Introduction
Rotating peanut with non-host crops such as cotton is a key component of a management system for nematodes and diseases for both crops (Rodriquez-Kabana and Ivey, 1986; Rodriquez-Kabana et al., 1987; 1991). In addition to agronomic and weed management benefits (York et al., 1994), crop rotation assists in disease management by reducing the initial inoculums (Sobers and Littrell, 1974). Volunteer peanut can emerge in crops planted in preceding season. Volunteer peanut harbor diseases that likely reduce the effectiveness of crop rotation and can interfere with cotton growth and production (York et al., 1994). The severity of infestation will depend on peanut lost at harvest, autumn or winter tillage programs, consumption by birds and other wildlife, winter moisture to help germinate and/or rot seed, and time of seedbed preparation for the rotational crop (York et al., 1994).
Preemergence (PRE) cotton herbicides are partially effective on planted peanut and the lack of volunteer peanut control with root and shoot-absorbed herbicides is probably due to their emergence from below the herbicide treated zone (York et al., 1994). Cotton herbicide registrations for bromoxynil, flumioxazin, glyphosate, glufosinate, and trifloxysulfuron (Askew et al., 2002; Blair et al., 2000; Culpepper and York, 1998; Reddy, 2001; Shaw and Arnold, 2002; Porterfield et al., 2002; Burke and Wilcut, 2004) have occurred since the work of York et al. (1994). However, evaluations of these herbicides for peanut response have not been conducted. Porterfield and Wilcut (2003) reported that trifloxysulfuron-sodium at 4 and 7 g ai/ha reduced peanut pod yield 73 and 98%, respectively, as compared with the nontreated check. In other work, pyrithiobac applied POST reduced peanut pod yield 8 to 22% (Jordan et al., 1993), whereas chlorimuron reduced peanut yield 18 to 27% (Sims et al., 1987). Also, new runner peanut cultivars have been released since 1992 and have been evaluated for response to peanut herbicides (Main et al. 2002, 2003; Richburg et al. 1995) but not cotton herbicides. Research to evaluate the response of Spanish peanut to cotton herbicides has not been conducted.
The objective of this study was to evaluate runner and Spanish peanut control with POST cotton herbicides, which may be used to control volunteer peanut in a peanut-cotton rotation. In order to evaluate cotton herbicides for volunteer peanut control, this study simulated volunteer peanut response using planted peanut.
Materials and Methods
Field studies were conducted from 2003 to 2005 at the Texas Agricultural Experiment Station research site near Yoakum in south-central Texas and at the Western Peanut Growers Research Farm (WPGRF) near Denver City. Soil type at Yoakum was a Hallettsville fine loamy sand (fine, montmorillonitic, thermic Udertic Paleustolls) with 1% organic matter and pH 7.0 and at WPGRF, the soil was a Brownfield fine sand (loamy, mixed, superactive, thermic Arenic Aridic Paleustalfs) with 0.1% organic matter, and pH 7.8. The experiments were conducted in conventionally planted peanut fields without cotton.
Spanish peanut was planted at the WPGRF location while runner type peanut was planted at the south Texas location (Yoakum). At the Yoakum location, runner peanut cultivars ‘Carver’, ‘OL01’, ‘Hull’, ‘Tamrun 96’, ‘Georgia 02C’, and ‘Georgia 01R’ were planted. At the WPGRF location, Spanish cultivar ‘Tamspan 90’ was planted. Peanut was planted at a rate of 100 kg/ha at each location. Spanish peanuts were planted April 29, 2003 and April 27, 2004 while at the Yoakum location, runner peanuts were planted June 22, 2004 and June 7, 2005.
Experimental design at Yoakum was a randomized complete block with treatments replicated three times. There was a factorial arrangement of cotton herbicides with six peanut cultivars. Plot size was two rows spaced 97 cm apart and 4 m long. Pendimethalin EC at 1.12 kg ai/ha was applied preplant incorporated to control annual grasses and broadleaf weed species.
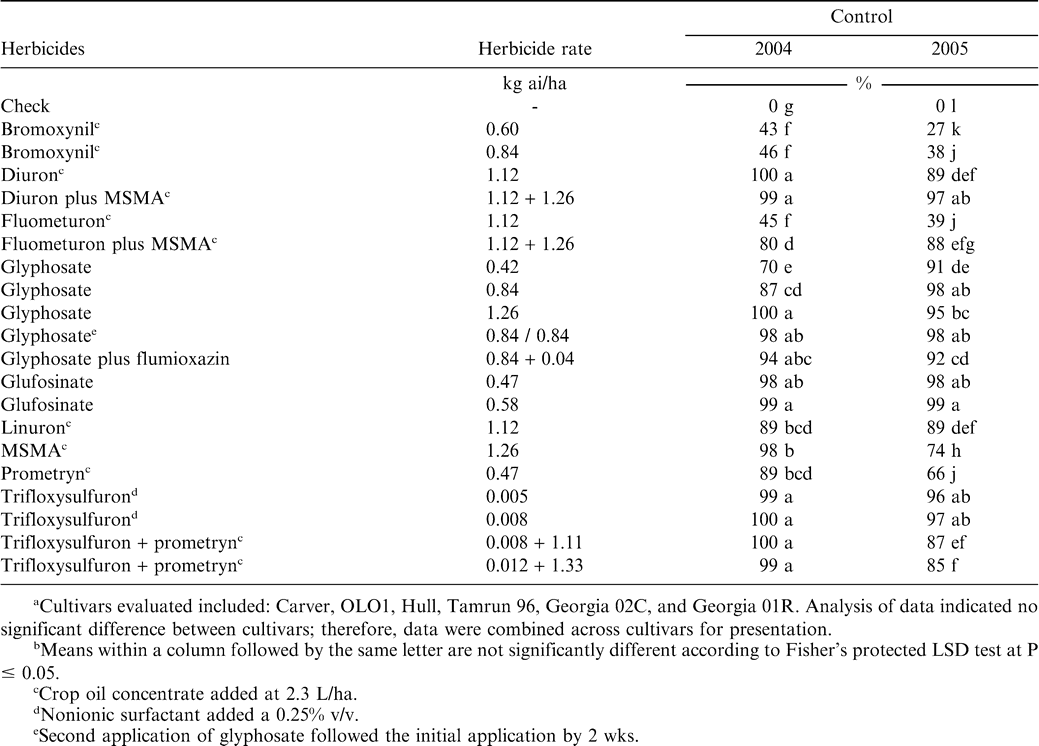
Treatments included bromoxynil at 0.6 and 0.84 kg ai/ha, diuron at 1.12 kg ai/ha, diuron at 1.12 kg ai/ha plus MSMA at 1.26 kg ai/ha, fluometuron at 1.12 kg ai/ha, fluometuron at 1.12 kg ai/ha plus MSMA at 1.26 kg ai/ha, glyphosate at 0.42, 0.84, and 1.26 kg ae/ha, glyphosate applied at 0.84 kg ae/ha followed by 0.84 kg/ha applied 2 wks later, glyphosate at 0.84 kg ae/ha plus flumioxazin at 0.04 kg ai/ha, glufosinate at 0.47 and 0.58 kg ai/ha, linuron at 1.12 kg ai/ha, MSMA at 1.26 kg ai/ha, prometryn at 0.47 kg ai/ha, and trifloxysulfuron at 5 and 8 g ai/ha, trifloxysulfuron at 8 g ai/ha plus prometryn at 1.11 kg ai/ha, and trifloxysulfuron at 12 g ai/ha plus prometryn at 1.33 kg ai/ha. A nontreated check was included for comparison. Glyphosate and glufosinate treatments did not include a surfactant, and trifloxysulfuron alone was applied with a nonionic surfactant (Induce, Helena Chemical Co., 6075 Poplar Ave., Memphis,TN 38137) at 0.25% v/v. All other herbicide treatments included a crop oil concentrate (Agridex, Helena Chemical Co.) at 2.3 L/ha.
Initial herbicide application in south Texas was to peanuts approximately 8 to 10 cm tall with the sequential glyphosate application approximately 2 wks later. Herbicides were applied with a compressed-air backpack sprayer equipped with Teejet 11002 DG flat fan spray tips (Spraying Systems Co., P.O. Box 7900, Wheaton, IL 60189) which delivered a spray volume of 187 L/ha at 180 kPa. In 2004, 122 mm of rainfall was received between peanut planting and herbicide application while in 2005 no rainfall was received between peanut planting and herbicide application. No supplemental irrigation was applied in either year.
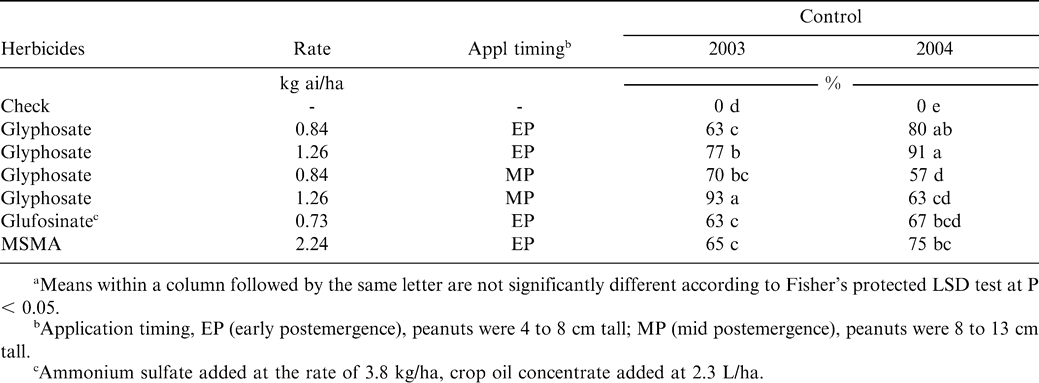
At the WPGRF location, the experimental design was a randomized complete block with three replications. Herbicides included glyphosate at 0.84 and 1.26 kg ae/ha applied early postemergence (early POST) and mid postemergence (mid POST), glufosinate at 0.73 kg ai/ha applied early POST, and MSMA at 2.24 kg ai/ha applied early POST. No surfactants were added to any glyphosate treatments while a crop oil concentrate (Agridex) was added to glufosinate at the rate of 2.3 L/ha. Ammonium sulfate at 3.8 kg/ha was also added to glufosinate. Plot size consisted of two rows spaced 97 cm apart and 7.6 m long. The initial applications (early POST) in 2003 and 2004 were to 4 cm and 8 cm tall peanuts, respectively, with subsequent applications (mid POST) when peanuts were 8 to 13 cm tall. Herbicides were applied with a compressed-air backpack sprayer equipped with Teejet 80015 Turbo Tee (TT) flat fan spray tips in a spray volume of 94 L/ha at 180 to 270 kPa depending on herbicide timing.
Peanut was not harvested for yield at either location. Visual estimates of peanut control (chlorosis, necrosis, stunting) were recorded approximately 4 and 8 wks after the initial herbicide application using a scale of 0 (no control) to 100% (complete control). Data were transformed to the arcsine square root before ANOVA by PROC GLM in SAS (2004); however, transformed data did not change the analysis and nontransformed means are presented. Data from the WPGRF location were subjected to analysis of variance and means were separated by Fisher's protected LSD at P ≤ 0.05. Data from the Yoakum location was subjected to ANOVA with partitioning appropriate for a twenty (cotton herbicide) by six (peanut cultivar) factorial arrangement. Means were separated with Fisher's Protected LSD test at the 0.05 level of probability. Analysis of peanut control did include the nontreated check.
Results and Discussion
It was assumed that the response of planted peanut to POST herbicides would be similar to the response of volunteer peanut (York et al. 1994), although emergence is more uniform in planted peanut. Since there was no peanut variety by cotton herbicide interaction for the south Texas location, all cultivar treatments were combined over herbicide treatments; however, years are presented separately due to a treatment by year interaction. For the WPGRF location, data are presented separately by years due to a treatment by year interaction. Since peanut control did not change drastically over the two rating dates, only the 4 wk after the initial herbicide application is presented.
South Texas
In 2004, bromoxynil and fluometuron failed to control peanut (< 50%) while glyphosate at 0.42 kg/ha provided only fair (70%) control (Table 1). Fluometuron plus MSMA controlled peanut 80% while glyphosate at 0.84 kg/ha, linuron, and prometryn controlled peanut 87 to 89%. All other herbicide treatments controlled peanut at least 94%. In 2005, bromoxynil, fluometuron, and prometryn failed to control peanut (27 to 66%). MSMA controlled peanut 74% while diuron, fluometuron plus MSMA, linuron, and trifloxysulfuron plus prometryn controlled peanut 85 to 89%. Diuron plus MSMA, all rates of glyphosate, glyphosate plus flumioxazin, glufosinate, and trifloxysulfuron controlled peanut 91 to 99%.
Bromoxynil has been evaluated in peanut (Grichar and Colburn, 1996). They reported no peanut injury but bromoxynil will cause peanut yellowing and stunting (authors personal observation). York et al. (1994) reported that fluometuron plus MSMA controlled peanut more effectively than MSMA alone. Our data showed inconsistent results. In 2004, MSMA alone controlled peanut better than fluometuron plus MSMA, while in 2005, fluometuron plus MSMA controlled peanut better than MSMA alone.
Peanut controlled by glufosinate was apparent 3 to 4 d after herbicide application and gradually increased over time (data not shown). Glyphosate can provide inconsistent peanut control (Baughman et al., 2001). In 2004, glyphosate at 0.42 and 0.84 kg/ha controlled peanut less than 90% while in 2005 control of peanut with glyphosate at these rates was at least 91%. Improved peanut control with glyphosate under drier conditions has been seen in previous years (Todd Baughman, personal communication). Also, York et al. (1994) reported that as the glyphosate rate increased peanut control generally increased.
Porterfield and Wilcut (2003) reported trifloxysulfuron applied POST at 3.75 to 7.5 g/ha controlled Virginia peanut similar to two applications of glyphosate at 1.12 kg/ha (York et al. 1994). Their data indicated that it was unlikely that trifloxysulfuron would be developed for use in peanut and their conclusion was that trifloxysulfuron applied POST would be an effective treatment for volunteer peanut control in cotton.
West Texas
All herbicides provided inconsistent Spanish peanut control (Table 2). In 2003, glyphosate at 0.84 kg/ha applied early-postemergence (EPOST) or mid-postemergence (MPOST) controlled peanut 63 to 70% while glyphosate at 1.26 kg/ha controlled peanut greater than 90% when applied MPOST but less than 80% when applied EPOST. Glufosinate and MSMA controlled peanut less than 70%. In 2004, glyphosate at 0.84 or 1.26 kg/ha applied EPOST controlled peanut 80 and 91%, respectively, while glyphosate applied MPOST controlled peanut less than 65%. Peanut control with MSMA was 75% but glufosinate controlled less than 70%. Initial herbicide applications were made when peanut was 4 to 8 cm tall with the subsequent application when peanut were 8 to 13 cm in height. York et al. (1994) reported poor control with glyphosate and attributed this to extreme moisture stress at the time of application. They also attributed inconsistent control to differences in peanut size at time of herbicide application and reported better control with glyphosate when applied late-POST.
In conclusion, the results of our study show that all peanut runner cultivars responded similar to the cotton herbicides. In south Texas, diuron plus MSMA, glyphosate at 0.84 kg/ha applied twice, glyphosate applied once at 1.26 kg/ha, glyphosate plus flumioxazin, glufosinate, and trifloxysulfuron controlled volunteer peanut at least 90%. Peanut response to glufosinate was quickest with injury within 3 d and complete peanut death within 5 to 7 d. In west Texas, results were inconsistent on Spanish peanut with all herbicides. While volunteer peanut and planted peanut behave differently with respect to time of emergence, growth, and development they are genetically the same and their response to POST cotton herbicides should be similar; therefore, these results should provide growers with the information to effectively control volunteer peanuts in cotton.
Acknowledgements
We gratefully acknowledge the Texas Peanut Producers Board for support of this research project.
Literature Cited
Askew S. D. , Wilcut J. W. , and Cranmer J. D. 2002 Cotton (Gossypium hirsutum) and weed response to flumioxazin applied preplant and postemergence directed. Weed Technol 16 : 184 – 190 .
Blair L. K. , Dotray P. A. , Keeling J. W. , Gannaway J. R. , Lyon L. L. , Quisenberry J. E. , and Oliver M. J. 2000 Crop tolerance and weed management in Liberty (glufosinate)-tolerant cotton. Proc. Beltwide Cotton Conf 2 : 1458 – 1459 .
Baughman T. A. , Porter B. L. , Grichar W. J. , Johnson W. C. , Dotray P. A. , Keeling J. W. , Karnei J. R. , Lemon R. G. , Besler B. A. , and Brewer K. D. 2001 Peanut tolerance to glyphosate spot applications. Proc. Amer. Peanut Res. Educ. Soc 33 : 62 (abstr.) .
Burke I. C. and Wilcut J. W. 2004 Weed management in cotton with CGA 362622, fluometuron, and pyrithiobac. Weed Technol 18 : 268 – 276 .
Culpepper A. S. and York A. C. 1998 Weed management in glyphosate-tolerant cotton. J. Cotton Sci 4 : 174 – 185 .
Grichar W. J. and Colburn A. E. 1996 Postemergence control of eclipta (Eclipta prostrate L.) in peanuts (Arachis hypogaea L.). Texas J. Agric. Nat. Resour 9 : 89 – 96 .
Jordan D. L. , Wilcut J. W. , and Richburg J. S. 1993 DPX-PE350 for weed control in peanut (Arachis hypogaea). Peanut Sci 20 : 97 – 101 .
Main C. L. , Tredaway-Ducar J. , and MacDonald G. E. 2002 Response of three runner market-type peanut cultivars to diclosulam. Weed Technol 16 : 593 – 596 .
Main C. L. , Tredaway-Ducar J. , and MacDonald G. E. 2003 Response of three runner-type peanut cultivars to flumioxazin. Weed Technol 17 : 89 – 93 .
Porterfield D. J. , Wilcut J. W. , and Askew S. D. 2002 Weed control with CGA-362622, fluometuron, and prometryn. Weed Sci 50 : 642 – 647 .
Porterfield D. and Wilcut J. W. 2003 Peanut (Arachis hypogaea L.) response to residual and in-season treatments of CGA-362622. Weed Technol 17 : 441 – 445 .
Reddy K. N. 2001 Broadleaf weed control in ultra narrow row bromoxynil-resistant cotton (Gossypium hirsutum). Weed Technol 15 : 497 – 504 .
Richburg J. S. , Wilcut J. W. , Culbreath A. K. , and Kvien C. K. 1995 Response of eight peanut (Arachis hypogaea) cultivars to the herbicide AC 263,222. Peanut Sci 22 : 76 – 80 .
Rodriquez-Kabana R. and Ivey H. 1986 Crop rotation systems for the management of Meloidogyne arenaria in peanut. Nematropica 16 : 53 – 63 .
Rodriquez-Kabana R. , Ivey H. , and Backman P. A. 1987 Peanut-cotton rotations for the management of Meloidogyne arenaria. J. Nematol 19 : 484 – 487 .
Rodriquez-Kabana R. , Robertson D. G. , Wells L. , Weaver C. F. , and King P. S. 1991 Cotton as a rotation crop for the management of Meloidogyne arenaria and Sclerotium rolfsii in peanut. J. Nematol 23 : 652 – 657 .
[SAS] Statistical Analysis Systems 2004 SAS/STAT User's Guide Release 9.0 Cary, NC Statistical Analysis Institute .
Shaw D. R. and Arnold J. C. 2002 Weed control from herbicide combinations with glyphosate. Weed Technol 16 : 1 – 6 .
Sims G. R. , Wehtje G. , McGuire J. A. , and Hicks T. V. 1987 Weed control and response of peanuts (Arachis hypogaea) to chlorimuron. Peanut Sci 14 : 42 – 45 .
Sobers E. K. and Littrell R. H. 1974 Pathogenicity of three species of Cylindrocladium to select hosts. Plant Dis. Reptr 58 : 1017 – 1019 .
York A. C. , Jordan D. L. , and Wilcut J. W. 1994 Peanut control in rotational crops. Peanut Sci 21 : 40 – 43 .