples studied (Table 3). The range of the oleic to linoleic ratios (O/L) was from 1.07 (PI 471954) to 3.47 (PI 468271). Only three of the varieties were above 3 (PI 274193, PI 290594, PI 468271). For comparison, the 11 samples of the Florunner variety tested as part of the 2006 United Peanut Performance Trials (UPPT, 2006) produced an average O/L value of 1.65 (± 0.27) which is an indication of how this parameter would be expected to change with environment. None of the samples from the mini core had any high oleic traits. Oil quality as defined by high O/L and low IV (Iodine Value) values has been found to be dependent upon cultivar rather than environment (Anderson and Gorbet, 2002). Some minor changes may occur as a result of soil temperature due to location or year to year weather variations, but they would not be expected to cause significant differences within each individual variety (Brown et al., 1975). Three accessions (PI 274193, PI 290594 and PI 468271) had linoleic acid concentrations below 20 g/100 g and they could be of interest in reducing levels of this more easily oxidized fatty acid through conventional breeding methods.
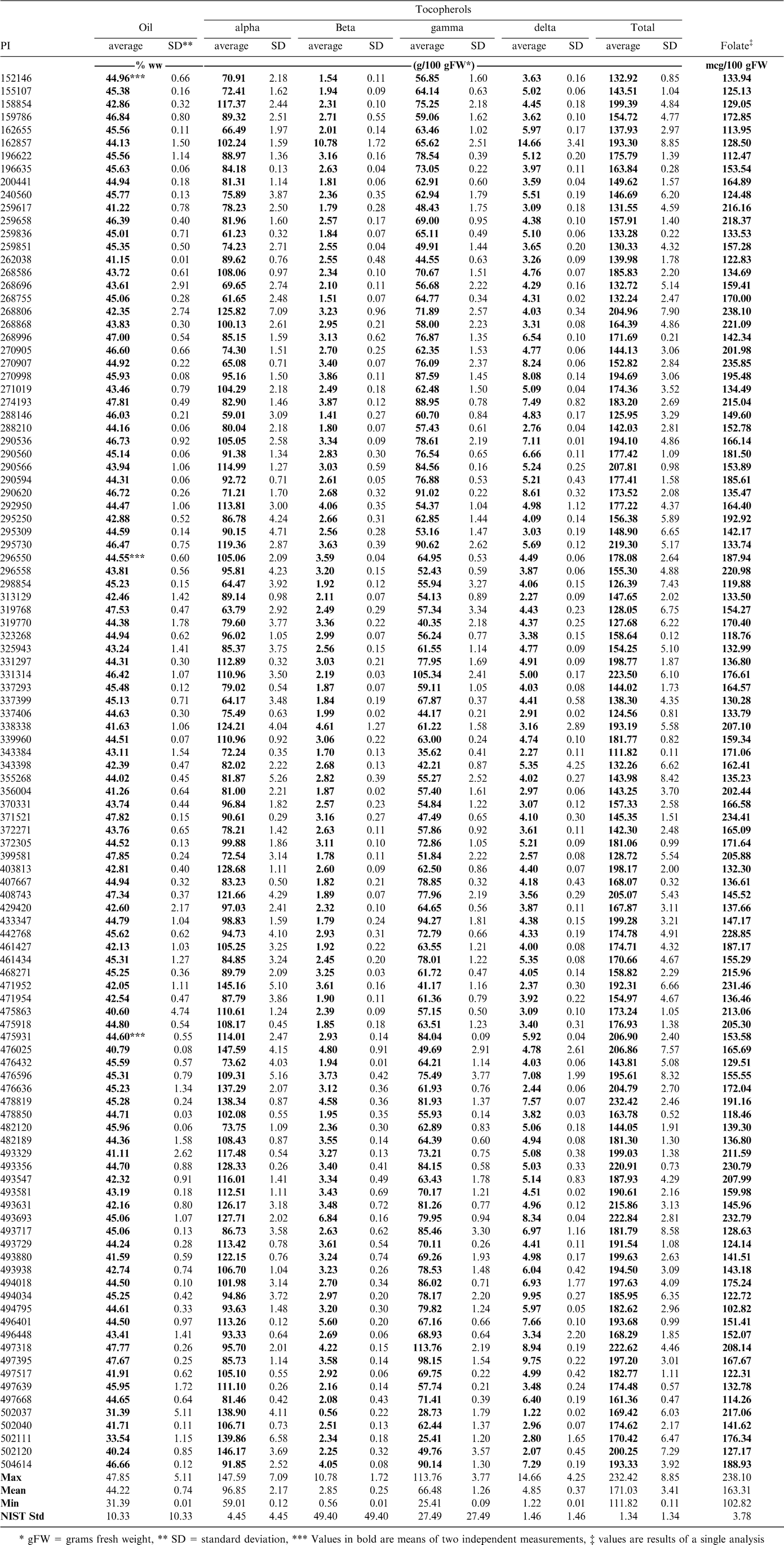
The total tocopherols in the oil expressed from the samples tested produced a range of values (Table 4). The range for the mini core samples was found to be from 112 g/100 FW (PI 343384) to 232 g/100 g FW (PI 478819). Most samples were rather low compared to Florunner with an average of 224 g/100 g FW (± 26.23 g/100 g FW) in the 2006 UPPT. A more important point is that the tocopherols that make up the total need to be considered. It is commonly found that peanuts contain nearly equal amounts of α and the less biologically active γ tocopherol with much smaller amounts of both β and δ tocopherols. Although tree nuts such as almonds contain similar amounts of total tocopherols, they are almost entirely in the α form, making these products nutritionally more advantageous to peanuts (Kornsteiner et al., 2006). Some samples did show relatively high levels of α-tocopherol. Two samples in particular (PI 502037 and PI 502111) both contained α-tocopherol at 82% of the total tocopherols present. The breeding potential of these should be investigated. Differences of this magnitude provide unique opportunities for use in breeding programs aimed at improving the α-tocopherol levels in peanuts.
Food sources of folates are limited to plant sources, of which legumes are a significant source (Dang et al., 2000). Although many commercial cereal and bread products are now fortified with folic acid, leafy greens and legumes are the best sources of naturally occurring folates. Currently the USDA National Nutrient Database lists a value of 145 mcg/100 g of folate equivalents for roasted peanuts (Gebhart et al., 2006). Analysis of the core of the core samples produced a range of 103 mcg/100 g (PI 494795) to 238 mcg/100 g (PI 268806). Georgia Green, a popular commercial variety was found to have a folic acid level of 111 mcg/100 g when measured with other samples in this study. Table 4 shows values for all the samples tested. There appear to be a number of samples that show potential for increased folate levels through breeding. Future work will determine if a change in methodology from the microbiological method to one utilizing high pressure liquid chromatography coupled with mass spectrometry (LC-MS) will reveal if the folate levels are being under reported (Phillips et al., 2006).
Conclusion
The nutrients measured in the core of the core of the peanut germplasm collection indicated large differences in values. This work is presented as an initial report on the nutritional components in the mini core and indicates that breeding potential does exist to increase positive nutrients such as α-tocopherol and folates. The fatty acid profile range was small with no samples showing a high oleic trait. Some samples have higher levels of certain amino acids which could possibly be used for increases. Increased levels of the limiting amino acids would be advantageous from a nutritional standpoint, in order to make peanut a more complete protein. Such a change could have a large effect on how peanuts are currently marketed and how peanut protein could be used functionally. As discussed previously, the selection of the present mini core was based on encompassing a range of morphological characteristics. The samples studied here possibly do not represent the entire range of nutritional characteristics for the germplasm collection. It may warrant that more samples outside of those selected for the “Core of Core” be studied in the future centered on their nutritional profiles.
Abbreviations Used in the Text and the Tables
AAamino acids
FWfresh weight
IVIodine value
OL ratioRatio of Oleic acid content to that of Linoleic acid
TEAATotal essential amino acids
TNEAATotal nonessential amino acids
SDstandard deviation
Acknowledgements
The authors would like to thank James Schaefer of the Market Quality and Handling Unit for technical support and Dr. Thomas Isleib of the Crop Science Department at North Carolina State University for advice with the statistical analysis. Mention of a trademark or proprietary product does not constitute a guarantee or warranty of the product by the U. S. Dept. of Agric. nor does it imply approval to the exclusion of other products that may be suitable.
Literature Cited
Anderson P. C. 1998 Fatty acid and amino acid profiles of selected peanut cultivars and breeding lines. J. Food Comp. Anal 11 : 100 – 111 .
Dang J. , Arcot J. , and Shrestha A. 2000 Folate retention in selected processes legumes. Food Chem 68 : 295 – 298 .
Firestone D. 2004 Official Methods and Recommended Practices of the American Oil Chemists Society. 5th edition Champaign, IL American Oil Chemists Society .
Gebhart S. E. , Cutrutelli R. L. , Howe J. C. , Haytowitz D. B. , Pehrsson P. R. , Lemar L. E. , Holcomb G. T. , Stup M. S. , Thomas R. G. , Exler J. , Showell B. A. , and Holden J. M. 2006 USDA National Nutrient Database for Standard Reference, Release 19 Washington DC USDA ARS. http://www.nal.usda.gov/fnic/foodcomp/Data/SR19/nutrlist/sr19w435.pdf
Gornik H. L. and Creager M. A. 2004 Arginine and endothelial and vascular health. J. Nutr 134 : 2880S – 2887S .
Hagen S. R. , Frost B. , and Augustin J. 1989 Precolumn phenylisothiocyanate derivatization and liquid chromatography of amino acids in foods. J. Assoc. Off. Anal. Chem 72 : 912 – 916 .
Hashim I. B. , Koehler P. E. , Eitenmiller R. R. , and Kvien C. K. 1993 Fatty acid composition and tocopherol content of drought stressed Florunner peanuts. Peanut Sci 20 : 21 – 24 .
Helrich K. 1990 Official Methods of Analysis of the Association of Official Analytical Chemists. 15th edition Arlington, VA Association of Official Analytical Chemists, Inc .
Holbrook C. C. , Anderson W. F. , and Pittman R. N. 1993 Selection of a core collection from the U. S. germplasm collection of peanut. Crop Sci 33 : 859 – 861 .
Holbrook C. C. and Dong W. 2005 Development and evaluation of a mini core collection for the U. S. peanut germplasm collection. Crop Sci 45 : 1540 – 1544 .
Kornsteiner M. , Wagner K-H. , and Elmadfa I. 2006 Tocopherols and total phenolics in 10 different nut types. Food Chem 98 : 381 – 387 .
Moriguti J. C. , Ferriolli E. , Donadi E. A. , and Marchini J. S. 2005 Effects of arginine supplementation on the humoral and innate immune response of older people. Eur. J. Clin. Nutr 59 : 1362 – 1366 .
Phillips K. M. , Ruffio D. M. , Ashraf-Khorassani M. , and Haytowitz D. B. 2006 Differences in folate content of green and red sweet peppers (Capsicum annuum) determined by liquid chromatography-mass spectrometry. J. Agric. Food Chem 54 : 9998 – 10002 .
Sarwar G. , Peace R. W. , Botting H. G. , and Brulé D. 1989 Relationship between amino acid scores and protein quality indices based on rat growth. Plant Foods for Human Nutrition 39 : 33 – 44 .
Uniform Peanut Performance Trial Data (UPPT) USDA, ARS, MQHRU Raleigh, NC http://152.1.118.33/downloads.htm
Young C. T. 1972 Free arginine content of peanuts (Arachis hypogaea L.) as a measure of seed maturity. J. Food Sci 37 : 722 – 725 .
Notes
Author Affiliations
1Market Quality and Handling Research Unit, USDA, ARS, SAA, Raleigh, NC 27695-7624.
2Crop Genetics and Breeding Research Unit, USDA, ARS, SAA, Tifton, GA 31793.
*Corresponding author: Lisa.Dean@ars.usda.gov