Introduction
Rainfall unpredictability for amount and distribution significantly reduces yield potential of agronomic crops in the USA and worldwide. To produce competitive crops, peanut (Arachis hypogaea) requires approximately 600 mm of water equally distributed from planting to harvest in weekly amounts of 25 to 50 mm, depending on the growth stage (Putnam et al., 2014; Rowland et al., 2012). However, achieving this distribution is hindered by irregular rainfall patterns and irrigation unavailability for over 65% of peanut acreage in the US. In Virginia and Carolinas (VC region) over 85% of peanut acreage is rainfed. Under this condition, peanut heavily relies on rainfall. For example, year 2014 was a good peanut year for the VC region with a state average yield in Virginia of 5040 kg/ha (USDA, 2019a). Precipitation of July and August combined in 2014 was 381 mm. Year 2010, on the other hand, only marked 15 mm of rain during July and August and the average yield was 2016 kg/ha. This is a 60% yield reduction under severe drought conditions in 2010 from the high yielding year 2014. In the sub-humid VC region, even in "rainy" years peanut yield can significantly decrease in absence of rain or irrigation for more than ten consecutive days in July and August (Singh et al., 2014). Virginia-type peanut is the predominant market type grown in this region; it has large seeds when compared with other types. For example, a hundred kernels of the virginia-type weigh approximately 100 g; the runners, and other types weigh 20 to 40% less (Branch et al., 2017). The large kernel size of this peanut type seems to require more water to fully develop then the runners, spanish or valencia types (Erickson and Ketring, 1985).
There is little research on how virginia-type peanut cultivars respond to water deficit in terms of quality characteristics of the pods and seeds. For example, work by Dang et al. (2013) clearly showed that the content of sound mature kernels (SMK) and total sound mature kernels (TSMK) were significantly reduced by a 3-wk drought in Georgia, USA. But little is known on water deficit effects on the extra large kernels (ELK), an important grade characteristic for the virginia-type peanut, and damaged kernels (DK) content. A DK content of over 3.49% declassifies farmers' stock peanut to segregation II that reduces the peanut economic value by 65% (USDA, 2019b). Pod color or brightness is a valuable characteristic for the in-shell peanut processors, and virginia-type is predominantly used for in-shell products. The higher the color (brightness) numbers, the more desirable pods are. Average Hunter L score numbers of 45 or higher denote "white" or "bright" pods, which is desired for the in-shell markets. Empirical observations showed that pod color decreased in dry soils, but there is no research relating water deficit to pod color and showing if genetic differences for this characteristic may exist. Finally, drought may delay peanut maturity from reduced growth rates (Reddy et al., 2003) and oleic fatty acid (C18:1) accumulation in the seeds. For example, C18:1 was low in immature peanut seed (Andersen and Gorbet, 2002; Hinds, 1995; Klevorn et al., 2016; Sanders et al., 1982). The C18:1 is an important quality characteristic for the new cultivars with high oleic oil chemistry because of positive effect of increased oil saturation levels on seed shelf life, i.e., delayed oxidative degradation, (Mozingo et al., 2004). There are no reports to show how seed C18:1 content varies among virginia-type cultivars under different soil moisture conditions.
In the VC environment, where large seeded and drought-prone virginia-type peanut is grown, understanding how different genotypes cope with soil moisture deficit in terms of yield and quality, and developing screening tools to allow precise phenotyping for improved resilience to soil moisture extremes is important. However, rainfall unpredictability makes field selection for tolerance to water deficit impossible in this environment. None the less, use of relatively simple rainout shelters, e.g., snow arch type on ground rails with forward and backward movability when pulled by tractors, could provide an economic solution for phenotyping peanut genotypes at multiple locations in a breeding program. They also offer an economical solution to identifying the most resilient cultivars to water deficit to help researchers make better recommendations for cultivar selection on dry-prone soils. The objectives of this work were 1) to evaluate eleven peanut cultivars and breeding lines for yield, grade, and oil profile in response to different levels of soil moisture using a simple rain exclusion setting, and 2) to assess the reliability of the snow arch rain exclusion shelter setting for screening of peanut tolerance to soil moisture deficit.
Materials and Methods
In 2013 and 2014, field experiments were performed at the Tidewater Agricultural Research and Extension Center near Holland, VA (36° 68' N, 76° 77' W, 18.9 m elevation) under three soil water regimes. The soil at this site is classified as Eunola (fine-loamy, siliceous, thermic Aquic Hapludults); 83.5% sand, 9.5% silt, 7% clay, 0.5 to 2.0 % organic matter, and 0.06 to 0.14 m3 m-3 available water capacity in the first 30 cm. Water regimes were well watered (WW), moderate soil moisture deficit (MD), and severe soil moisture deficit (SD). These regimes were achieved by covering the plots from mid-July to early Sep, i.e. beginning pod to beginning maturity growth stages, with three rain exclusion shelters and applying overhead irrigation under each shelter (Fig. 1). Each shelter included an aluminum frame of 22 m long, 9 m wide, and 4 m high in the center of the frame set on rails provided with skid plates at each end (Fig 1). Each shelter was covered with plastic allowing 95% light penetration (Atlas Manufacturing Inc., Alapaha, GA). To allow proper crop rotation and plot coverage as needed, shelters were moved along the field by two tractors pulling forward or backward the rails. A reel line system (AMADAS Industries, Suffolk, VA) delivered a total of 220 mm water for the WW and 90 mm for the MD regime in 2013. These amounts were delivered in five equally distributed irrigations on July 25, Aug 08, Aug 19, Aug 27, and Sep 05 in 2013, of approximately 44 mm per irrigation for the WW and 18 mm per irrigation for the MD. In 2013, the SD received a total of 40 mm on Aug 25. In 2014, 246 mm were applied to the WW regime and 104 mm to the MD on July 24, Aug 04, Aug 11, Aug 19, Aug 26, and Sep 01; 41 mm per irrigation for the WW and 17 mm for the MD. In 2014, the SD plots only received 40 mm on Aug 19. Before coverage and after shelters removal, plots of all water regimes received equal and plentiful amounts of rainfall; in 2013, 303 mm from planting to mid-Jul and 42 mm after removal to digging; in 2014, 230 mm from planting to mid-Jul and 163 mm after removal to digging. To set these water regimes, plant water requirement, i.e. total water (irrigation and precipitation) needed to produce high yields, was used. Peanut needs 600 mm for the season and at least 40 mm weekly from beginning pod to beginning maturity growth stages to produce high yields (Putnam et al., 2014; Rowland et al., 2012). This agrees with the multi annual (30 years) weekly average precipitation in SE Virginia. By applying 40 mm weekly, the WW regime ensured optimum growth and yield; this amount was also in agreement with the soil water holding capacity of the field so no runoff could have happened. The MD was set to approximately 40% of the WW, which is standard for studding drought effects on plants in controlled conditions (Kapanigowda et al., 2014). Lastly, the water applied to the SD was only a salvage irrigation.
Under each shelter and water regime, eleven peanut cultivars and breeding lines were planted in 1.7 m long and 0.9 m wide 2-row plots on May 17 in 2013 and May 19 in 2014. Table 1 includes the names, market type, and reason for inclusion of these genotypes.
Cultural practices were performed according with the Virginia Peanut Production Guide's recommendations (Balota et al., 2013). Plots were dug on Sep 26 in 2013 and Sep 30 in 2014 at harvest maturity (Boote, 1982). After a few days of windrow drying, pods were combined. Pod yield was determined from plot weight and adjusted to 7% seed moisture and percent foreign material in a 500 g sub-sample.
The same sub-sample was further used to determine pod color or brightness and farmer stock grades. Pod color was measured using Hunter L, a, b, color scale by a colorimeter (model D25LT Hunter Associates Lab., Inc. Reston, VA). After shelling, kernels were sorted by size. The ELK were kernels that did not pass a 25.4 mm (1-in) × 8.5 mm (21.5/64) screen; the mediums passed the larger screen but did not pass a 25.4 mm × 7.1 mm (18/64-in) screen; and number 1's passed the larger screens but did not pass a 25.4 mm × 5.9 mm (15/64-in) screen. The DK content included decayed, molded, sprouted, and discolored kernels; the sound splits (SS) were halved and broken kernels; and the other kernels (OK) were kernels passing through a 25.4 mm × 5.9 mm (15/64-in) screen also known as fall through. Percent of SMK was determined as the sum of ELK, mediums and number 1's. Total kernels (TK) was the sum of SMK, DK, SS, and OK. These grade standards were used to determine the crop value using the USDA Agricultural Marketing Service approach (USDA, 2019b). After grading, ELKs, mediums, number 1's, and fall through, i.e., kernels that pass through the screens as shown above, from the WW and SD regimes were finely ground and 2 to 5 g samples from each group were shipped to the Virginia Tech's Department of Dairy Science, Blacksburg, VA, for oil profile analysis by the GC method. Kernels were sorted by size for this analysis in order to determine if oil profile was affected by kernel size. For the oil profile analysis, only the high oleic genotypes were used, i.e., Florida 07 (Gorbet and Tillman, 2009), N08082olJCT, Spain, and Wynne. Reported in this paper are the oleic (C18:1), linoleic (C18:2) and total saturated fatty acids, i.e., sum of palmitic (C16:0), stearic (C18:0), arachidic (C20:0), behenic (C22:0), and lignoceric (C24:0) fatty acids.
Each rain exclusion shelter was considered an environment, different from each other by the water regime. In each environment, i.e., WW, MD, and SD, the experimental design was a randomized complete block with genotype as the only treatment. Environment (water regime), year, and kernel size were treated as fixed effects for combined analysis. In both years, genotypes were replicated three times under each rain shelter. Water regimes were not replicated within each year. ANOVA was performed using SYSTAT® 10.2 (2002, SYSTAT Software Inc, Richmond, CA). Means were separated by Fisher least significant difference (LSD) test at 5% (P ≤ 0.05) probability level.
Results
Weather conditions
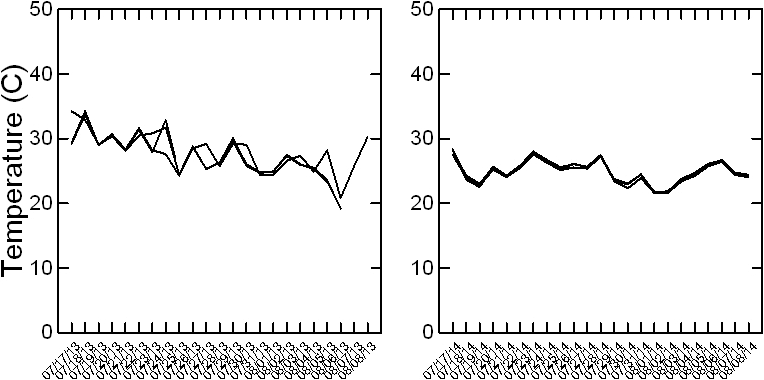
Even though years 2013 and 2014 were similar in terms of weather conditions, i.e., from May to October average temperature was 23 C in both years, relative humidity (RH) was 75% in 2013 and 80% in 2014, and rainfall totaled 719 mm in 2013 and 592 mm in 2014 with uniform distribution in both years, under the rainout shelters there were differences among years for temperature and RH. Under rainout shelters, in 2013, average temperature from mid-July to mid-August was 28 C (≤0.4 C difference among the water regimes) (Fig. 2) and RH 73% (≤1.5% difference among the water regimes). In 2014, average temperature during the same time frame was 25 C (≤0.7 C difference among the water regimes) and RH 82% (≤1.6% difference among the water regimes). This was because 2013 was sunnier than 2014 during July and August. For example, the daily average (from 9:00 to 18:00) of the photosynthetic active radiation (PAR) measured under the rainout shelters was 1008 μmol m-2 s-1 in 2013 vs. only 880 μmol m-2 s-1 in 2014. Sunnier days in 2013 increased the temperature under the shelters by 5 C from the ambient; while in 2014 temperature was only 2 C over the ambient.
Combined analysis of genotype, water regime, and year
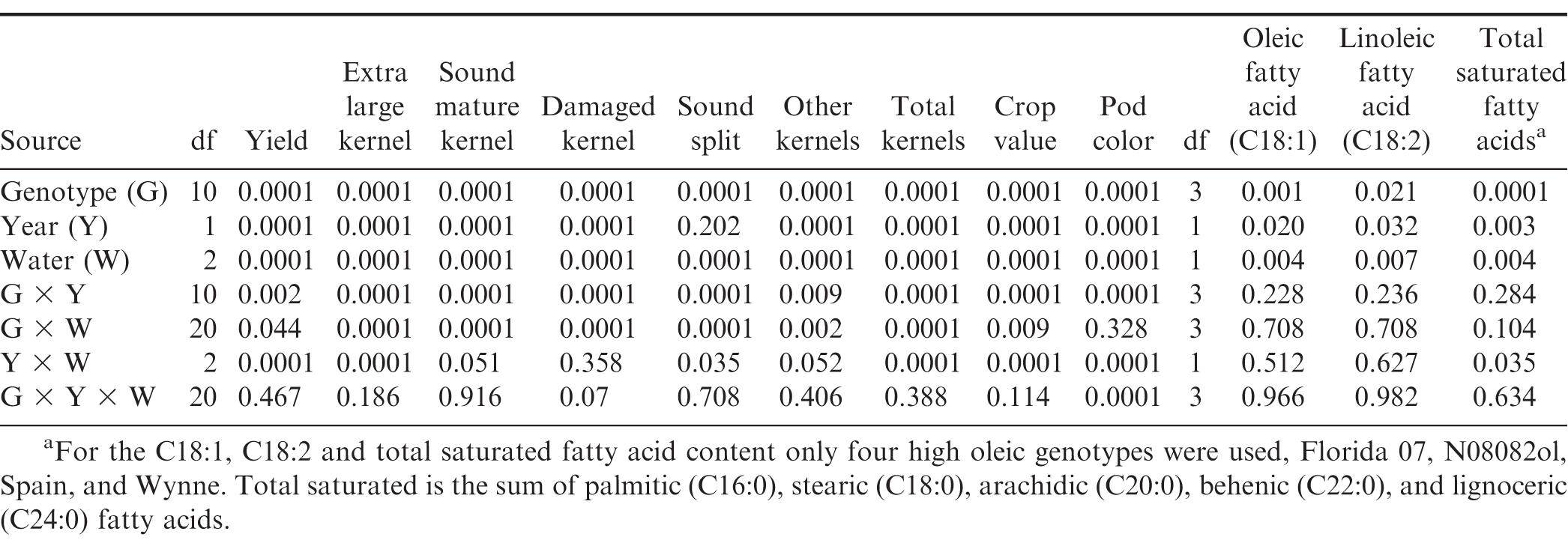
To asses water regime effect, and because within years water regime was not replicated, water regimes were treated as independent environments. Because temperature and RH were the same across water regimes including when shelters covered the plots (Fig. 2), and same soil type, i.e. the total area occupied by all three rainout shelters was less than 800 m2 or 0.08 ha within the same field, environmental effect was safely attributed to the water regime differences. ANOVA identified significant main effects for genotype, environment/water regime, and year for all measured characteristics with the exception of year main effect for the SS content (Table 2). The genotype × water regime × year (G × W × Y) interaction was not significant with the exception of pod color; but the genotype × water regime (G × W), genotype × year (G × Y), and water regime × year (W × Y) interactions were significant for yield, value, and almost all grade characteristics (Table 2). Because of this, data on yield, value and grade factors were further analyzed and mean comparisons presented within each environment/water regime. Within water regime analysis also provided better insight into usability of the rainout shelter setting for genotypic screening, i.e. what water regime maximized genotypic effect.
Yield, crop value and grade characteristics
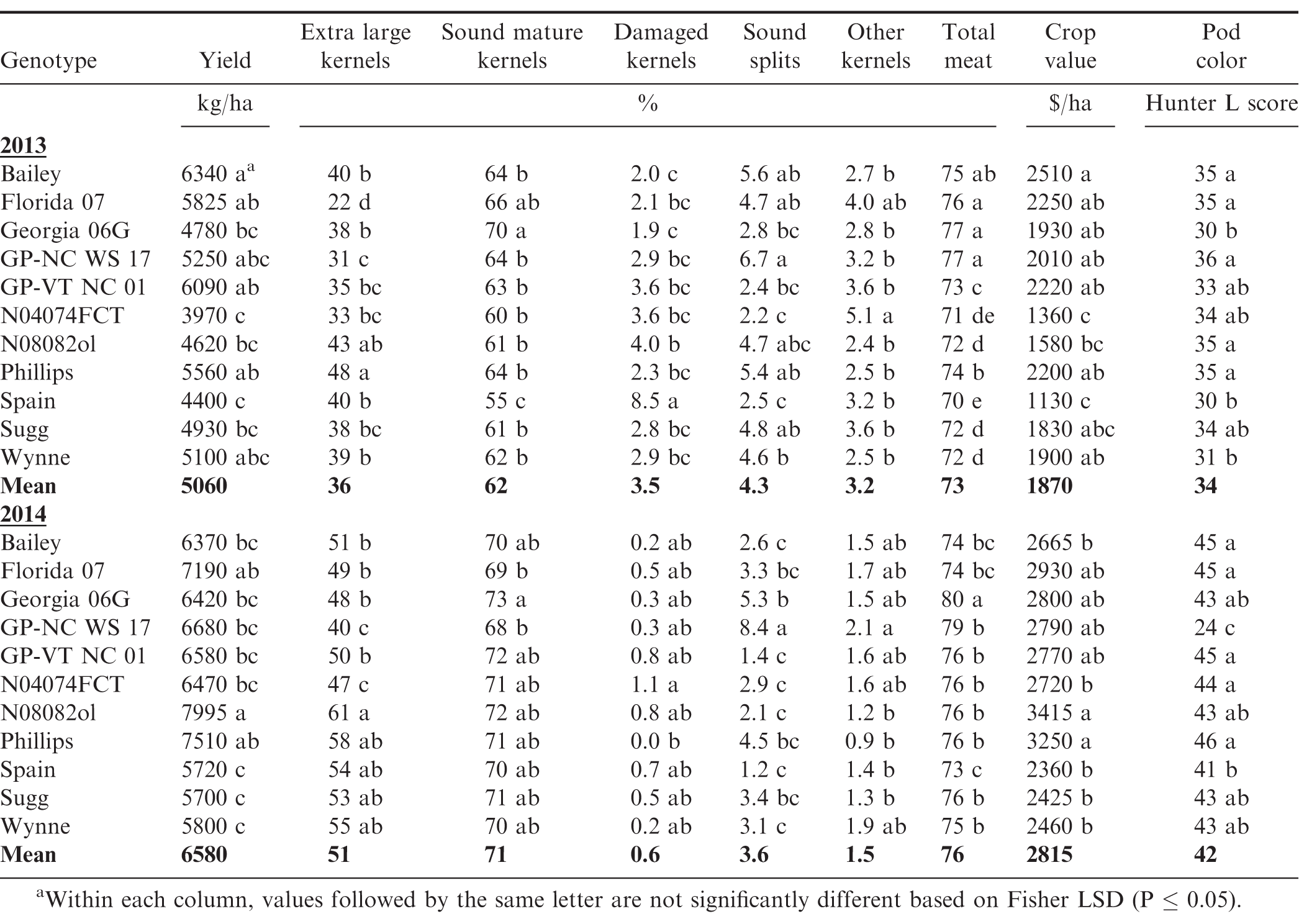
Within the WW water regime, genotype (G), year (Y), and the G × Y interaction were significant for yield, crop value and all grade characteristics (Table 3). In average of genotypes, yield, ELK, SMK, OK, TK and pod color were significantly lower in 2013 vs. 2014, while DK was higher in the hotter 2013 vs. cooler 2014 (Table 4). Therefore, crop value, as an integrative measure of yield and grade, was less in 2013 than in 2014; it was $1870 ha-1 in 2013 vs. $2815 in 2014. The G × Y interaction was driven by the genotypic response to the year when water was unlimited. For example, line N08082ol produced the highest yield in 2014 (7995 kg/ha), but yielded 40% less in the hot 2013 (4620 kg/ha); while Bailey, Florida 07, Phillips, and GP-VT NC 01, a germplasm jointly released in 2016 by the Virginia Tech and North Carolina State University (Balota & Isleib, 2020), showed comparable, relatively high yields in both years, i.e. yield ranged from 5560 to 7510 kg/ha (Table 4). Similarly, the drought susceptible check N04074FCT showed the least yield in 2013 (3970 kg/ha), but yielded slightly higher than Bailey in 2014 (6470 kg/ha) under WW water regime. Spain, Sugg, and Wynne had comparable, relatively low yields in both years, i.e. yield ranged from 4400 to 5800 kg/ha.
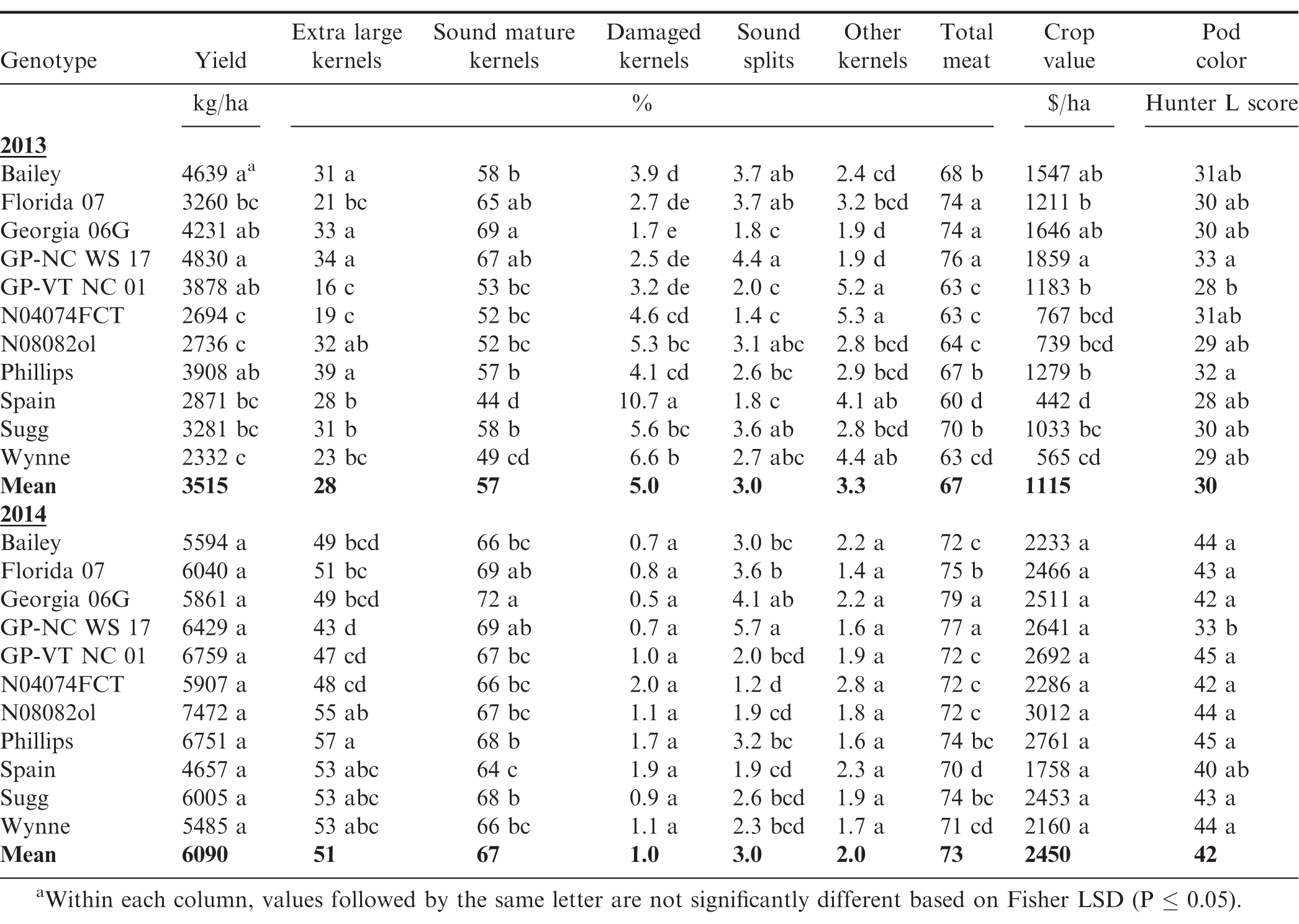
Within the MD water regime, G and Y effects were significant for all characteristics, except SS, for which Y effect was not significant. The G × Y interaction was not significant for yield, crop value and SS; but it was for all the other grade factors (Table 3). This showed that under moderate soil water deficit, genotypic response for yield and value was not affected by year; even though the hot year 2013 affected average yield and grade in a similar manner as under the WW water regime, i.e. yield, ELK, SMK, OK, TK and pod color decreased and DK increased in 2013 vs. 2014 (Table 5). For example, the overall genotypic value was $1115 ha-1 in 2013 vs. $2450 in 2014 under MD. Genotypes with consistent high yield under MD were Bailey, Florida 07, Georgia 06G, GP-VT NC 01, Phillips, and the exotic-derived line GP-NC WS 17, which was released for disease resistance, and improved tolerance to heat and drought (Tallury et al., 2014).
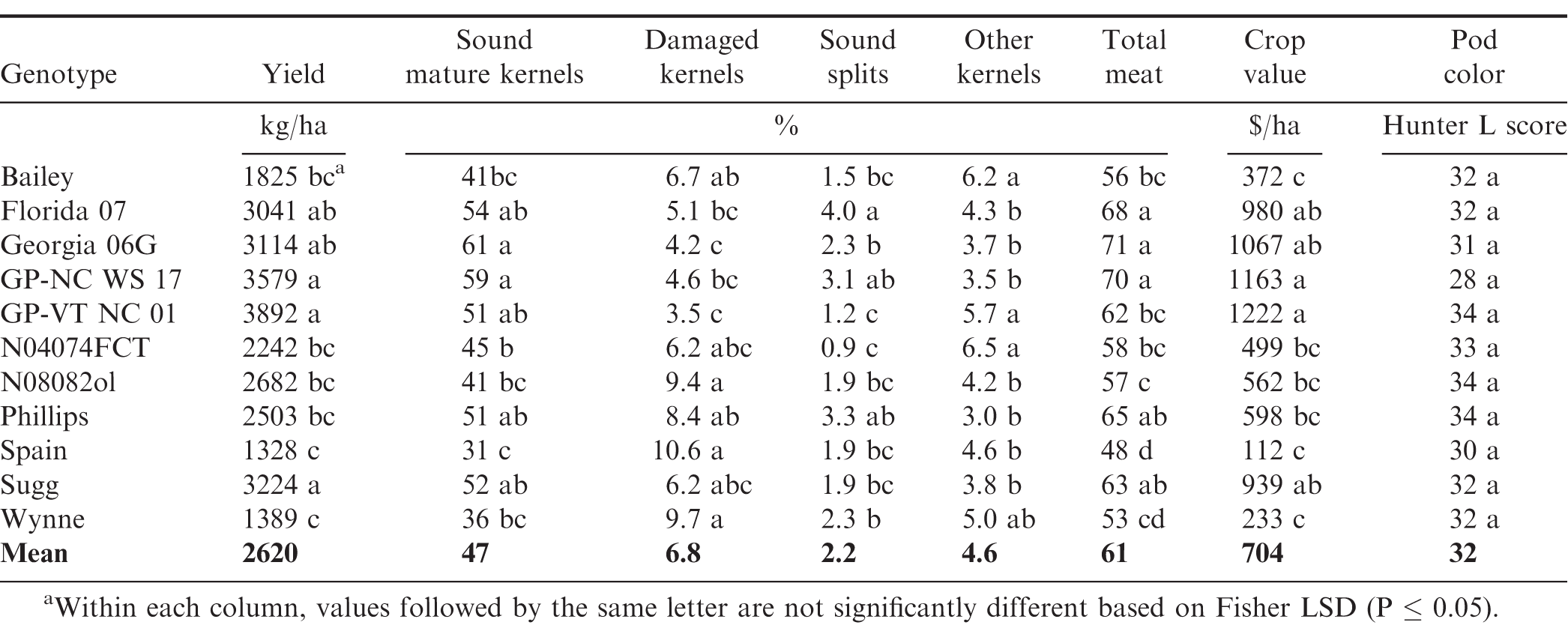
Under SD water regime, G and Y effects were significant for all characteristic, excepting SS and OK, for which Y effect was not significant. Year affected average yield and grade in a similar manner as under WW and MD regimes, which resulted in a crop value of $418 ha-1 in the hot 2013 vs. $1068 in 2014 under SD. More importantly, the G × Y interaction was not significant for yield, crop value and all grade factors, except ELK content (Table 3). This showed that the rainout shelter SD-induced water regime maximized genotypic effect clearly sorting out genotypes with highest stability across years. In average of the two years, the highest yielding genotypes under SD were GP-VT NC 01, GP-NC WS 17, Florida 07, Georgia 06G, and Sugg, with yields ranging from 3041 to 3892 kg/ha (Table 6). For the ELK content, even though the G × Y interaction was significant, Phillips and Sugg had the greatest ELK in both years, where Spain and Wynne had the least (Fig. 3). Similarly, Phillips and Sugg, along with Florida 07, Georgia 06G, GP-NC WS 17 and GP-VT NC 01 maintained the highest SMK under SD, while Spain and Wynne had the lowest. In both years, OK highest levels were observed for Bailey, GP-VT NC 01, N04074FCT and Wynne.
C18:1, C18:2, and total saturated fatty acids
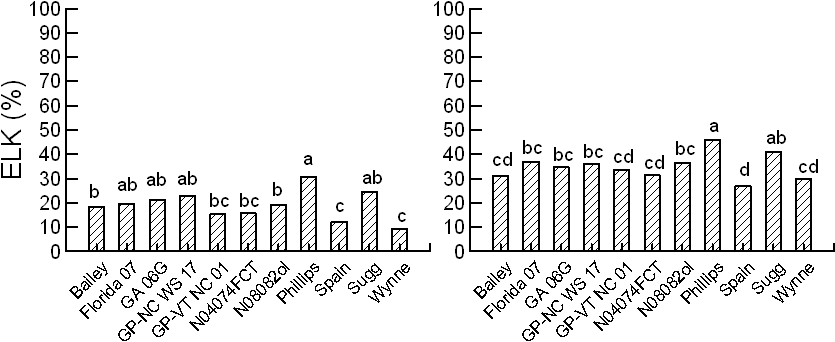
ANOVA from the farmer stock grade samples showed significant main effects of genotype, year, and water regime on the C18:1, C18:2 and total saturated fatty acids; no interactions of these factors were significant (Table 2). In the hotter environment, 2013 vs. 2014, C18:1 was significantly higher while the C18:2 and total saturated fatty acids were lower. Specifically, in average of genotypes and water regimes, C18:1 increased 4%, C18:2 decreased 4%, and saturated fatty acids decreased 0.5% in 2013 vs. 2014. When years were combined, variation of the oil profile for the farmer stock kernels due to genotype and water regime, i.e. only WW and SD water regimes were available for this analysis, is presented in Fig. 4. While DS reduced the C18:1 content in all genotypes, Spain and N08082ol showed a more drastic reduction than the other genotypes; they also showed greater increases of the C18:2 and total saturated oils under DS that the other genotypes (Fig. 4).
Effect of genotype and water regime on oil profile in peanut kernels. Years 2013 and 2014 are combined. Total saturated fatty acids are the sum of palmitic (C16:0), stearic (C18:0), arachidic (C20:0), behenic (C22:0), and lignoceric (C24:0) fatty acids. Within each genotype, significant changes among water regimes are marked with * for P ≤ 0.05, ** for P ≤ 0.01, and *** for P ≤ 0.001. The error bars are ± SD.
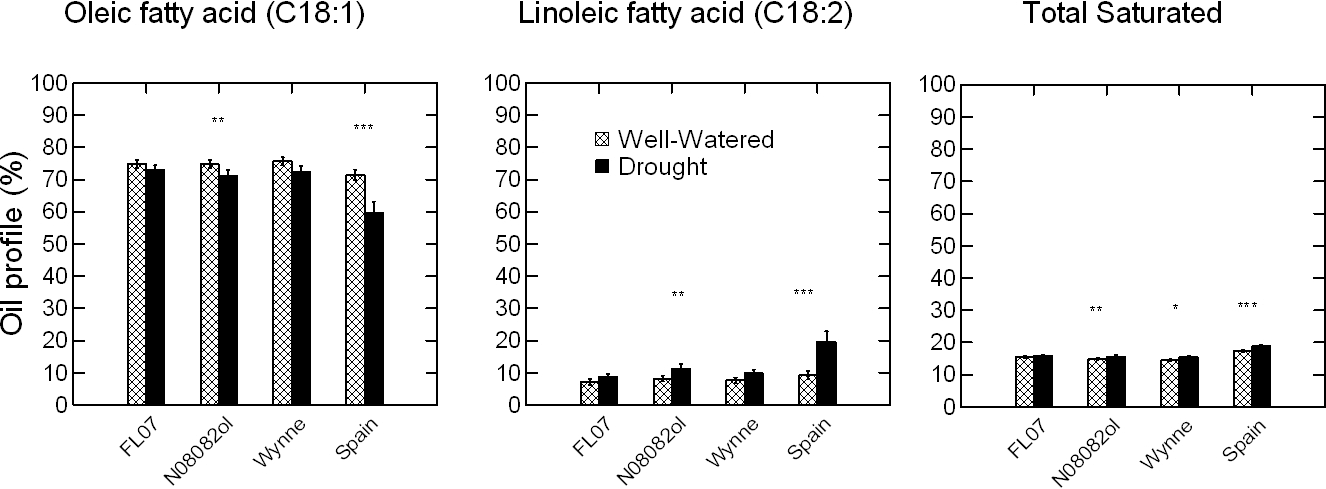
ANOVA for the effect of Y, W, G, and kernel size (K) on the oil profile of peanut kernels identified significant main effects of these factors on the C18:1, C18:2, and total saturated oils; the W × K, G × K, K × Y, G × W × K, and K × G × Y interactions were not significant with the exception of K × Y for the total saturated oils (Table 7). As anticipated, the C18:1 decreased, and C18:2 and total saturated oils increased with decreasing kernel size, under both WW and SD water regimes (Fig. 5). The K × Y interaction was significant because of dramatic increase of total saturated oils in response to DS in 2014 as compared to 2013. Implications of seed oil alteration from kernel size will be further discussed.
Effect of kernel size and water regime on oil profile in peanut kernels. Years 2013 and 2014 are combined. Total saturated is the sum of palmitic (C16:0), stearic (C18:0), arachidic (C20:0), behenic (C22:0), and lignoceric (C24:0) fatty acids. Within each kernel size, significant differences (P ≤ 0.05) were noted for the oleic, linoleic and total saturated fatty acids. Extra large kernels (ELK) are kernels that do not pass a 25.4 (1-in) × 8.5 mm (21.5/64-in) screen; mediums (Med) pass the larger screen but do not pass a 25.4 mm × 7.1 mm (18/64-in) screen; number 1's (No 1) pass the larger screens but do not pass a 25.4 × 5.9 mm (15/64-in) screen, and fall through (Fall) passing all screens. The error bars are ± SD.
Discussion
Under all water regimes, yield and grade characteristics of the peanut genotypes in this study were less in the hotter 2013 than in cooler 2014. For example, crop value, as an integrative measure of yield and grade, was 34% less under WW in 2013 vs. 2014, 57% under MD, and 61% under SD. Possible cause could be pollen sterility and disturbance of the reproductive process under high temperature, which was well documented in peanut (Prasad et al., 2003). As global temperature is expected to increase by 4 C by the end of this century (Wheeler and von Broun, 2013), our data suggest that heat stress, in addition to water scarcity, may challenge peanut production in the VC in the future; but further, more detailed research is needed to clarify this aspect.
In both years, water shortage decreased yield, ELK, SMK, TK, pod color, and C18:1; while DK, SS, C18:2 and total saturated oils were increased. Consequently, crop value was reduced. For example, in average of both years, yield was 19% less (4746 kg/ha) under MD and 54% less (2667 kg/ha) under SD than under WW regime (5822 kg/ha). While irrigated yields of the contest winners in Virginia were around 6000 kg/ha (D. Cotton, personal communication, 2018), state average from over 85% rainfed farms, in "rainy years", was around 4500 kg/ha; and in dry years, like 2010, average yield was around 2000 kg/ha (USDA, 2019a). This would seem to suggest that the rainout shelter setting used in this study provided comparable results with real farm peanut production in the region; and it could be used successfully in screening for peanut response to water deficit stress.
Regardless the year, MD and SD had negative impact on yield, grade characteristics, and C18:1 in all genotypes used in this study. This is an important aspect of peanut production, as farmers are paid not just by yield but a complex formula including all grade standards reported in this study (USDA, 2019b). Clearly, our results suggest that improving solely for yield is not sufficient for achieving positive economic impact in rainfed peanut production. Grade and quality are equally important for this commodity, and breeding should consider simultaneous improvement of yield and grade factors.
Currently, Bailey is the mostly grown virginia-type cultivar in the VC region. Sugg and Phillips were grown until recently. Since there is a push for growing "high oleic" cultivars in the VC region, and Bailey, Sugg and Phillips are not, future acres will be planted more with cultivars like Wynne, which was among the least resilient to soil moisture deficit in this study. Runners like Florida 07 and Georgia 06G, which here showed relatively good yield and grade under water deficit, are also grown in the VC region but only on reduced acreage; runners are not preferred by farmers and the local industries that evolved mainly around virginia-type peanut. Of the virginia-type cultivars, only Sugg was comparable for yield under MD and SD with the drought tolerant checks used in this study, GP-VT NC 01 and GP-NC WS 17. Sugg also maintained relatively high ELK and SMK under SD. Bailey and Phillips had comparable yields with the drought tolerant checks but only under MD but not SD, and grades were low under stress. This confirms the need for development of new cultivars with improved resilience to soil moisture extremes for the VC region. This task may be relatively easy achievable, as the mechanisms conferring high yield under MD, and high yield and grades under SD seem to have high heritability, i.e., the G × Y interaction was not significant for these traits under MD and SD regimes.
Physiological studies associated with drought tolerance have been done on some genotypes included in this study, either in laboratory or open field settings; and some agree with the responses to MD and SD described here. For example, Bailey, Sugg, and Phillips, were studied by Rosas-Anderson et al. (2014a, 2014b) and Shekoofa et al. (2015) in relation with low epidermal conductance, limited transpiration under high VPD, and fast transpiration recovery from severe soil drying as tolerance mechanisms to water deficit stress. They showed that Bailey seedlings, while having substantial recovery from one day exposure to severe soil drying, did not recover after prolonged stress in laboratory. Under field conditions, Bailey, Sugg and Phillips also showed rapid recovery of CO2 assimilation (A) and transpiration efficiency (TE), i.e., ratio of A and stomatal conductance, after moderate drought stress (Balota et al., 2012a, 2012b). Here, Bailey and Phillips had high yield under MD but low under SD; Sugg had relatively high yields under MD and SD. Balota et al. (2012a, 2012b) showed that N04074FCT and GP-VT NC 01, tested as line N05006, had high A under optimum soil moisture, which decreased under drought, but only GP-VT NC 01 returned to the original A levels after irrigation while maintaining relatively high TE. Similarly, N04074FCT did not recover from either short or prolonged dry conditions in laboratory testing; but GP-VT NC 01 maintained highest TE and A under ambient VPD, and had the greatest stomatal recovery in the field, along with GP-NC WS 17, tested as line SPT 06-07 by Rosas-Anderson et al. (2014a) and Shekoofa et al. (2015). In other studies, GP-NC WS 17 showed low epidermal conductance, and had minimal changes of the Fv/Fm ratio and metabolite levels in rainfed vs. irrigated plants and under heat stress (Rosas-Anderson et al, 2014b; Singh et al., 2014, 2016). GP-NC WS 17 line derived from interspecific hybridization with Arachis cardenasii Krapov. & W.C. Gregory. In 2014, GP-NC WS 17 was released as a germplasm line for multiple biotic and abiotic stress resistance (Tallury et al., 2014). In 2016, GP-VT NC 01 was released as germplasm with improved drought tolerance (Balota and Isleib, 2020). Here, N04074FCT had among the lowest yield and grade under MD and SD, and it was used as drought susceptible check. GP-VT NC 01 and GP-NC WS 17 had the greatest yield under MD and SD and were used as drought tolerant checks. Finally, Florida 07 and N08082olJCT were used as parents to develop 16 RIL populations for molecular genotyping (Holbrook et al., 2013). These genotypes were compared by Shekoofa et al. (2013) for the transpiration response to soil drying in laboratory. Conclusions from these authors showed that Florida 07 had faster response than N08082olJCT to soil drying, indicating possible better tolerance to water deficit stress. Here, both genotypes possessed some tolerance, showed as high pod yield under water deficit, but N08082olJCT had poor grades under SD, for which the value was among the lowest. In addition, under WW regime, N08082olJCT had the highest yield in the cool 2014, but not in the hot 2013; and, unlike Florida 07, it had a significant decrease of C18:1 under SD, for which N08082olJCT appears to be sensitive to high temperature stress. While genotypic differences for transpiration rate and other physiological traits were documented in peanut, finding the traits with significant contribution to improved yield and grade under water deficit in the field is still needed.
The C18:1 content was significantly higher in the hotter than in the cooler year under both water regimes, 78 vs. 75% (p=0.0003) in WW and 75 vs. 70 % (p=0.070) in SD. The C18:2 significantly decreased in 2013 vs. 2014 only under WW, and the total saturated increased only in SD. Changes in fatty acid composition and saturation levels under stress have been well studied in leaves and roots, but little in seeds (Alfonso et al., 2003; Liu and Huang, 2004; Yan et al., 2012). Recent work by Zhou et al. (2018) showed that high night temperature decreased the relative proportion of C18:1 and increased C18:2 in rape seeds. Similarly, for peanut seed from dry fields, decrease of C18:1 and increase of C18:2 is expected; but this is based on rather indirect than direct effect of water stress. Drought usually reduces seed development and increases the proportion of immature seeds in the seed sample, i.e., more mediums, number 1's, and fall through than ELK (Dang et al., 2013); and under-developed and immature seeds have less C18:1 content (Andersen and Gorbet, 2002; Hinds, 1995; Klevorn et al., 2016; Sanders et al., 1982). But it is not clear if drought can affect C18:1 directly. To test this, in this study, peanut seeds were grouped by size, i.e., ELK, mediums, number 1's, and fall through, and oil profile analysis was performed on each group individually. Both seed size and water deficit stress had significant effects on C18:1, C18:2 and saturated oils. In agreement with earlier studies, C18:1 decreased progressively from 76% in ELK to 74% in mediums, 70% in number 1's, and 65% in fall through, indicating that a sample with a higher proportion of mediums and number 1's will have less C18:1 than a sample with a higher proportion of ELK and mediums. At the same time, SD caused a significant decrease of C18:1, and increased C18:2 and total saturated, uniformly for all seed sizes, i.e. the interaction of G × K, W × K, G × W × K, and K × G × Y was not significant (Table 7). This shows that under water deficit stress, oil profile of peanut kernels may be affected directly, from drought, and indirectly, from increased proportion of immature seeds.
In conclusion, this study showed that soil moisture deficit negatively impacted peanut yield, grade and fatty acids with intensities depending on stress severity, year, and genotype; hot years appeared to exacerbate drought effect. Runner cultivars, Florida 07 and Georgia 06G were, indeed, more drought tolerant than virginia-type cultivars, in particular under SD. Among the virginia-type, Sugg had highest yields under SD but was out yielded by Bailey and Phillips when moisture stress was mild. Wynne and Spain have the largest kernels in this set; and they both performed poorest for yield and grade, confirming that larger kernels require more water to be filled. Finally, the rainout setting used in this study provided comparative results with real farm peanut production in the region; this is important for breeding programs in the VC region, where rain unpredictability does not allow for drought screening in an open field setting.
Acknowledgements
The author thanks Dr. Tom Isleib for providing seeds of the breeding lines. This work was supported with funding from the National Peanut Board, Virginia Peanut Board, and Virginia Crop Improvement Association.
Literature Cited
M.I., Alfonso, I Yruela, A Almarcegui, E Torrado, M.A Perez, and R Picorel (2001). Unusual tolerance to high temperatures in a new herbicide-resistant D1 mutant from Glycine max (L.) Merr. Cell structure deficient in fatty acid desaturation. Planta 212: 573- 582.
Andersen, P.C., and D. W Gorbet 2002 Influence of year and planting date on fatty acid chemistry of high oleic acid and normal peanut genotypes J. Agri Food Chem 50: 1298- 1305 doi:10.1021/jf0113171 .
Balota, M., 2011 Peanut Variety and Quality Evaluation results I: 2010 agronomic and grade data. Virginia Tech and Virginia Coop Ext. Publ. 3101-1523. 81 p. http://pubs.ext.vt.edu/3101/3101-1523/3101-1523.html
Balota, M., Isleib, T.G and Tallury, S., 2012 a. Variability for drought related traits of Virginia-type peanut cultivars and advanced breeding lines Crop Sci 52 (6): 2702- 2713.
Balota, M., McGrath, S., Isleib, T. G and Tallury, S., 2012 b. Transpiration response to vapor pressure deficit in field grown peanut Peanut Sci 39 (1): 53- 61.
Balota, M., Monfort, W. S and Isleib, T. G., 2013 Peanut Variety and Quality Evaluation results I: 2012 agronomic and grade data*. Virginia Tech and Virginia Coop Ext. Publ. AREC-32NP. 63 p. http://pubs.ext.vt.edu/AREC/AREC-32/AREC-32-PDF.pdf.
Balota, M., 2013 Agronomic recommendations and procedures In 2013 Peanut Production Guide Virginia Tech and Virginia Coop Ext. Publ. AREC-31NP. 112 p.
M., Balota, T.G Isleib (2020). Registration of GP-VT NC 01 peanut germplasm. J. Plant Registrations, https://doi.org/10.1002/plr2.20028 .
K.J Boote, (1982). Growth stages of peanut (Arachis hypogaea L.). Peanut Sci 9: 35- 40.
W. D Branch, (2007). Registration of 'Georgia-06G' peanut. J. Plant Registrations 2 ((1)): 120.
Branch, W., Balota, M., Baring, M., Bostick, J., Burow, M., Chapin, J., Chamberlain, K., Isleib, T. G., Tillman, B and Simpson, C., 2017 Uniform Peanut Performance Tests in 2016. Agronomic and grading factors University of Georgia, Coastal Plain Experiment Station, Research Progress Report No. 4-17 25 p.
P.M., Dang, C.Y Chen and C.C Holbrook (2013). Evaluation of five peanut (Arachis hypogaea) genotypes to identify drought responsive mechanisms utilizing candidate-gene approach. Functional Plant Biol 40: 1323- 1333.
P.I., Erickson, and D.L Ketring (1985). Evaluation of genotypes for resistance to water stress in situ. Crop Sci 48: 125- 133.
D.W., Gorbet, and B.L Tillman, (2009). Registration of 'Florida-07' peanut. J. Plant Registrations , 3 ((1)): 14- 18.
Hinds, M.J 1995. Fatty acid composition of Caribbean-grown peanuts (Arachis hypogaea L.) at three maturity stages Food Chem 53: 7- 14 doi:10.1016/0308-8146(95)95779-6 .
C.C., Holbrook, T.G Isleib, P Ozias-Akins, Y Chu, S.J Knapp, B Tillman, B Guo, R Gill, and M.D Burow (2013). Peanut Sci. 40: 89- 90.
M.H., Kapanigowda, W.A Payne, W.L Rooney, J.E Mullet, and M Balota (2014). Quantitative trait locus mapping of the transpiration ratio related to preflower drought tolerance in sorghum (Sorghum bicolor). Func. Plant. Biol 41: 1049- 1065 http://dx.doi.org/10.1071/FP13363
C.M., Klevorn, K.W Hendrix, T.H Sanders, and L.L Dean (2016). Differences in development of oleic and linoleic acid in high- and normal-oleic virginia and runner-type peanuts. Peanut Sci 43: 12- 23 doi.org/10.3146/0095-3679-43.1.12 .
T. G., Isleib, S. R Milla-Lewis, H.E Pattee, S.C Copeland, M.C Zuleta, B.B Shew, J.E Hollowell, T.H Sanders, L.O Dean, K.W Hendrix, M Balota, and J.W Chapin (2011). Registration of 'Bailey' peanut. J. Plant Registrations, 2011 , 5: 27- 39.
T. G., Isleib, P.W Rice, R.W.II Mozingo, S.C Copeland, J.B Graeber, H.E Pattee, T.H Sanders, R.W Mozingo, D.L Coker (2006). Registration of 'Phillips' peanut. Crop Sci 46: 2308- 2309.
Isleib, T. G., Milla-Lewis, S. R., Pattee, H. E., Copeland, S. C., Zuleta, M. C., Shew, B. B., Hollowell, J. E., Sanders, T. H., Dean, L. O., Hendrix, K. W., Balota, M., Chapin, J. W., and Monfort, W.S 2015 Registration of 'Sugg' peanut J. Plant Reg. 9: 44- 52 doi:10.3198/jpr2013.09.0059crc .
Z Liu, and B Huang (2004). Changes in fatty acid composition and saturation in leaves and roots of creeping bentgrass exposed to high soil temperature. J. Amer. Soc. Soc. Hort. Sci 129 ((6)): 795- 801.
R.W., Mozingo, S.F O'Keefe, T.H Sounders, and K.W Hendrix (2004). Improving shelf life of roasted and salted inshell peanuts using high oleic fatty acid chemistry. Peanut Sci 31: 40- 45.
P.V., Prasad, K.J Boote, L. A Hartwell, and J.M.G Thomas (2003). Super-optimal temperatures are detrimental to peanut (Arachis hypogaea L.) reproductive processes and yield at both ambient and elevated carbon dioxide. Global Change Biol 9: 1775- 1787.
Putnam, D. H., E. S Oplinger, T. M Teynor, E.A Oelke, K.A Kelling and J.D Doll 2014 Peanut alternative field crops manual http://www.hort.purdue.edu/newcrop/afcm/peanut.html (accessed Oct 2019).
T.H., Sanders, J.A Lansden, R.L Greene, J.S Drexler, and E.J Williams (1982). Oil characteristics of peanut fruit separated by a nondestructive maturity classification method. Peanut Sci 9: 20- 23.
T.Y., Reddy, V.R Reddy, and V Anbumozhi (2003). Physiological responses of groundnut (Arachis hypogea L.) to drought stress and its amelioration: a critical review. Plant Growth Regul 41: 75- 88.
Rosas-Anderson, P., Shekoofa, A., Sinclair, T.R., Balota, M., Isleib, T.G., Tallury, S and Rufty, T 2014 a Genetic variation in peanut leaf maintenance and transpiration recovery from severe soil drying Field Crops Res 158: 65- 72.
Rosas-Anderson, P., Sinclair, T.R., Balota, M., Tallury, S., Isleib, T.G and Rufty, T 2014 b Genetic variation for epidermal conductance in peanut Crop Sci 54 (2): 730- 737.
D.L., Rowland, W.H Faircloth, P Payton, D.T Tissue, J.A Ferrell, R.B Sorensen, and C.L Butts (2012). Primed acclimation of cultivated peanut (Arachis hypogaea L.) through the use of deficit irrigation times to crop developmental periods. Agric. Water Management 113: 85- 95.
A., Shekoofa, J.M Devi, T.R Sinclair, C.C Holbrook, and T.G Isleib (2013). Divergence in drought-resistance traits among parents of recombinant peanut inbred lines. Crop Sci 53: 2569- 2576.
A., SheKoofa, P., Rosas-Anderson, T.R., Sinclair, M Balota and T.JG Isleib, (2015). Measurement of limited-transpiration trait under high vapor pressure deficit for peanut in chambers and in field. Agron. J 107 ((3)): 1019- 1024.
D., Singh, M., Balota, T.G., Isleib, E Collakova, and G.E., Welbaum, (2014). Suitability of canopy temperature depression, specific leaf area, and SPAD chlorophyll reading for genotypic comparison of peanut grown in a sub-humid environment. Peanut Sci 41: 100- 110.
D., Singh, M Balota, E Collakova, T.G Isleib, G.E Welbaum, and S.P Tallury (2016). Heat Stress Related Physiological and Metabolic Traits in Peanut Seedlings. Peanut Sci 43: 24- 35.
S.P., Tallury, T.G Isleib, S.C Copeland, P Rosas-Anderson, M Balota, D Singh, and H.T Stalker (2001). Registration of two multiple disease-resistant peanut germplasm lines derived from, Arachis cardenasii Krapov. & W.C. Gregory, GKT 10017. J. Plant Registrations 8: 86- 89.
U.S. Department of Agriculture 2019 a. National Agricultural Statistics Service. Agricultural Statistics, U.S. Government Printing Office. Washington, DC. Retrieved 02/15, 2019, from https://quickstats.nass.usda.gov/ .
U.S. Department of Agriculture 2019 b. Agricultural Marketing Service. U.S. Government Printing Office. Washington, DC. Retrieved 10/23, 2019, from https://www.ams.usda.gov/sites/default/files/media/FarmersStockPeanutsInspectionInstructions.pdf
T., Wheeler, and J von Broun, (2013). Climate change impacts on global food security. Science 341: 508- 513.
P., Yan, C., Xu, L Xu, and B Huang (2012). Improved heat tolerance through drought precondition associated with changes in lipid composition, antioxidant enzymes, and protein expression in Kentucky bluegrass. Crop Sci 52: 807- 817.
L., Zhou, T Yan, X Chen, Z Li, D Li, D Wu, S Hua, and L Jiang (2018). Effect of night temperature on storage lipids and transcriptome changes in developing seeds of oilseed rape. J. Exp. Bot 69 ((7)): 1721- 1733.
Notes
- 1Associate Professor, Tidewater Agric. Res. & Ext. Ctr., Va. Polytechnic Inst. & State Univ., 6321 Holland Rd., Suffolk, VA 23437. [^] *Corresponding author Email: mbalota@vt.edu
Author Affiliations